Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9680
Peer-review started: April 6, 2022
First decision: June 16, 2022
Revised: July 14, 2022
Accepted: August 15, 2022
Article in press: August 15, 2022
Published online: September 26, 2022
Processing time: 157 Days and 16.7 Hours
Heart rate variability (HRV) and pulse-wave velocity (PWV), indicators of cardiac function, are altered in patients with spinal cord injury (SCI), suggesting that autonomic cardiac function and arterial stiffness may underlie the high risk of cardiovascular complications in these patients. No study has simultaneously investigated HRV and PWV in the same patients.
To evaluate cardiovascular complications in SCI patients by comparing HRV and PWV between patients with and without SCI.
In this cross-sectional pilot study, patients with (n = 60) and without SCI (n = 60) were recruited from December 7, 2019 to January 21, 2020. Each participant received a five-minute assessment of HRV and the cardiovascular system using the Medicore HRV Analyzer SA-3000P. Differences in HRV and PWV parameters between participants with and without SCI were statistically examined.
We observed a significant difference between participants with and without SCI with respect to the standard deviation of all normal-to-normal intervals, square root of the mean sum of squared successive risk ratio interval differences, physical stress index, total power, very-low frequency, low frequency, high frequency, and arterial elasticity.
Patients with SCI have weaker sympathetic and parasympathetic activity as well as lower arterial elasticity compared to those without, suggesting that SCI may increase cardiac function loading.
Core Tip: Noninvasive, simultaneous assessment of heart rate variability and pulse-wave velocity showed that patients with spinal cord injury have weaker sympathetic and parasympathetic activity and lower arterial elasticity compared to those without. These findings indicate that increased cardiac function loading may underlie the high risk of cardiovascular complications in patients with spinal cord injury. Continuous dynamic monitoring of heart rate variability and pulse-wave velocity could be integrated into care programs for spinal cord injury patients to inform the development of measures to reduce stress and increase vitality.
- Citation: Tsou HK, Shih KC, Lin YC, Li YM, Chen HY. Altered heart rate variability and pulse-wave velocity after spinal cord injury. World J Clin Cases 2022; 10(27): 9680-9692
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9680.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9680
Spinal cord injury (SCI) that causes total or partial disability is a catastrophic event that often leads to multiple complications[1,2]. Patients with high-level SCI commonly have sympathetic nervous system hypofunction, resulting in cardiovascular complications including hypotension, cardiac dysrhythmias, orthostatic hypotension, and autonomic dysreflexia[3,4]. As a result, coronary artery disease is the leading cause of death among people with SCI[5]. Over a decade of research on the effects of SCI on the cardiovascular system[6-8] has revealed a clear correlation between cardiovascular disorders and SCI. However, the physiological mechanisms underlying cardiovascular disorders associated with SCI vary between patients, and their elucidation requires further investigation[9]. Numerous recent studies of cardiac function in SCI patients have investigated heart rate variability (HRV), the change in time between heartbeats. The complex oscillations of the heart allow the cardiovascular system to rapidly adjust to sudden physical and psychological challenges to homeostasis[10]. HRV is an indicator of cardiac autonomic activity, as cardiac function is directly related to sympathetic and parasympathetic nervous system activity[11]. Measurable HRV parameters are divided into frequency and time domains. Frequency domain analysis separates HRV into its component rhythms that operate within different frequency ranges: ultra-low frequency (ULF), very-low frequency (VLF), low frequency (LF), and high frequency (HF)[12,13]. The ratio of LF to HF power (LF/HF ratio) estimates the ratio sympathetic to parasympathetic nervous system activity, and the total power (TP) is the sum of the energy in the VLF, LF, and HF bands in short-term recordings[12]. Time domain parameters include the mean normal-to-normal (NN) intervals during the entire recording and the standard deviation between NN intervals (SDNN), which reflects the ebb and flow of all the factors that contribute to heart rate variability[12]. The root mean square of successive differences between normal heartbeats (RMMSD) reflects the beat-to-beat variance in heart rate and is used to estimate the vagally-mediated changes reflected in HRV[12]. These HRV parameters have been investigated in SCI patients to better understand the factors underlying the association between SCI and cardiovascular complications.
Compared to able-bodied control subjects, patients with cervical SCI were found to have reduced supine and upright power in the LF range, while those with thoracic SCI had reduced supine HF power[8]. Compared to subjects without SCI, thoracic SCI patients had altered HRV parameters, including the SDNN, TP, VLF power, and LF power[14]. In another study evaluating changes in HRV and skin perfusion in response to postural changes, no changes were found in individuals with SCI, while healthy individuals exhibited significant changes in the sympathovagal balance[15]; these observations suggest SCI-induced impairment of microvascular function. In addition, the higher level of neurological impairment in patients with SCI suggests reduced sympathetic activity due to disordered cardio
Pulse-wave velocity (PWV), the velocity of the blood pressure wave as it travels between 2 sites within the arterial system, is a measure of arterial stiffness[18]. This parameter can be assessed noninvasively using acceleration plethysmography. PWV was first used to evaluate cardiac function in SCI patients 2009[5] and since has been widely used to evaluate arterial parameters in SCI patients[19-21]. In particular, significant elevations in PWV appear to indicate greater cardiovascular risk in patients with cervical and thoracic SCI[21]. However, to the best of our knowledge, HRV and PWV have not been evaluated simultaneously in individuals with SCI. Therefore, this cross-sectional study aimed to measure both HRV and PWV at the same time in people with and without SCI to comprehensively assess the influence of SCI on cardiac function and arterial stiffness.
This cross-sectional study recruited patients diagnosed with SCI (SCI group) and those without history of neurological injury (non-SCI group) between December 7, 2019 and January 21, 2020. Participants in the non-SCI group were patients admitted to the study hospital for causes other than neurological injury. Inclusion criteria for all participants, regardless of group, were age 20–80 years and without bipolar disorder or psychosis. Only patients with SCI who were able to maintain basic life functions independently or with the assistance of a caregiver 6 mo after SCI were included. Patients with SCI with severe spasticity or brain trauma or who were undergoing mechanical ventilation or oxygen therapy were excluded.
The study protocol was approved by the Institutional Review Board of Taichung Jen-Ai Hospital (JAHIRB-108-73). All participants provided signed informed consent prior to participating in the study.
All participants were first asked to complete a questionnaire to provide their demographic and clinical characteristics, as described previously[22]. Subsequently, the HRV and PWV of each participant were measured simultaneously using the Medicore HRV Analyzer SA-3000P (Medicore, Seoul, Korea) according to the manufacturer’s instructions [23] in a room that was maintained with bright indoor lighting, no external noise, and a comfortable ambient temperature (20 °C–25 °C). Participants were instructed to avoid consuming coffee, tea, alcohol, and other potentially irritating foods for 2 h before the measurement and not to wear any metal objects during the measurement. A fingertip sensor was attached to the participant’s left index finger, held at the same height as the heart. Participants were instructed to sit upright in a chair or wheelchair with backrest, to avoid closing their eyes or falling asleep, and to maintain normal breathing throughout the 5-minute measurement period.
The SA-3000P analyzer measures time and frequency domain HRV parameters[23]. Time domain parameters assessed include the mean HRT over a given time period, SDNN, RMSSD (which reflects parasympathetic regulation of the heart), and the physical stress index (PSI). Frequency domain parameters included TP (< 0.4 Hz) (which reflects sympathetic nervous system activity), VLF (0.0033 – 0.04 Hz), LF (0.04 – 0.15 Hz), and HF (0.15–0.4 Hz). Frequency domain parameters assessed include the LF norm [calculated as LF/ (TP−VLF) × 100], HF norm [calculated as HF/(TP−VLF) × 100], and LF/HF ratio, as previously described[23].
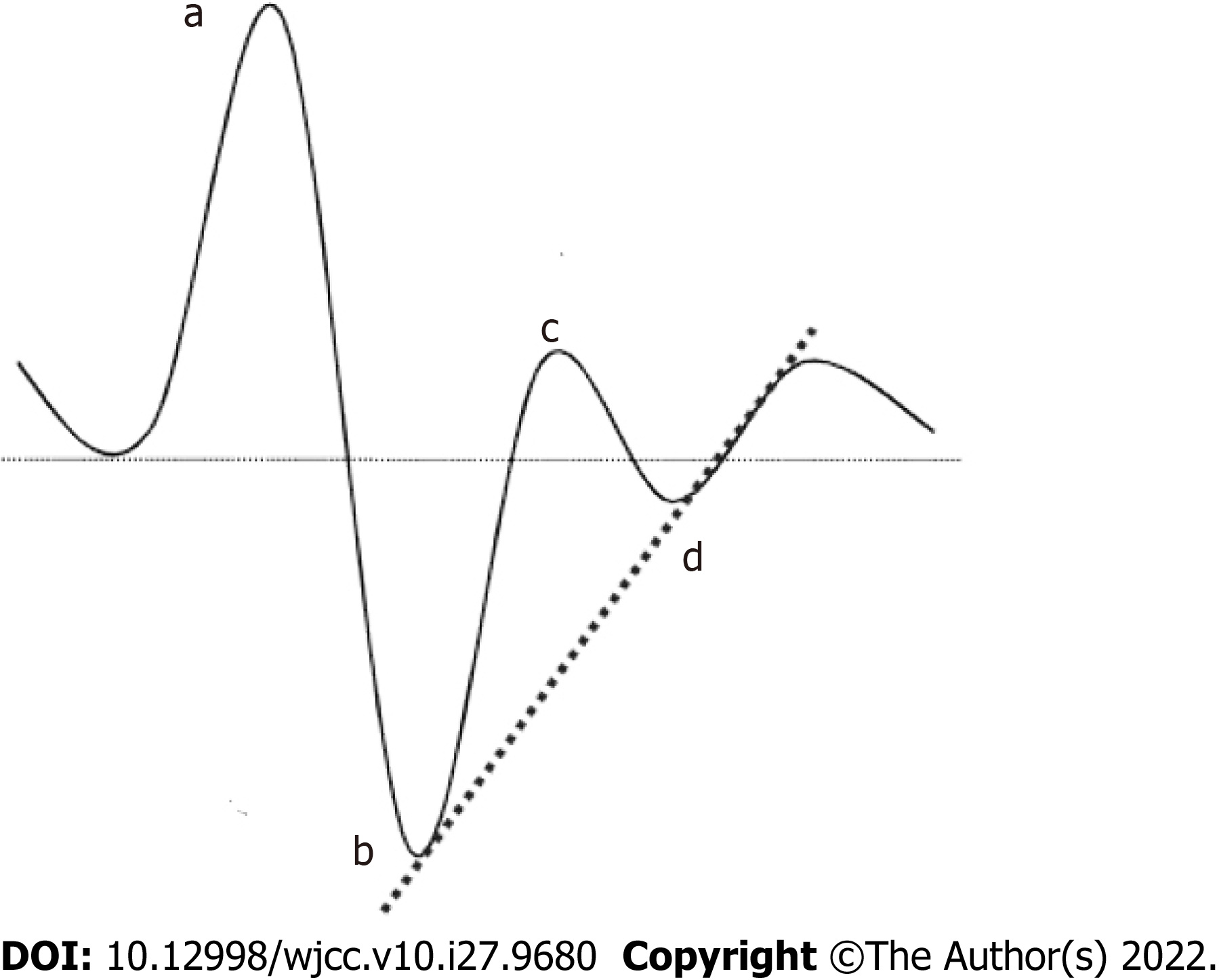
The SA-3000P analyzer performs acceleration plethysmography to measure PWV, which reflects arterial elasticity[23]. The pulse waves generated by cardiac constriction travel to different parts of the body at different rates. Infrared sensors detect these differences in the rate of arterial pulse wave travel (Figure 1). Increases in PWV reflect greater arterial wall thickness and stiffness[23]. Parameters calculated from PWV include the differential pulse wave index (DPI) [calculated as (b−c−d)/a], eccentric constriction power (EC) (b/a), arterial elasticity (AE) (c/a), and remaining blood volume (RBV) (d/a) (Figure 1)[23]. In addition, vascular age was estimated based on the wave pattern detected, with each of 7 wave patterns corresponding to an arterial age level (1–7)[20]. Only the number of patients with each type of wave pattern was determined in this study; no actual ‘vascular age’ can be calculated using the SA-3000P data.
The SA-3000P analyzer converted the original data files to CSV format, which then were imported into SPSS 24.0 (IBM Corp., Armonk, NY, United States) for all statistical analyses. Descriptive statistics of the study population were performed. Continuous variables are expressed as the mean ± SD, and categorical variables are presented as n (%). For normally distributed data, the t-test was conducted to examine differences between groups. Non–normally-distributed data were analyzed using Fisher’s exact test and the Mann–Whitney U test. P < 0.05 was established as statistically significant.
A total of 120 participants [60 patients with SCI (SCI group) and 60 without (non-SCI group)] were included in this cross-sectional pilot study. The demographic and clinical characteristics of the two groups are presented in Table 1. The mean ages of the SCI and non-SCI groups were comparable (P = 0.09). The SCI group had 45 males and 15 females, and the non-SCI group had 15 males and 45 females. Regardless of SCI status, the majority of all participants (82.5%) had a high school, university, or graduate school diploma, worked as freelancers (30.83%), were married or cohabiting (50.83%), and practiced a combination of Taoism and Buddhism (40.00%). At least one comorbidity was present in 29 patients (48.3%) in the SCI group, including hypertension (20%), diabetes (8.3%), and pneumonia (6.7%) (Table 1). In contrast, 17 patients (28.3%) in the non-SCI group had at least one comorbidity, including hypertension (13.3%), asthma (5.0%), and hyperlipidemia (5.0%). In the SCI group, 36 patients (60.0%) were taking at least one medication, including a hypoglycemic, antihypertensive, and anticoagulant agents and others. In contrast, 11 patients (18.3%) in the non-SCI group were taking at least one medication, including hypoglycemic and antihypertensive agents and others (Table 1). The clinical characteristics of the SCI group are summarized in Table 2, in which patients with SCI were further divided into two subgroups: paraplegia (n = 30) and tetraplegia (n = 30). For all included patients with SCI, the mean SCI duration was 16.73 years, and the main cause of SCI was automobile accident (51.67%) (Table 2). Most patients had cervical spine injuries (47.30%), followed by thoracic spine injuries (31.08%), lumbar (20.27%), and sacral injuries (1.35%). The majority of patients with paraplegia had thoracic spine injuries (46.34%), while most patients with tetraplegia had cervical spine injuries (81.82%). Four patients had incomplete paraplegia, 26 patients had complete paraplegia, 22 patients had incomplete tetraplegia, and 8 patients had complete tetraplegia (Table 2).
| SCI (n = 60) | Non-SCI (n = 60) | P value | |
| Age, yr | 49.92 ± 11.61 | 45.93 ± 13.99 | 0.09 |
| Sex | |||
| Male | 45 (75.0%) | 15 (25.0%) | < 0.001a |
| Female | 15 (25.0%) | 45 (75.0%) | |
| Education level | |||
| Elementary school | 2 (3.3%) | 1 (1.7%) | < 0.001a |
| Junior high school | 6 (10.0%) | 0 (0.0%) | |
| Senior high/vocational school | 32 (53.3%) | 6 (10.0%) | |
| Five-year junior college | 6 (10.0%) | 6 (10.0%) | |
| University | 14 (23.3%) | 21 (35.0%) | |
| Graduate school | 0 (0.0%) | 26 (43.3%) | |
| Occupation | |||
| Freelancer | 37 (61.7%) | 0 (0.0%) | < 0.001a |
| Teacher | 1 (1.7%) | 17 (28.3%) | |
| Public servant | 1 (1.7%) | 15 (25.0%) | |
| Service industry | 4 (6.7%) | 10 (16.7%) | |
| Others | 17 (28.3%) | 18 (30.0%) | |
| Marital status | |||
| Unmarried | 27 (45.0%) | 20 (33.3%) | 0.01a |
| Married/cohabiting | 24 (40.0%) | 37 (61.7%) | |
| Divorced | 8 (13.3%) | 1 (1.7%) | |
| Widowed | 1 (1.7%) | 2 (3.3%) | |
| Religion | |||
| None | 13 (21.7%) | 22 (36.7%) | 0.14 |
| Taoism/Buddhism | 24 (40.0%) | 24 (40.0%) | |
| Christianity/Catholicism | 14 (23.3%) | 6 (10.0%) | |
| Folk belief | 9 (15.0%) | 8 (13.3%) | |
| Comorbidity | |||
| No | 31 (51.7%) | 43 (71.7%) | 0.04a |
| Yes | 29 (48.3%) | 17 (28.3%) | |
| Heart attack | 3 (5.0%) | 1 (1.7%) | 0.62 |
| Diabetes | 11 (8.3%) | 2 (3.3%) | 0.02a |
| Kidney disease | 2 (3.3%) | 2 (3.3%) | 1.00 |
| Pneumonia | 4 (6.7%) | 0 (0.0%) | 0.12 |
| Hypertension | 12 (20.0%) | 8 (13.3%) | 0.46 |
| Asthma | 1 (1.7%) | 3 (5.0%) | 0.62 |
| Hyperlipidemia | 0 (0.0%) | 3 (5.0%) | 0.24 |
| Malignant tumor | 0 (0.0%) | 1 (1.7%) | 1.00 |
| Medication | |||
| No | 24 (40.0%) | 49 (81.7%) | < 0.001a |
| Yes | 36 (60.0%) | 11 (18.3%) | |
| Hypoglycemic agent | 11 (18.3%) | 2 (3.3%) | 0.02a |
| Antihypertensive agent | 12 (20.0%) | 6 (10.0%) | 0.20 |
| Anticoagulant | 2 (3.3%) | 0 (0.0%) | 0.50 |
| Others | 21 (35.0%) | 5 (8.3%) | 0.001a |
| Variables | SCI patients (n = 60) | Subgroup | |
| Paraplegia (n = 30) | Tetraplegia (n = 30) | ||
| Age, yr | 49.92 ± 11.61 | 51.53 ± 12.39 | 48.30 ± 10.73 |
| Duration of injury, years | 16.73 ± 11.05 | 17.67 ± 12.96 | 15.80 ± 8.88 |
| Cause of injury | |||
| Car accident | 31 (51.67%) | 12 (40.00%) | 19 (63.33%) |
| Fall from high place | 11 (18.33%) | 5 (16.67%) | 6 (20.00%) |
| Sports injury | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Hit by a heavy object | 1 (1.67%) | 1 (3.33%) | 0 (0.00%) |
| Lesions | 6 (10.00%) | 4 (13.33%) | 2 (6.67%) |
| Other | 11 (18.33%) | 8 (26.67%) | 3 (10.00%) |
| Injured region | |||
| Cervical | 35 (47.30%) | 8 (19.51%) | 27 (81.82%) |
| Thoracic | 23 (31.08%) | 19 (46.34%) | 4 (12.12%) |
| Lumbar | 15 (20.27%) | 13 (13.71%) | 2 (6.06%) |
| Sacral | 1 (1.35%) | 1 (2.44%) | 0 (0.00%) |
| Level of injury | |||
| Complete tetraplegia | 8 (13.33%) | - | 8 (26.67%) |
| Incomplete tetraplegia | 22 (36.67%) | - | 22 (73.33%) |
| Complete paraplegia | 26 (43.33%) | 26 (86.67%) | - |
| Incomplete paraplegia | 4 (6.67%) | 4 (13.33%) | - |
All HRV parameters were treated as continuous variables (Table 3). Subsequently, the mean HRV parameters were compared between patients with and without SCI. The mean SDNN, RMSSD, PSI, TP, VLF, LF, and HF differed significantly between SCI and non-SCI groups (all P < 0.05). In contrast, no significant between-group differences were observed in the mean HRT, LF/HF ratio, LF norm, or HF norm (Table 3). The HRT, SDNN, RMSSD, PSI, TP, and LF/HF ratio also were treated as categorical variables and compared between the 2 groups (Table 3). The SDNN, RMSSD, PSI, and TP differed significantly between the SCI and non-SCI groups (all P < 0.05). No significant between-group differences were observed in the HRT or LF/HF ratio (Table 3). Most participants in both groups had normal mean HRT (SCI, 75%; non-SCI, 81.67%) (Table 3). SDNN was low in most SCI patients (75%) and normal in most non-SCI participants (66.67%). RMSSD was very low in the majority of SCI patients (68.33%)and normal in most non-SCI patients (65%). PSI was very high in most SCI patients (70%) and normal in most non-SCI patients (65%). Very low TP was observed in 86.67% SCI patients and 53.33% of non-SCI patients. A normal LF/HF ratio was observed in 50% of SCI patients and 55% of non-SCI patients (Table 3).
| SCI (n = 60) | Non-SCI (n = 60) | P value | |
| Continuous variables | |||
| HRT | 79.93 ± 13.19 | 77.03 ± 9.90 | 0.18b |
| SDNN | 27.11 ± 18.45 | 34.30 ± 11.18 | < 0.001a,c |
| RMSSD | 21.54 ± 17.65 | 25.19 ± 9.51 | < 0.001a,c |
| PSI | 125.42 ± 126.02 | 58.68 ± 63.64 | < 0.001a,c |
| TP | 813.78 ± 2211.10 | 989.19 ± 671.65 | < 0.001a,c |
| LF/HF ratio | 1.80 ± 2.52 | 1.98 ± 2.68 | 0.20c |
| VLF | 519.54 ± 1903.98 | 443.89 ± 372.20 | 0.001a,c |
| LF | 179.09 ± 319.20 | 299.85 ± 292.49 | < 0.001a,c |
| HF | 115.16 ± 133.82 | 245.45 ± 276.53 | < 0.001a,c |
| LF norm | 51.33 ± 20.21 | 55.78 ± 18.33 | 0.21a |
| HF norm | 48.67 ± 20.21 | 44.22 ± 18.33 | 0.21a |
| Categorical variables | |||
| HRT | |||
| Low (≤ 60) | 1 (1.7%) | 2 (3.3%) | 0.51d |
| Normal (60–90) | 45 (75.0%) | 49 (81.7%) | |
| High (> 90) | 14 (23.3%) | 9 (15.0%) | |
| SDNN | |||
| Low (≤ 30) | 45 (75.0%) | 20 (33.3%) | < 0.001a,d |
| Normal (> 30) | 15 (25.0%) | 40 (66.7%) | |
| RMSSD | |||
| Low (≤ 20) | 41 (68.3%) | 21 (35.0%) | < 0.001a,d |
| Normal (> 20) | 19 (31.7%) | 39 (65.0%) | |
| PSI | |||
| Normal (≤ 50) | 18 (30.0%) | 39 (65.0%) | < 0.001a,d |
| High (> 50) | 42 (70.0%) | 21 (35.0%) | |
| TP | |||
| Low (≤ 1000) | 52 (86.7%) | 32 (53.3%) | < 0.001a,d |
| Normal (> 1000) | 8 (13.3%) | 28 (46.7%) | |
| LF/HF ratio | |||
| Low (≤ 0.5) | 15 (25.0%) | 9 (15.0%) | 0.42d |
| Normal (0.5-2.0) | 30 (50.0%) | 33 (55.0%) | |
| High (> 2.0) | 15 (25.0%) | 18 (30.0%) | |
The PWV parameters, including DPI, EC, AE and RBV, were treated as continuous variables and expressed as the mean ± SD (Table 4). The mean AE differed significantly between the SCI and non-SCI groups (p < 0.05). No significant between-group differences were observed in the mean DPI, EC, or RBV (Table 4). Vascular age was assessed based on a 7-level scale[23]. In Table 4, vascular age, HRT, SDNN, RMSSD, PSI, TP, and LF/HF ratio were also treated as categorical variables and compared between patients with and without SCI (Table 4). Vascular age and AE differed significantly different between patients with and without SCI (P < 0.05). No significant between-group differences were observed in the DPI, EC, or RBV (Table 4). The majority of patients in the SCI group (55.00%) and non-SCI group (33.3%) had a Level 2 vascular age (Table 4). The DPI was normal or good in nearly all patients in the SCI and non-SCI groups (86.6% and 96.7%, respectively). The AE was normal in most patients in the SCI and non-SCI groups (55% and 51.67%, respectively).
| SCI (n = 60) | Non-SCI (n = 60) | P value | |
| Continuous variables | |||
| DPI | -63.57 ± 44.51 | -75.12 ± 46.32 | 0.17 |
| EC | -88.59 ± 16.95 | -88.67 ± 18.05 | 0.98 |
| AE | -21.17 ± 15.27 | -7.18 ± 17.29 | < 0.001a |
| RBV | -27.65 ± 17.88 | -30.08 ± 17.67 | 0.38 |
| Categorical variables | |||
| Vascular age | |||
| Level 1 | 6 (10.0%) | 18 (30.0%) | 0.01a |
| Level 2 | 33 (55.0%) | 20 (33.3%) | |
| Level 3 | 4 (6.7%) | 11 (18.3%) | |
| Level 4 | 15 (25.0%) | 10 (16.7%) | |
| Level 5 | 1 (1.7%) | 1 (1.7%) | |
| Level 6 | 1 (1.7%) | 0 (0.0%) | |
| DPI | |||
| Poor | 8 (13.3%) | 2 (3.3%) | 0.12 |
| Normal | 26 (43.3%) | 25 (41.7%) | |
| Good | 26 (43.3%) | 33 (55.0%) | |
| EC | |||
| Poor | 12 (20.0%) | 6 (10.0%) | 0.24 |
| Normal | 28 (46.7%) | 28 (46.7%) | |
| Good | 20 (33.3%) | 26 (43.3%) | |
| AE | |||
| Poor | 24 (40.0%) | 12 (20.0%) | 0.001a |
| Normal | 33 (55.0%) | 31 (51.7%) | |
| Good | 3 (5.0%) | 17 (28.3%) | |
| RBV | |||
| Poor | 8 (13.3%) | 6 (10.0%) | 0.56 |
| Normal | 21 (35.0%) | 27 (45.0%) | |
| Good | 31 (51.7%) | 27 (45.0%) | |
In this pilot study assessing HRV parameters and vessel conditions of patients with and without SCI, we observed significantly lower SDNN, RMSSD, TP, LF, and HF and significantly higher PSI and VLF in those with SCI. These results suggest that patients with SCI have weaker cardiac load function, higher pressure, lower vitality, and weaker sympathetic and parasympathetic activity compared to those without SCI. Accordingly, the SCI patients were more prone to mental and physical fatigue and abdominal discomfort. Overall, simultaneous decreases in the HRV frequency domains TP, LF, and HF were more likely in people under great long-term cardiovascular pressure, demonstrating a relationship between autonomic function and fatigue status. The relationship between autonomic function and fatigue was demonstrated previously in populations of different ages[24]. In the present pilot study, vascular age assessment showed that arterial elasticity was generally poorer among those with SCI compared to those without, suggesting inferior vasculature in SCI patients. Correspondingly, SCI patients exhibited relatively weaker cardiac function and vascular conditions, higher remaining blood volume and vessel resistance, and poorer circulation and metabolism than did those without SCI. A previous study also showed that chronic SCI correlates with changes in vascular structure that result in lower elasticity of the blood vessel walls, in turn increasing cardiovascular risk[25]. Lee et al[26] reported that elevated PWV in SCI patients indicates that central arterial stiffness is a powerful index of cardiovascular health associated with accelerated cardiovascular decline. The authors concluded that the higher risk of arterial stiffness resulted from autonomic dysfunction, vascular remodeling, and low physical activity levels over time. In addition to pharmacological treatments, lifestyle and dietary interventions are suggested to ameliorate these developments in people with SCI.
An Australian study by Craig et al[27] used HRV assessment to explore the relationship between daytime sleepiness and autonomic dysfunction in people with and without SCI. The authors observed weak cardiac function and reduced daytime sympathetic nervous system activity in those with SCI, causing them to tire readily and become sleepy during the day. However, the authors observed no significant difference in parasympathetic activity between people with and without SCI[27]. The present study supports these previous findings of low cardiac function and sympathetic activity in those with SCI. Specifically, we found in our Taiwanese cohort that SCI patients were also characterized by low parasympathetic activity, suggesting that they were not only prone to insomnia, neurosis, and metabolic syndrome, but also vulnerable to complications such as diabetes.
Our results also are consistent with those of many previous studies assessing HRV and PWV parameters. However, while the present study used simultaneous measurement of HRV and PWV using an HRV analyzer[23,24], previous studies applied various techniques to simultaneously measure HRV[13,15-17] and PWV parameters[5,19-21] separately. The SA-3000P HRV analyzer is a non-invasive and quick evaluation tool that can detect cardiovascular complications after SCI, facilitating appropriate early intervention. In the present and previous studies, HRV alterations (particularly lower SDNN) were predictive of progressive coronary artery disease in people with SCI and were found more frequently in those with a sedentary lifestyle and without regular physical exercise[14]. Physical exercise after SCI is shown to increase sympathetic activity and may help minimize the risk of cardiac arrhythmias and prevent sudden cardiac death in those with cervical SCI[28]. Fatigue is also associated with altered HRV parameters and greater risk of cardiovascular sequelae after SCI; appropriate levels of exercise as well as sufficient sleep and dietary measures are recommended as anti-fatigue and anti–cardiovascular-risk strategies[16]. Regarding PVW, the present study found significant differences in the AE between patients with and without SCI but not in the vascular age, DPI, EC, or RBV. The results of other studies, however, are inconsistent regarding the cause-and-effect relationship between hypertension and arterial stiffening in SCI patients[20]. Although repeated episodes of hypertension over time are recognized as stimuli for vascular remodeling, some authors found no relationship between autonomic dysreflexia and aortic augmentation indices[21].
Karri et al[29] developed a novel modality for diagnosing neuropathic pain after SCI, finding that people with SCI and chronic neuropathic pain demonstrated significantly lower SDNN, RMSSD, HF, and LF than did able-bodied adults. This finding is also consistent with the findings of the present study. El-Kotob et al[30] used HRV as a surrogate measure of cardiac autonomic function in chronic traumatic SCI patients in Canada, showing a positive relationship between HF and LF. The present study also identified this relationship in Taiwanese people with SCI. While we found that most previous studies focused on HRV analysis, we also noted that they rarely discussed vascular age. The present pilot study found that comprehensive evaluation of HRV and vascular age indicators addresses the full spectrum of poor AE, SDNN, and RMSSD in those with SCI. Studies have shown that physical activity, painting, respiratory training, music therapy, and nature therapy have the potential to reduce stress and improve HRV-related disorders and blood vessel structure[28,31-34]. Changes in the autonomic system, for example, are readily observed in response to physical exercise in individuals with SCI. Adjustments occur in the cardiac autonomic system as a result of exercise-induced remodeling of damaged axons, which increases sympathetic activity and may help to minimize risk of arrhythmia or sudden cardiac death in those with cervical SCI[28]. Kyriakides et al[31] examined the effects of regular physical workouts (four-hour sessions once a week for three months) on reducing the HRV of people with SCI, reporting that all HRV metrics improved in SCI patients who engaged in this exercise program. Other interventions have applied various relaxation techniques to reduce HRV. Chang et al[32] used painting as an intervention to help participants relax the mind, relieve stress, and stabilize the HRV. According to Ditterline et al[33], respiratory training performed 5 d a week for 4 wk effectively improved both sympathetic and parasympathetic nervous system function and further resulted in improved HRV in individuals with chronic SCI. Audio stimulation with music was used as a therapeutic approach to reduce fatigue, increase comfort and relaxation, and induce sympathetic and parasympathetic activity in cancer survivors, confirming the effectiveness of music in improving HRV parameters in this patient population[34]. Nature therapy was also evaluated in adult male SCI patients; visual stimulation with bonsai trees induced mental and physical relaxation, reduced stress, promoted parasympathetic activity, and ultimately improved HRV in these patients[35]. Self-management strategies are also suggested to assist people with SCI in improving self-care skills that may ultimately help to prevent complications after SCI[22]. Together, the results of these studies suggest the importance of developing a care program that can reduce stress, increase vitality, and improve cardiovascular function in SCI patients. Implementing such a program may help people with SCI to improve cardiac function and load, reduce stress, and increase vitality, autonomic function, and vascular health. Based on the results of the present study and those in the related literature, our follow-up study will seek to develop an evidence-based care program that can reduce stress, increase vitality, and support cardiovascular health in patients with SCI.
The present pilot study has several limitations. All data analyzed were from Taiwanese subjects; similar studies of patients in other countries are needed to confirm the applicability of these findings to other populations. Because the study was closed, we were not able to conduct sex-matched sampling. Therefore, the unmatched sex ratio is a limitation of this pilot study. We did not perform subgroup analysis based on the level of injury (e.g., tetraplegia vs. paraplegia) to address differences between types of SCI. The large standard deviation in the time elapsed after SCI may affect study results to some degree. Continuous monitoring of dynamic HRV and PWV would be of greater clinical significance than the assessments employed in this pilot study, and continuous monitoring will be included in our future research. Large-scale, cohort-matched multi-center studies in different countries are needed to expand and confirm the findings of the present study.
Analysis of HRV and PWV parameters showed that cardiac function loading is elevated in SCI patients, resulting in stress and a decline in vitality. SCI patients have weaker sympathetic and parasympathetic activity than do those without SCI, increasing their risk of mental and physical fatigue and abdominal discomfort. Cardiovascular assessment demonstrates that SCI patients also have lower arterial elasticity. HRV and PWV data can be obtained non-invasively, such that continuous dynamic monitoring of HRV and PWV could be integrated into care programs for SCI patients, along with measures aiming to reduce stress and increase vitality.
While a clear correlation has been established between spinal cord injury and cardiovascular disorders, the underlying mechanism is not fully understood. Heart rate variability (HRV) and pulse-wave velocity (PWV), indicators of cardiac function, are altered in patients with spinal cord injury, implicating autonomic cardiac function and arterial stiffness in this mechanism.
While studies have independently assessed HRV or PWV in patients with spinal cord injury, simultaneous assessment to gain a broader view of their cardiovascular condition has not been reported.
The study objective is to elucidate the mechanism underlying cardiovascular complications in spinal cord injury (SCI) patients
Short-term HRV and PWV parameters were compared between patients with and without spinal cord injury. All assessments were made using the Medicore HRV Analyzer SA-3000P, which measures HRV time and frequency domain parameters and uses acceleration plethysmography to measure PWV.
Factors that differed significantly between participants with and without spinal cord injury included the standard deviation of all normal-to-normal intervals, square root of the mean sum of squared successive RR interval differences, physical stress index, total power, very-low frequency, low frequency, high frequency, and arterial elasticity.
Patients with spinal cord injury have weaker sympathetic and parasympathetic activity as well as lower arterial elasticity compared to those without, suggesting that SCI may increase cardiac function loading.
Further investigation is needed using multi-center, cohort-matched studies with continuous assessment of HRV and PWV. This non-invasive assessment could be integrated into care programs for SCI patients as an indicator of the need for measures to reduce stress and increase vitality.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Nursing
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lakusic N, Croatia; Taheri M, Iran; Zharikov YO, Russia S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Boldt C, Velstra IM, Brach M, Linseisen E, Cieza A. Nurses' intervention goal categories for persons with spinal cord injury based on the International Classification of Functioning, Disability and Health: an international Delphi survey. J Adv Nurs. 2013;69:1109-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Chang MY, Chen HY, Cheng ML, Liu HY. Rebuilding Life: Investigating the Long-Term Homecare Needs of Clients With Spinal Cord Injuries. J Nurs Res. 2017;25:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Hou S, Rabchevsky AG. Autonomic consequences of spinal cord injury. Compr Physiol. 2014;4:1419-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Partida E, Mironets E, Hou S, Tom VJ. Cardiovascular dysfunction following spinal cord injury. Neural Regen Res. 2016;11:189-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 5. | Miyatani M, Masani K, Oh PI, Miyachi M, Popovic MR, Craven BC. Pulse wave velocity for assessment of arterial stiffness among people with spinal cord injury: a pilot study. J Spinal Cord Med. 2009;32:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Sezer N, Akkuş S, Uğurlu FG. Chronic complications of spinal cord injury. World J Orthop. 2015;6:24-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 267] [Cited by in RCA: 316] [Article Influence: 31.6] [Reference Citation Analysis (3)] |
| 7. | La Fountaine MF, Cirnigliaro CM, Hobson JC, Dyson-Hudson TA, Mc Kenna C, Kirshblum SC, Spungen AM, Bauman WA. Establishing a threshold to predict risk of cardiovascular disease from the serum triglyceride and high-density lipoprotein concentrations in persons with spinal cord injury. Spinal Cord. 2018;56:1051-1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Claydon VE, Krassioukov AV. Clinical correlates of frequency analyses of cardiovascular control after spinal cord injury. Am J Physiol Heart Circ Physiol. 2008;294:H668-H678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Sisto SA, Lorenz DJ, Hutchinson K, Wenzel L, Harkema SJ, Krassioukov A. Cardiovascular status of individuals with incomplete spinal cord injury from 7 NeuroRecovery Network rehabilitation centers. Arch Phys Med Rehabil. 2012;93:1578-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Shaffer F, Ginsberg JP. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health. 2017;5:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1840] [Cited by in RCA: 3137] [Article Influence: 392.1] [Reference Citation Analysis (0)] |
| 11. | Mejía-Mejía E, May JM, Torres R, Kyriacou PA. Pulse rate variability in cardiovascular health: a review on its applications and relationship with heart rate variability. Physiol Meas. 2020;41:07TR01. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart's anatomy and heart rate variability. Front Psychol. 2014;5:1040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 673] [Cited by in RCA: 1021] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 13. | Sharif H, Wainman L, O'Leary D, Ditor D. Cardiac parasympathetic activity and ventricular diastolic interactions in individuals with spinal cord injury. Spinal Cord. 2019;57:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Serra-Añó P, Montesinos LL, Morales J, López-Bueno L, Gomis M, García-Massó X, González LM. Heart rate variability in individuals with thoracic spinal cord injury. Spinal Cord. 2015;53:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Jan YK, Anderson M, Soltani J, Burns S, Foreman RD. Comparison of changes in heart rate variability and sacral skin perfusion in response to postural changes in people with spinal cord injury. J Rehabil Res Dev. 2013;50:203-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Rodrigues D, Tran Y, Guest R, Middleton J, Craig A. Influence of neurological lesion level on heart rate variability and fatigue in adults with spinal cord injury. Spinal Cord. 2016;54:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Fang X, Goh MY, O'Callaghan C, Berlowitz D. Relationship between autonomic cardiovascular control and obstructive sleep apnoea in persons with spinal cord injury: a retrospective study. Spinal Cord Ser Cases. 2018;4:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003;23:554-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 569] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 19. | Miyatani M, Alavinia SM, Szeto M, Moore C, Craven BC. Association between abnormal arterial stiffness and cardiovascular risk factors in people with chronic spinal cord injury. Eur J Prev Cardiol. 2017;24:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Currie KD, Hubli M, MacDonald MJ, Krassioukov AV. Associations between arterial stiffness and blood pressure fluctuations after spinal cord injury. Spinal Cord. 2019;57:1057-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Katzelnick CG, Weir JP, Pinto Zipp G, LaFountaine MF, Bauman WA, Dyson-Hudson TA, Wecht JM. Increased pulse wave velocity in persons with spinal cord injury: the effect of the renin-angiotensin-aldosterone system. Am J Physiol Heart Circ Physiol. 2021;320:H272-H280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Lin PY, Wang HC, Chen HY. Examination of the effectiveness of self-management program for spinal cord injury patients. Chengqing Yihu Guanli Zazhi. 2020;16:40. |
| 23. | Medicore. Heart rate variability analysis system. SA-3000P clinical manual version 3.0 [cited 2020 12/29]. Available from: https://medi-core.com/download/HRV_clinical_manual_ver3.0.pdf. |
| 24. | Okawa N, Kuratsune D, Koizumi J, Mizuno K, Kataoka Y, Kuratsune H. Application of autonomic nervous function evaluation to job stress screening. Heliyon. 2019;5:e01194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Ong B, Wilson JR, Henzel MK. Management of the Patient with Chronic Spinal Cord Injury. Med Clin North Am. 2020;104:263-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Lee AH, Phillips AA, Krassioukov AV. Increased Central Arterial Stiffness after Spinal Cord Injury: Contributing Factors, Implications, and Possible Interventions. J Neurotrauma. 2017;34:1129-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Craig A, Rodrigues D, Tran Y, Guest R, Middleton J. Daytime sleepiness and its relationships to fatigue and autonomic dysfunction in adults with spinal cord injury. J Psychosom Res. 2018;112:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Martins EW, Magalhães R, Marocolo M, Maior AJASHS. Cardiac autonomic profile in cervical spinal cord injury subjects practitioners of the physical exercise. 2018; 40: 33469. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Karri J, Zhang L, Li S, Chen YT, Stampas A. Heart Rate Variability: A Novel Modality for Diagnosing Neuropathic Pain after Spinal Cord Injury. Front Physiol. 2017;8:495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | El-Kotob R, Craven BC, Mathur S, Ditor DS, Oh P, Miyatani M, Verrier MC. Assessing Heart Rate Variability As a Surrogate Measure of Cardiac Autonomic Function in Chronic Traumatic Spinal Cord Injury. Top Spinal Cord Inj Rehabil. 2018;24:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Kyriakides A, Poulikakos D, Galata A, Konstantinou D, Panagiotopoulos E, Chroni E. The effect of level of injury and physical activity on heart rate variability following spinal cord injury. J Spinal Cord Med. 2019;42:212-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. |
Chang K-Y, Lian C.
The Study of Stress Relief |
| 33. | Legg Ditterline BE, Aslan SC, Randall DC, Harkema SJ, Castillo C, Ovechkin AV. Effects of Respiratory Training on Heart Rate Variability and Baroreflex Sensitivity in Individuals With Chronic Spinal Cord Injury. Arch Phys Med Rehabil. 2018;99:423-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Chuang CY, Han WR, Li PC, Young ST. Effects of music therapy on subjective sensations and heart rate variability in treated cancer survivors: a pilot study. Complement Ther Med. 2010;18:224-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Ochiai H, Song C, Ikei H, Imai M, Miyazaki Y. Effects of Visual Stimulation with Bonsai Trees on Adult Male Patients with Spinal Cord Injury. Int J Environ Res Public Health. 2017;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |