Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9727
Peer-review started: May 18, 2022
First decision: June 19, 2022
Revised: June 24, 2022
Accepted: August 14, 2022
Article in press: August 14, 2022
Published online: September 26, 2022
Processing time: 121 Days and 2.9 Hours
Dermatomyositis (DM) is a rare autoimmune disease involving the connective tissue. The association between DM and gastric cancer remains unclear. Patients with DM have an increased risk of cancer and higher mortality. It requires immu
Two cases of gastric cancer with DM as the first symptom in Zhongshan Hospital, Fudan University (Shanghai, China) were reported. Two patients had a typical skin rash. The rash in the first patient involved mainly bilateral upper limbs and neck, while the second patient manifested rash associated mainly with the face, neck, and back. Both manifested muscle weakness in the extremities and elevated serum creatine kinase. Radical resection of the tumor dramatically improved DM-related symptoms in the two patients. The literature review showed that gastric cancer is more commonly associated with DM in middle-aged and older male populations.
The findings suggest the need for comprehensive screening for malignant tumors in patients with DM refractory to long-term pharmacotherapy or hormone manip
Core Tip: Dermatomyositis is a rare autoimmune disease. Limited knowledge of the relationship between the disease and gastric cancer may complicate the diagnosis of gastric cancer. In this study, we report 2 cases of gastric cancer with dermatomyositis as the first symptom. A literature review was performed to further explore the relationship between dermatomyositis and gastric cancer.
- Citation: Sun XF, Gao XD, Shen KT. Treatment of gastric cancer with dermatomyositis as the initial symptom: Two case reports and review of literature. World J Clin Cases 2022; 10(27): 9727-9733
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9727.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9727
Dermatomyositis (DM) is a rare autoimmune disease involving the connective tissue. It is associated with inflammatory myopathy and a characteristic rash[1]. The incidence of DM is 0.6-1.0 per 100000[2]. Patients with DM have an increased risk of cancer and a higher mortality[3-5]. Immunosuppressive therapy, heightened surveillance, and immunologic response to internal malignancy are some of the options available[6]. Recent studies reported that the symptoms of DM are closely related to the progression of a malignant tumor, and effective treatment of the malignant tumor can alleviate the symptoms of DM[7]. This paper reports 2 cases of gastric cancer with DM as the first symptom.
Case 1: A 67-year-old female complained about persistent rash in her bilateral upper limbs for 2 mo.
Case 2: A 67-year-old female had systemic skin erythema (purplish-red edematous erythema) for 1 year.
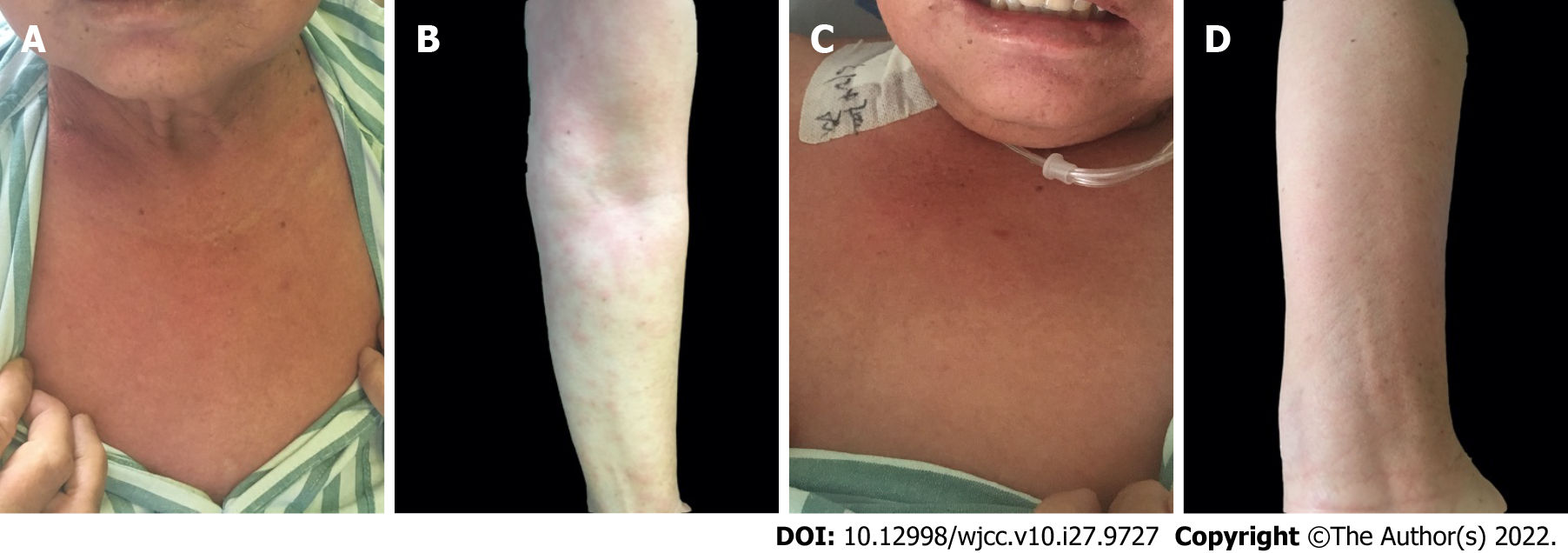
Case 1: The rash then progressed to her neck and bilateral lower limbs (Figure 1A and B).
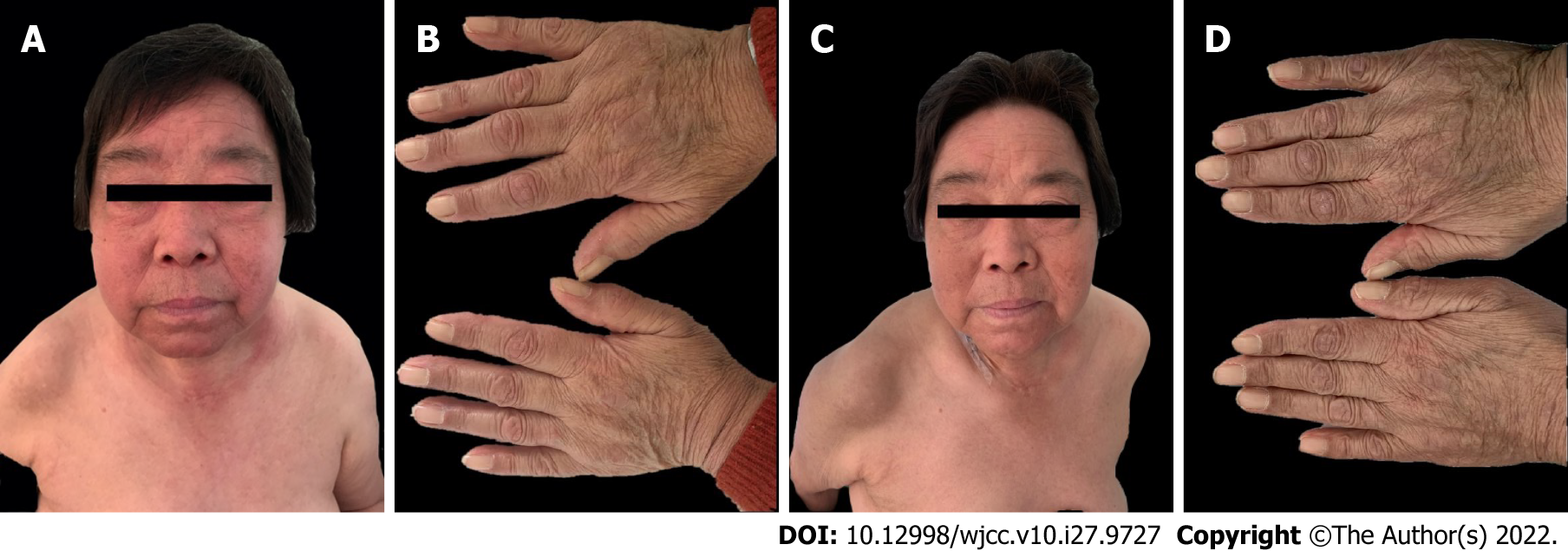
Case 2: The patient had systemic skin erythema (purplish-red edematous erythema) on both eyelids and patchy rash on the face, neck, abdomen, and limbs for 1 year (Figure 2A and B).
Case 1: Her major medical history was a 5-year history of hypertension.
Case 2: Her major medical history was a 10-year history of hypertension.
All patients had no special personal and family history.
Case 1: Significant findings of the physical examinations were V-neck sign (+), muscle soreness, and weakness in the extremities (Figure 1A and B).
Case 2: Significant findings of the physical examinations were shoulder shawl sign (+), V-neck sign (+), Gottron’s papule (+), muscle soreness, and weakness in the extremities (Figure 2A and B).
Case 1: Laboratory tests showed that the anti-nuclear antibodies were negative. The levels of C-reactive protein, erythrocyte sedimentation rate, complement 3, and complement 4 were in the normal range. The following serum indicators were markedly elevated, including creatine kinase (CK) (344 U/L, normal: 26-140 U/L), CK-MB (30 U/L, normal: 0-23U/L), CK-MM (314 U/L, normal:16-140 U/L), aspartate aminotransferase (40 U/L, normal: 13-35 U/L), and lactate dehydrogenase (441 U/L, normal: 109-245 U/L).
Case 2: Laboratory examination revealed normal ranges of serum autoimmune disease-related markers and CK. Blood examination showed increased levels of tumor markers: carbohydrate antigen-199 (68.0 U/mL, normal range < 34 U/mL) and carbohydrate antigen-724 (27.7 U/mL, normal range < 10.0U/mL).
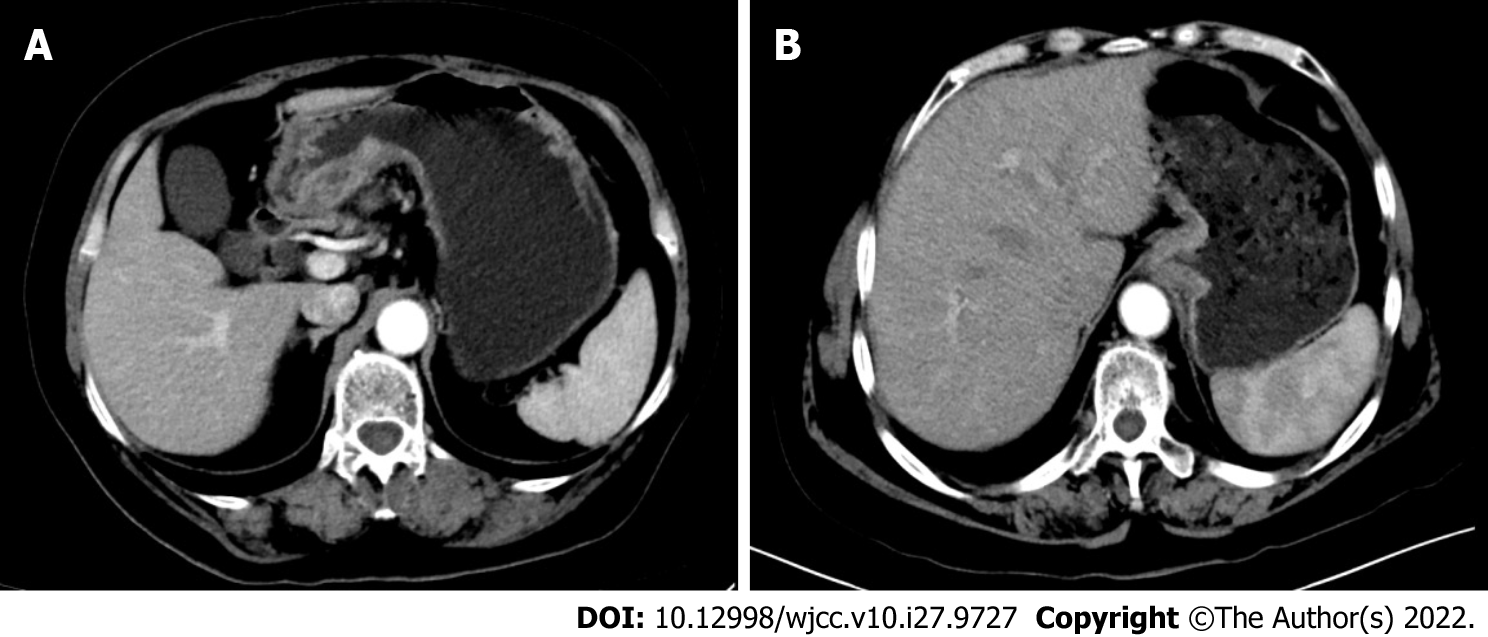
Case 1: Electronic gastroscopy showed huge irregular ulcers about 5 cm × 4 cm in the gastric corner, and biopsy revealed adenocarcinoma. An enhanced computed tomography (CT) scan of the abdomen and pelvis showed that the gastric wall at the antrum was clearly thickened and enhanced in the arterial phase (Figure 3A).
Case 2: Electromyography showed myogenic muscle damage. Endoscopic examination showed mucosal eminence of 2 cm × 2 cm in the lower esophagus and cardia. An enhanced CT scan of the abdomen and pelvis showed thickening of the wall of the gastric cardia, involving the lower esophagus, and enlarged lymph nodes were found in the lesser curvature of the stomach (Figure 3B).
The main diagnosis was gastric cancer and DM.
Systemic glucocorticoid therapy was administered with hydrocortisone oral prednisolone acetate tablets (30 mg, qd, po), followed by hydroxychloroquine sulfate (100 mg, tid, po). However, no alleviation of the symptoms was observed. After 3 mo, the patient was hospitalized in Wuxi No. 2 People’s Hospital for epigastric discomfort. To facilitate the adjustment of drug dosage and suppress autoimmunity temporarily, hydrocortisone sodium succinate (100 mg, qd, ivgtt) was used to replace prednisone for 3 d in the preoperative period, without symptom alleviation. The patient underwent radical distal gastrectomy plus D2 lymphadenectomy in our hospital. Postoperative pathological examination confirmed pT3N1M0 (stage III B) gastric adenocarcinoma.
Prednisone was orally administered (10 mg, qd, po) combined with azathioprine (50 mg, bid, po) and hydroxychloroquine (100 mg, bid, po). The rash was slightly alleviated; however, muscle soreness and weakness did not improve. After 2 mo, the patient was hospitalized because of epigastric discomfort. Similar to the first case, hydrocortisone sodium succinate (100 mg, qd, ivgtt) was used to replace prednisone during the preoperative period. The patient underwent radical distal gastrectomy combined with D2 lymphadenectomy in our hospital. Postoperative pathological examination confirmed pT4aN3M0 (stage III B) gastric adenocarcinoma.
To our surprise, the rash on the arms disappeared dramatically on the 2nd day after the operation (Figure 1C and D). The constellation of signs and symptoms was thought to constitute a paraneoplastic syndrome associated with gastric cancer. The patient was followed up for 54 mo, and no recurrence of the rash or tumor was observed.
A systematic search of PubMed and Embase was performed. We listed 15 reports published from 2000 to date in Table 1[8-22]. We summarized the clinical characteristics of a total of 18 patients including 14 males and 4 females to assess the biological behavior of DM associated with gastric cancer. The 18 cases included 16 reported cases and 2 of our cases. The median age of patients was 60 (51-90) years. The diagnosis of gastric cancer in 16 cases was later than that of DM, while in 1 case it was before and in another case it was concomitant with DM. In 16 cases of gastric cancer with DM as the first symptom, the average time interval between the diagnosis of gastric cancer and DM was 11 mo. The diagnostic methods include endoscopy, CT, and positron emission tomography/CT. Among the total of 18 patients, 5 (27.8%) developed metastasis and 6 (33.3%) died.
| Ref. | Case, n | Sex | Age | Cancer diagnosis | Diagnostic method | Interval time (mo) | Metastasis | Prognosis |
| Our cases | 2 | F | 67 | After | Endoscopy | 2 | No | Survival |
| F | 67 | After | Endoscopy | 12 | No | Survival | ||
| Pozharashka et al[8], 2020 | 1 | M | 58 | After | CT | 3 | Yes | Death |
| Li et al[9], 2020 | 1 | M | 51 | After | PET/CT | NA | Yes | NA |
| Shibata et al[10], 2019 | 1 | M | 71 | Before | CT | 9 | Yes | Death |
| Nishikawa et al[11], 2016 | 1 | M | 70 | Same time | CT | 0 | Yes | Death |
| Ge et al[12], 2014 | 2 | M | 57 | After | Endoscopy | 1 | No | Survival |
| M | 66 | After | Endoscopy | 12 | No | Survival | ||
| Yamaoka et al[13], 2014 | 1 | F | 64 | After | CT | 1 | Yes | Death |
| Ito et al[14], 2013 | 1 | M | 59 | After | CT | 1 | No | Survival |
| Nogi et al[15], 2012 | 1 | F | 59 | After | Endoscopy | 2 | No | Survival |
| Asadi et al[16], 2010 | 1 | M | 58 | After | Endoscopy | 1 | No | Survival |
| Kim et al[17], 2009 | 1 | M | 90 | After | Endoscopy | 60 | No | Death |
| Castro et al[18], 2008 | 1 | M | 60 | After | PET/CT | 12 | No | Death |
| Sugihara et al[19], 2002 | 1 | M | 60 | After | Endoscopy | 2 | No | Survival |
| Nakaya et al[20], 2002 | 1 | M | 53 | After | Endoscopy | 56 | No | Survival |
| Tonouchi et al[21], 2001 | 1 | M | 62 | After | NA | 5 | No | Survival |
| Yamashita et al[22], 2001 | 1 | M | 59 | After | Endoscopy | 2 | No | Survival |
The mechanism of DM is not clear and is generally believed to be related to heredity and viral infection. However, a strong correlation with a variety of malignant tumors has been reported, including ovary, lung, pancreas, stomach, colorectal cancer, and non-Hodgkin’s lymphoma[23-26]. DM is generally a chronic disease associated with low mortality. The clinical manifestations of DM include typical rash, such as Gottron macular papule and dark purplish-red rash on the eyelids. Muscle discomfort is also the main manifestation of DM[27,28]. However, when DM is complicated with gastric cancer, it is often associated with tumor metastasis and poor prognosis. A total of 18 patients were analyzed in our study, of which 5 had distant metastasis and 6 died. According to the diagnostic criteria of polymyositis and DM[2,29,30], sensitive laboratory indices such as blood parameters, including autoantibodies and CK, changes in electromyography, and biopsy of diseased muscles are all helpful for the diagnosis of DM.
In this case report, 2 patients manifested typical skin rash. The first patient mainly involved bilateral upper limbs and neck, while the second patient showed rash mainly in the face, neck, and back. Both experienced muscle weakness in the extremities and elevated serum CK. However, no muscle biopsy was performed due to invasiveness.
According to previous studies, the diagnostic sequence of tumors and DM is uncertain. Tumors can be detected earlier or at the same time as DM[10,11]. Differential diagnosis of tumors is important for those diagnosed earlier than DM. The case study[10] involved a 71-year-old Japanese man who developed clinical symptoms such as muscle pain and weak myositis after receiving nivolumab for gastric cancer, so the patient was initially diagnosed with nivolumab-induced myositis. However, based on his serum markers, electromyography, and magnetic resonance imaging, he was eventually diagnosed with paraneoplastic DM. The symptoms were gradually improved after treatment with intravenous corticosteroids, immunoglobulin injection, and tacrolimus. However, 142 d after taking the medicine, he died of rapid deterioration of gastric cancer. The differential diagnosis of paraneoplastic DM or drug-associated myositis caused by immune checkpoint inhibitors is essential because it determines the need for immunosuppressive therapy for autoimmune diseases and the appropriateness of continuing immunosuppressive therapy for primary cancer.
The diagnosis of tumors in most studies (88.9%) was later than that of DM, and the average time interval between the diagnosis of gastric cancer and DM was 11 mo, which often led to a missed tumor diagnosis. These 2 patients were not treated for a long time, which may have led to tumor progression. Paraneoplastic DM can be regarded as an early manifestation of gastric cancer, and tumor screening can be performed by gastroscopy, CT, and positron emission tomography/CT. Therefore, a high index of clinical suspicion is needed to detect malignancies in patients with DM.
However, the exact mechanism of the association between DM and gastric cancer is unknown. Further analysis of such cases is required to elucidate the underlying mechanism.
Although DM associated with gastric cancer is extremely rare, patients with DM require comprehensive screening for malignant tumors if long-term pharmacotherapy or hormone manipulation is ineffective. Patients diagnosed with DM and gastric cancer should be treated for cancer first, which can improve the symptoms of DM and avoid tumor progression.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gálvez Salazar P, Ecuador; Kamran M, Pakistan S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yan JP
| 1. | Kamperman RG, van der Kooi AJ, de Visser M, Aronica E, Raaphorst J. Pathophysiological Mechanisms and Treatment of Dermatomyositis and Immune Mediated Necrotizing Myopathies: A Focused Review. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1000] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 3. | Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, Evans SR, Felson DT. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 675] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 4. | Song M, Latorre G, Ivanovic-Zuvic D, Camargo MC, Rabkin CS. Autoimmune Diseases and Gastric Cancer Risk: A Systematic Review and Meta-Analysis. Cancer Res Treat. 2019;51:841-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Dani L, Ian Che W, Lundberg IE, Hellgren K, Holmqvist M. Overall and site-specific cancer before and after diagnosis of idiopathic inflammatory myopathies: A nationwide study 2002-2016. Semin Arthritis Rheum. 2021;51:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Leatham H, Schadt C, Chisolm S, Fretwell D, Chung L, Callen JP, Fiorentino D. Evidence supports blind screening for internal malignancy in dermatomyositis: Data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97:e9639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Chen LF, Chao CT, Chen CJ. Inflammatory Muscle Diseases. N Engl J Med. 2015;373:393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 8. | Pozharashka J, Dourmishev L, Rusinova D, Balabanova M, Miteva L. Paraneoplastic Dermatomyositis in a Patient with Metastatic Gastric Carcinoma. Acta Dermatovenerol Croat. 2020;28:120-122. [PubMed] |
| 9. | Li X, Tan H. Value of 18F-FDG PET/CT in the detection of occult malignancy in patients with dermatomyositis. Heliyon. 2020;6:e03707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Shibata C, Kato J, Toda N, Imai M, Fukumura Y, Arai J, Kurokawa K, Kondo M, Takagi K, Kojima K, Ohki T, Seki M, Yoshida M, Suzuki A, Tagawa K. Paraneoplastic dermatomyositis appearing after nivolumab therapy for gastric cancer: a case report. J Med Case Rep. 2019;13:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Nishikawa K, Matsuda C, Kawada J, Fujitani K, Endo S, Hirao M, Yamamoto K, Maeda S, Uemura M, Miyake M, Hama N, Miyamoto A, Ikeda M, Nakamori S, Sekimoto M. [A Case of Rectal Metastasis of Gastric Cancer Associated with Dermatomyositis]. Gan To Kagaku Ryoho. 2016;43:2401-2403. [PubMed] |
| 12. | Ge W, Teng BW, Yu DC, Chen G, Zheng LM, Ding YT. Dermatosis as the initial presentation of gastric cancer: two cases. Chin J Cancer Res. 2014;26:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Yamaoka T, Doi C, Yokomi A, Tanemura A, Murota H, Tani M, Saruban H, Hamaguchi Y, Fujimoto M, Katayama I. Anti-MDA5 antibody-positive dermatomyositis with lethal progressive interstitial lung disease and advanced gastric cancer. Eur J Dermatol. 2014;24:490-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Ito K, Imafuku S, Hamaguchi Y, Fujimoto M, Nakayama J. Case report of anti-transcription intermediary factor-1-γ/α antibody-positive dermatomyositis associated with gastric cancer and immunoglobulin G4-positive pulmonary inflammatory pseudotumor. J Dermatol. 2013;40:567-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Nogi S, Hashimoto A, Iwata K, Futami H, Takaoka H, Arinuma Y, Shimada K, Nakayama H, Matsui T, Komiya A, Furukawa H, Tamama S, Kinoshita S, Moriya H, Tohma S. [Case of immunosuppressant-resistant amyopathic dermatomyositis with rapidly progressive interstitial pneumonia ameliorated after resection of gastric cancer]. Nihon Rinsho Meneki Gakkai Kaishi. 2012;35:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Asadi M, Javanbakht M, Khajavian S, Zand S. Rapidly progressive myositis as the sole manifestation of a concurrent gastric carcinoma. BMJ Case Rep. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Kim SW, Kang YS, Park SH, Lee UH, Park HS, Jang SJ. A case of erythrodermic dermatomyositis associated with gastric cancer. Ann Dermatol. 2009;21:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 18. | Castro C, Khan Y, Awasum M, Belostocki K, Rosenblum G, Belilos E, Carsons S. Case report: primary gastric melanoma in a patient with dermatomyositis. Am J Med Sci. 2008;336:282-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Sugihara T, Imai Y, Sakurai T. [Case of hemophagocytic syndrome associated with active dermatomyositis]. Nihon Rinsho Meneki Gakkai Kaishi. 2002;25:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Nakaya I, Iwata Y, Sugiyama Y, Abe T, Nomura G. Dermatomyositis relapse complicated with gastric carcinoma and lupus nephritis five years after the initial diagnosis of dermatomyositis. Intern Med. 2002;41:502-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Tonouchi H, Miki C, Masato K. [Gastric cancer associated with dermatomyositis accompanied by photoallergy]. Gan To Kagaku Ryoho. 2001;28:689-691. [PubMed] |
| 22. | Yamashita K, Hosokawa M, Hirohashi S, Arimura Y, Endo T, Denno R, Ikeda T, Imai K. Epstein-Barr virus-associated gastric cancer in a patient with dermatomyositis. Intern Med. 2001;40:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Zádori N, Szakó L, Váncsa S, Vörhendi N, Oštarijaš E, Kiss S, Frim L, Hegyi P, Czimmer J. Six Autoimmune Disorders Are Associated With Increased Incidence of Gastric Cancer: A Systematic Review and Meta-Analysis of Half a Million Patients. Front Immunol. 2021;12:750533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Chen C, Chen Y, Huang Q, Hu Q, Hong X. Case Report: Rapidly Progressive Interstitial Lung Disease in A Pregnant Patient With Anti-Melanoma Differentiation-Associated Gene 5 Antibody-Positive Dermatomyositis. Front Immunol. 2021;12:625495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Tome J, Kamboj AK, Leggett CL. Dysphagia in Dermatomyositis Due to Esophageal Adenocarcinoma. Clin Gastroenterol Hepatol. 2022;20:A15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Bowerman K, Pearson DR, Okawa J, Werth VP. Malignancy in dermatomyositis: A retrospective study of 201 patients seen at the University of Pennsylvania. J Am Acad Dermatol. 2020;83:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | DeWane ME, Waldman R, Lu J. Dermatomyositis: Clinical features and pathogenesis. J Am Acad Dermatol. 2020;82:267-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 236] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 28. | Dalakas MC. Inflammatory disorders of muscle: progress in polymyositis, dermatomyositis and inclusion body myositis. Curr Opin Neurol. 2004;17:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Waldman R, DeWane ME, Lu J. Dermatomyositis: Diagnosis and treatment. J Am Acad Dermatol. 2020;82:283-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 30. | Sasaki H, Kohsaka H. Current diagnosis and treatment of polymyositis and dermatomyositis. Mod Rheumatol. 2018;28:913-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |