Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9670
Peer-review started: May 27, 2022
First decision: June 27, 2022
Revised: July 5, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: September 26, 2022
Processing time: 112 Days and 4.6 Hours
Solitary fibrous tumor (SFT) is predominant within the pleura but very rare in the orbit, which is why the diagnosis of orbital SFT poses challenges in clinical practice. Accordingly, an integrated approach that incorporates specific clinical features, histological, histopathological, and immunohistochemical (IHC) examinations, and molecular analyses is warranted.
To retrospectively explore the clinical and imaging characteristics, treatment, outcomes of a series of patients with orbital SFT.
We conducted a retrospective review of a series of patients diagnosed with a histopathologic orbital SFT treated at a single institution. All data on demogra
In total, 13 patients were enrolled, 7 (53.8%) of whom had the tumor located in the superomedial quadrant of the orbit. Computed tomography revealed a solitary ovoid lesion in 10 (76.9%) patients and irregular lesion in 3 (23.1%) patients. Magnetic resonance imaging results were as follows: On T1 weighted images, 3 (23.1%) patients had hypointense mixed signals, whereas 10 (76.9%) patients showed isointense mixed signals; on T2 weighted images (T2WI), 3 (23.1%), 4 (30.8%), and 6 (46.2%) patients exhibited hypointense mixed, isointense mixed, and hyperintense signals, respectively. Notably, 12 (92.3%) patients showed significant enhancement, whereas there were patchy slightly enhanced areas in the tumor. All patients were treated by surgery. IHC analysis demonstrated that the tumor cells were immunoreactive for CD34, CD99, STAT-6, and vimentin in all patients. The lesions showed Ki-67 positivity < 5% in 1 (7.7) patient, 5%-10% in 10 (76.9%), and > 10% in 2 (15.4%). Two (15.4%) patients exhibited tumor recurrence.
The clinical manifestations and radiologic characteristics of orbital SFT are diverse and not specific. Accurate diagnosis and treatment require detailed radiological and histopathological/ IHC evaluation.
Core Tip: The clinical manifestations of orbital solitary fibrous tumor (SFT) are diverse and not specific. In most cases, the lesions occur outside the muscular cone, are localized at the superomedial quadrant and inferomedial quadrant of the orbit. The mean computed tomography values of lesions are variable, and the signal of lesions on magnetic resonance imaging is uncertain. Contrast-enhanced imaging showed that most part of the lesions was significantly enhanced, whereas there were patchy slightly enhanced areas in them. Delineating SFT from histologic mimics requires nuclear staining for STAT6 as a diagnostic adjunct in conjunction with CD34 positivity. Ki-67 labelling index may be extremely low, and malignant forms with an enhanced propensity for local recurrence have been reported.
- Citation: Ren MY, Li J, Wu YX, Li RM, Zhang C, Liu LM, Wang JJ, Gao Y. Clinical characteristics and prognosis of orbital solitary fibrous tumor in patients from a Chinese tertiary eye hospital. World J Clin Cases 2022; 10(27): 9670-9679
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9670.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9670
Solitary fibrous tumor (SFT) is a rare spindle-cell tumor of mesenchymal origin, first described in the pleura by Klemperer and Rabin in 1931[1]. Original reports demonstrated that SFT is of submesothelial origin. However, there is controversy regarding its histogenesis and the etiology of the neoplasm remains largely unknown. Although the majority are benign, increasing evidence indicates the aggressive nature of the tumor. SFT is predominant within the pleura but very rare in the orbit[2], which is why the diagnosis of orbital SFT poses challenges in clinical practice. Therefore, an integrated approach that incorporates specific clinical features, radiological findings, histopathological and immunohistochemical (IHC) examinations, and molecular analyses is warranted[3]. Since this disease is relatively rare, most scholars focus on case reports or serial case studies. Due to the relatively limited number of cases, understanding this disease may be limited. The present work reviews the clinical characteristic features and treatment experience of 13 orbital SFT patients in a Chinese tertiary eye hospital, aiming to improve the accuracy of its diagnosis and treatment.
A retrospective review was conducted on subjects with orbital SFT. A total of 13 patients diagnosed with orbital SFT via postoperative histopathological and IHC examinations were identified between January 2012 and March 2019 at Hebei Eye Hospital. Patient charts and medical information were retrieved from the electronic medical records of Hebei Eye Hospital. Data on demographics, relevant medical and family history, clinical presentation, radiological findings, histopathological/IHC evaluation, treatments, and prognosis were reviewed for all the subjects. The study followed the tenets of the Declaration of Helsinki. The ethics committee of the Hebei Eye Hospital approved all study protocols. All patients gave written informed consent before the procedures. They were fully informed about the study and provided data voluntarily for analysis. All data were recorded and stored in compliance with ethical and data protection guidelines. All imaging examinations were performed under uniform conditions. An ophthalmologist who specialized in orbital diseases and a general neuroradiologist reviewed the computed tomography (CT) and magnetic resonance imaging (MRI) data at a conference. Two senior pathologists reviewed the data of pathological examination. Patients with severe MRI artifacts due to the presence of metal implants in adjacent sites and incomplete clinical case data were excluded.
Thirteen patients were reviewed, including 7 (53.8%) males and 6 (46.2%) females. The mean and median age of subjects at initial presentation was 45.2 (range 19-72) years and 43.0 years, respectively. The patients presented with the following systemic conditions: Hypertension (n = 3, 23.1%), iron deficiency anemia (n = 1, 7.7%), chronic bronchitis (n = 1, 7.7%), and sinusitis (n = 1, 7.7%). Their family members had no history of this disease (Table 1).
| Characteristic | |
| Gender | |
| Male (n, %) | 7 (53.8) |
| Female (n, %) | 6 (46.2) |
| Age (yr) | range 19-72 |
| Mean age (yr) | 45.2 |
| Median age (yr) | 43.0 |
| Systemic conditions | |
| Hypertension (n, %) | 3 (23.1) |
| Iron deficiency anemia (n, %) | 1 (7.7) |
| Chronic bronchitis (n, %) | 1 (7.7) |
| Sinusitis (n, %) | 1 (7.7) |
The patients predominantly presented with proptosis (n = 7, 53.8%). Subsequently, other symptoms appeared, including eyelid swelling (n = 2, 15.4%), painful mass (n = 2, 15.4%), epiphora (n = 1, 7.7%), and visual disturbances (n = 1, 7.7%). Nine (69.2%) patients exhibited visual acuity above 20/60, 1 (7.7%) showed 20/100 visual acuity, and 3 (23.1%) had visual acuity lower than 20/200. Hertel exophthalmometry readings showed that the median proptosis of the affected eye was 4 mm (range 1-6 mm), towered over the contralateral eye. Seven (53.8%) patients showed non-axial proptosis as follows: Inferolateral (n = 3, 23.1%), inferomedial (n = 1, 7.7%), superomedial (n = 1, 7.7%), superior (n = 1, 7.7%), and inferior (n = 1, 7.7%). Six (46.2%) patients presented with restricted extraocular muscle movements. Nine (69.2%) patients exhibited a palpable mass. One (7.7%) patient had noticeable tenderness. Six (46.2%) patients had secondary lesions. Two (15.4%) recurrent patients had optic nerve atrophy. Two (15.4%) patients exhibited nasolacrimal duct. One (7.7%) patient had corneal perforation secondary to exposure keratitis due to severe proptosis (Table 2).
| Characteristic | |
| Complaints | |
| Proptosis (n, %) | 7 (53.8) |
| Eyelid swelling (n, %) | 2 (15.4) |
| Painful mass (n, %) | 2 (15.4) |
| Epiphora (n, %) | 1 (7.7) |
| Visual disturbances (n, %) | 1 (7.7) |
| Exhibited visual acuity | |
| Above 20/60 (n, %) | 9 (69.2) |
| 20/100 (n, %) | 1 (7.7) |
| Lower than 20/200 (n, %) | 3 (23.1) |
| Proptosis of the affected eye towered over the contralateral eye | |
| Range proptosis (mm) | 1-6 |
| Median proptosis (mm) | 4 |
| Non axial proptosis | |
| Inferolateral (n, %) | 3 (23.1) |
| Inferomedial (n, %) | 1 (7.7) |
| Superomedial (n, %) | 1 (7.7) |
| Superior (n, %) | 1 (7.7) |
| Inferior (n, %) | 1 (7.7) |
| Others | |
| Restricted extraocular muscle movements (n, %) | 6 (46.2) |
| Exhibited palpable masses (n, %) | 9 (69.2) |
| Tenderness (n, %) | 1 (7.7) |
| Optic nerve atrophy (n, %) | 1 (7.7) |
| Nasolacrimal duct (n, %) | 2 (15.4) |
| Corneal perforation (n, %) | 1 (7.7) |
Orbital CT and MRI were applied to identify the location of the lesions. With the optic nerve as the original point, we classified the orbit into four quadrants. The mass was located in the superomedial quadrant (n = 7, 53.8%), the inferomedial quadrant (n = 3, 23.1%), the inferolateral quadrant (n = 2, 15.4%), and the superolateral quadrant (n = 1, 7.7%) (Table 3). The lesions: (i) Adhered closely to the extraocular muscles (n = 7, 53.8%); (ii) adhered to the optic nerve (n = 3, 23.1%), with 1 patient being a recurrent case; (iii) compressed the lacrimal sac (n = 2, 15.4%); or (iv) spread to the brain, nasal cavity, and eyelids (n = 1, 7.7%; this patient was a recurrent case).
| Location of the lesions | |
| Superomedial quadrant (n, %) | 7 (53.8) |
| Inferomedial quadrant (n, %) | 3 (23.1) |
| Inferolateral quadrant (n, %) | 2 (15.4) |
| Superolateral quadrant (n, %) | 1 (7.7) |
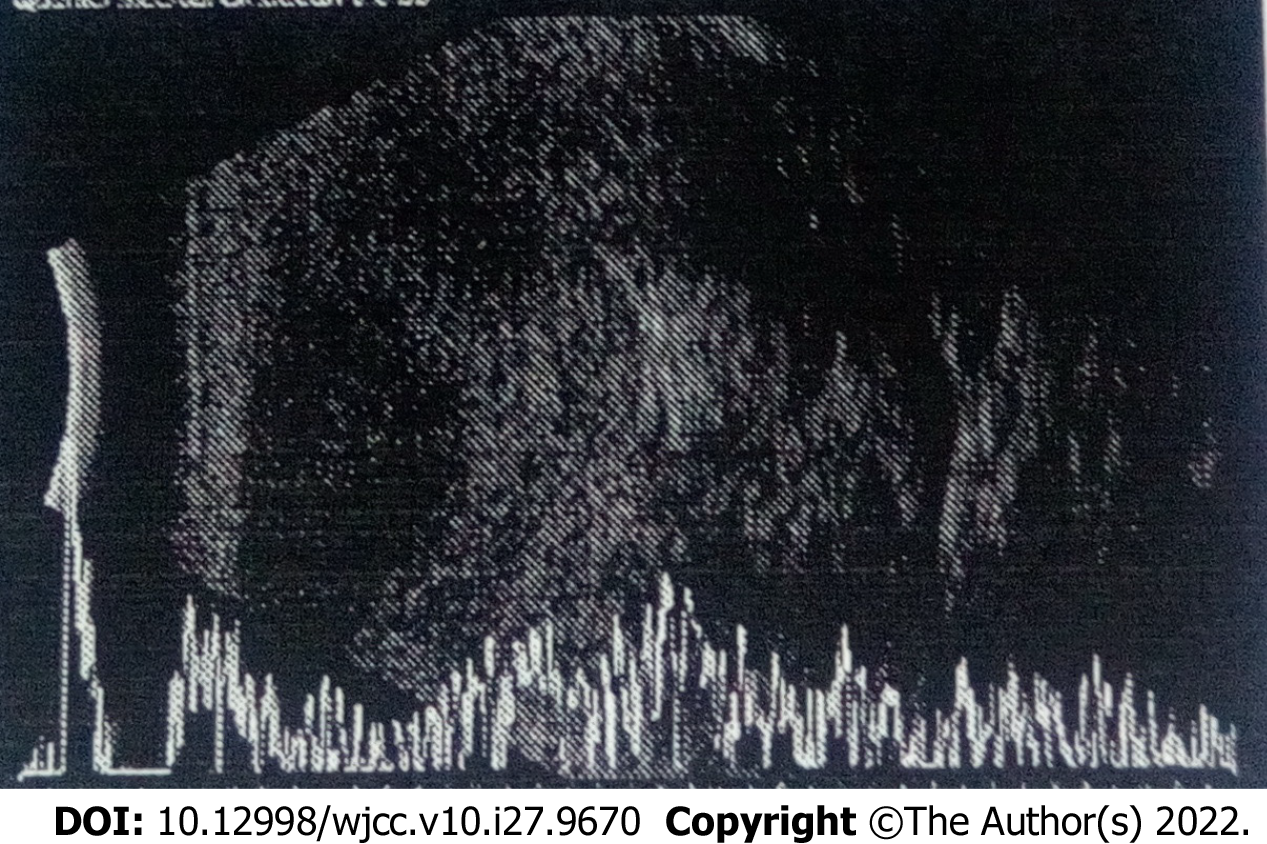
The results of ultrasonographic manifestations are as follows: 1 (7.7%) patient had a hypoechoic mass, 3 (23.1%) had a middle-echoic mass, and 9 (69.2%) had a mixed echogenic mass; 11 (86.4%) had a non-uniform echo, while 2 (15.4%) had relatively uniform echo; 9 (69.2%) had a clear boundary of the lesion, whereas 4 (30.8%) had an ill-defined boundary of the lesion; 10 (76.9%) exhibited ovoid masses, and 3 (23.1%) had irregular masses; 12 (92.3%) had abundant branching blood flow signals in the mass, including 1 (7.7%) recurrent case, and another (7.7%; recurrent patient) had small flaky blood flow signals in the mass (Figure 1).
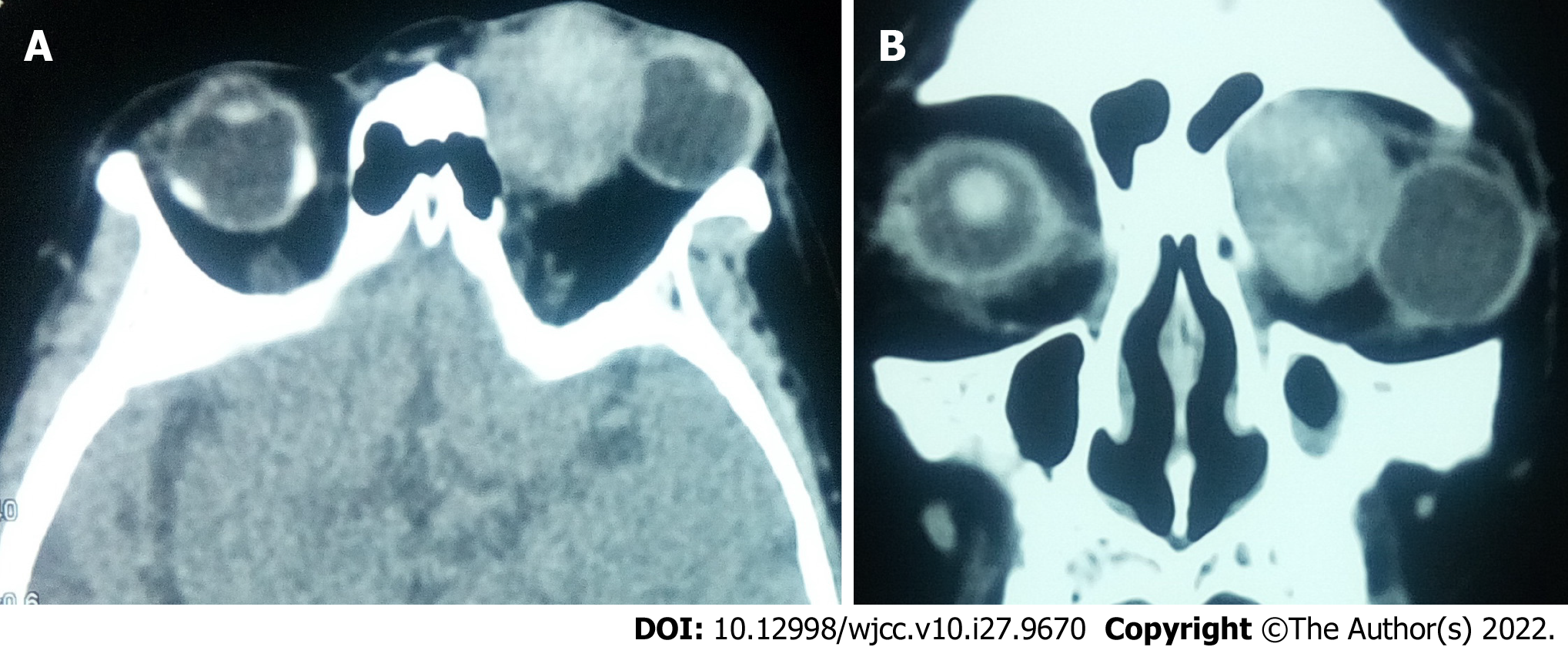
According to the morphology of lesions, 10 (76.9%) patients had a solitary ovoid mass, whereas 3 (23.1%) had an irregular mass. Nine (69.2%) patients had well-defined lesion boundaries, whereas 4 (30.8%) had ill-defined lesion boundaries. The arithmetic mean CT value of the tumor was 45.9 Hu (range 22.8-64.4Hu). Regarding the standard deviation of CT value, 4 (30.8%) and 9 (69.2%) patients showed a lower and higher value than 10% of their own CT value, respectively. The largest and the smallest lesions measured 2.7 cm*2.9 cm*4.1 cm and 1.0 cm*1.3 cm*1.4 cm, respectively (Figure 2). Nine (69.2%) patients showed compressive bone changes.
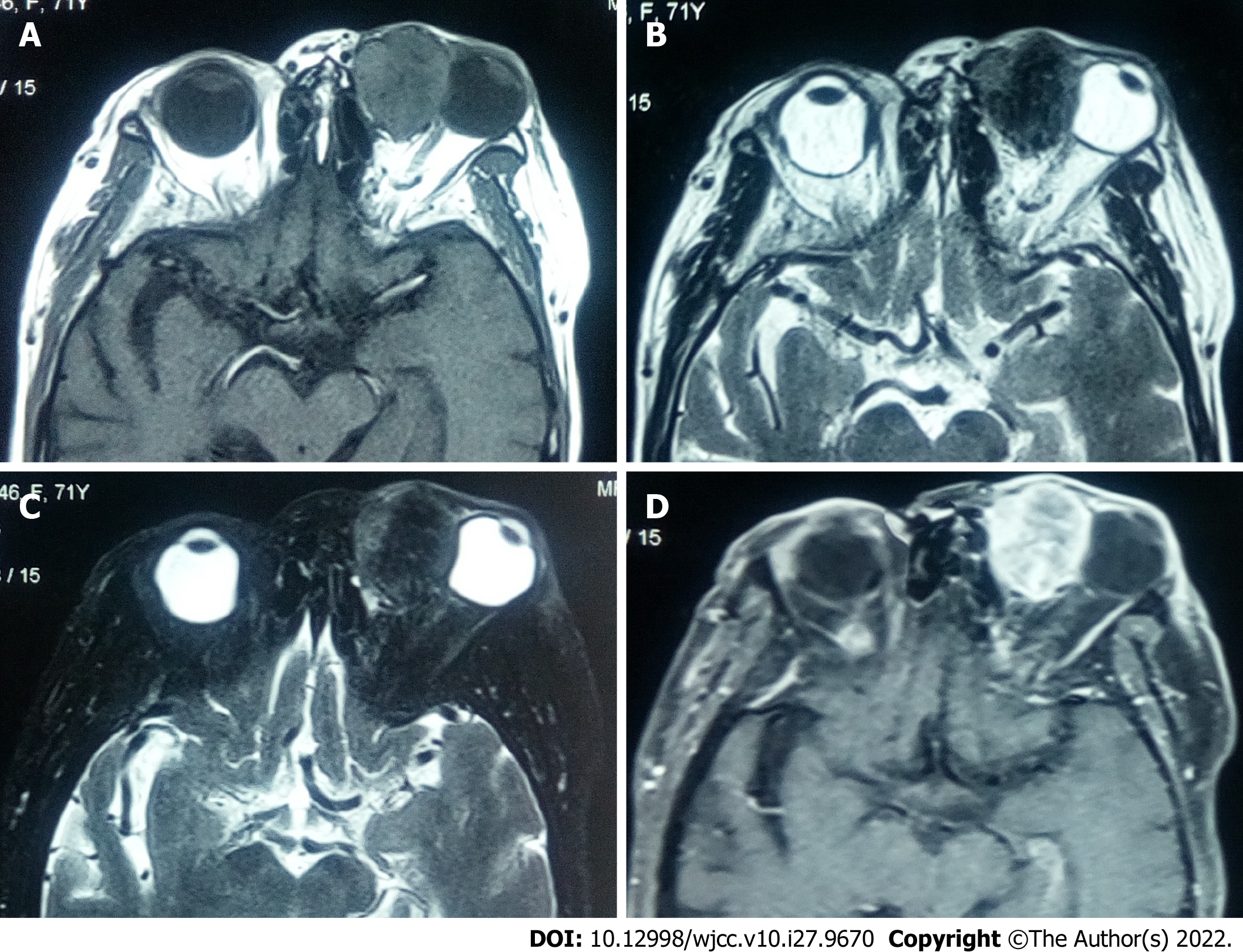
On T1 weighted images (T1WI), 3 (23.1%) patients showed hypointense mixed signals, whereas 10 (76.9%) showed isointense mixed-signals; 10 (76.9%) and 3 (23.1%) patients had homogeneous and uneven signal intensity, respectively; 4 (30.8%) patients showed vessel-emptied signals. On T2 weighted images (T2WI), 3 (23.1%) patients showed hypointense mixed signals, 4 (30.8%) showed isointense mixed signals, and 6 (46.2%) showed hyperintense signals; 2 (15.4%) patients showed relatively homogeneous signals, whereas 11 (86.4%) showed uneven signals. Contrast-enhanced imaging showed that most part of the tumors was significantly enhanced, whereas there were patchy slightly enhanced areas in those tumors in 12 (92.3%) patients. One recurrent patient presented with significant enhancement at the edges of the lesion and in tissues surrounding the lesion (Figure 3).
All patients were managed by surgery. The choice of the surgical approach was determined by the location, size, and relationship to surrounding tissue of the lesions. Two (15.4%) patients underwent lateral orbitotomy, both of whom had the lesions adhering to the optic nerve. Two (15.4%) patients underwent lateral orbitotomy-medial conjunctival procedure, of whom one had the lesion adhering to the optic nerve and one had the lesion spreading to the brain, nasal cavity, and eyelids. In addition, 9 (69.2%) patients were managed as follows: 1 (7.7%) via transcutaneous superolateral routes; 1 (7.7%) via transcaruncular medial orbitotomy; 1 (7.7%) via transconjunctival orbitotomy; 3 (23.1%) via transcutaneous superomedial routes; 3 (23.1%) via frontoethmoidal medial orbitotomy, of whom two had the lesions compressing the lacrimal sac. Eleven (86.4%) patients had the lesions completely removed. However, one recurrent patient who had the lesion adhering to the optic nerve underwent major resection, whereas another recurrent patient who had the lesion spreading to the brain, nasal cavity, and eyelids underwent mass resection combined with orbital exenteration.
Postoperative complications were found in 4 (30.8%) patients; 2 (15.4%) presented with restricted extraocular muscle movements, 1 (7.7%) had impaired visual function, and 1 (7.7%) presented with facial disfigurement due to orbital exenteration.
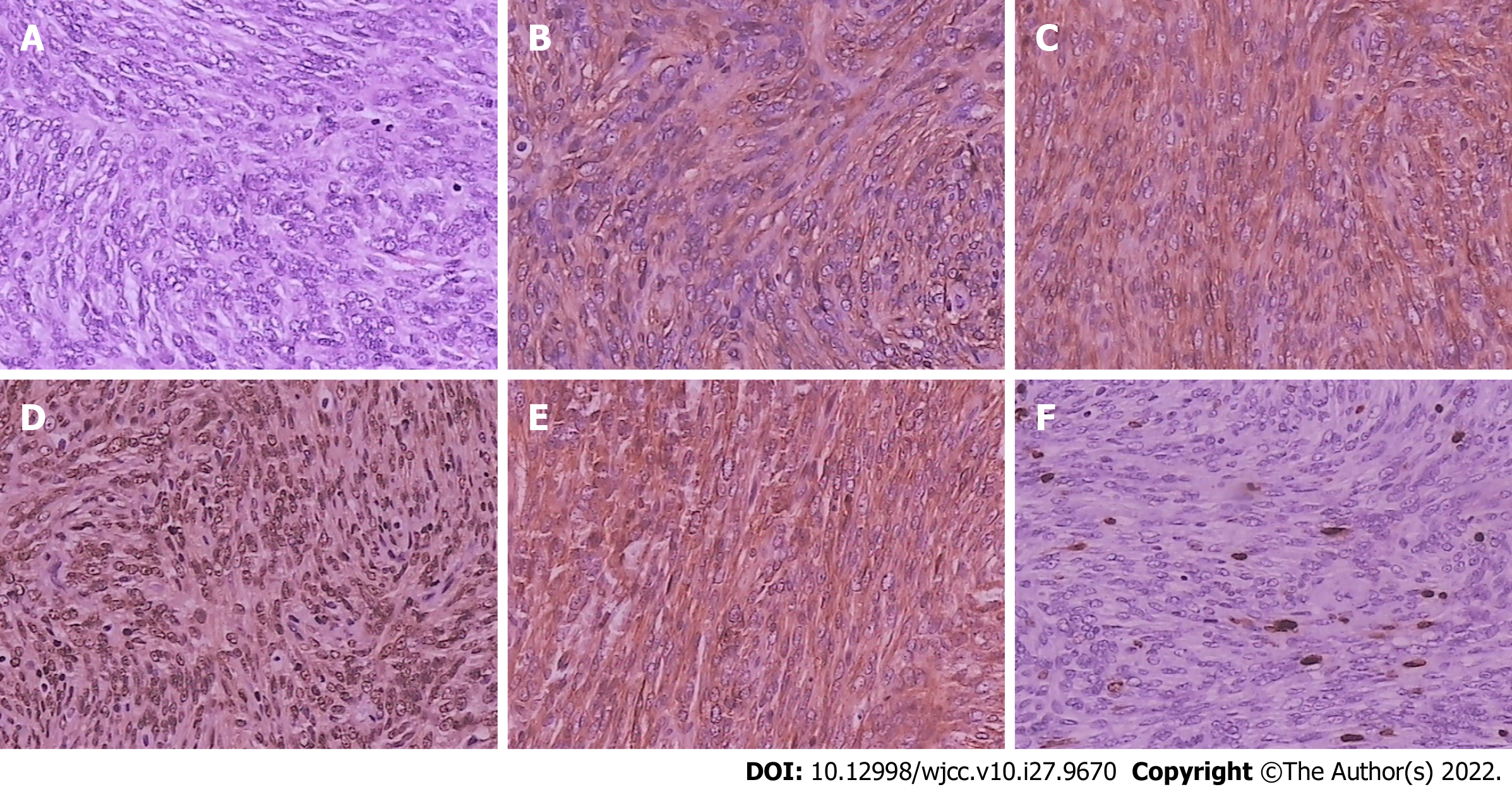
Among the excised tumors, 8 (61.5%) had capsules or pseudocapsules, whereas 5 (38.5%) had no capsules. The tumor sections were gray-white in 6 (46.2%) and gray-red in 7 (53.8%) cases. Eight (61.5%) patients had brittle tumors, while 5 (38.5%) had tough tumors. Histopathological examination showed that the tumor comprised haphazardly arranged spindle cells with bland nuclei and inconspicuous nucleoli. IHC examination showed that all patients had tumor cells with diffuse immunoreactivity for CD34 and CD99. Tumor cells from all patients tested positive for STAT-6 and vimentin. The lesions showed Bcl-2 positivity in 11 (86.4%) patients, S-100 negativity in 13 (100%), and SMA negativity in 11 (86.4%). Ki-67 positivity of the lesions was < 5% in 1 patient, 5%-10% in 10 (76.9%), and > 10% in 2 (15.4%) (Figure 4).
Median follow-up was 36 (range 18-96) mo. Two (15.4%) patients exhibited tumor recurrence, which occurred at 36 mo and 28 mo post-surgery, respectively. No patient died.
Recent reports have found that SFT can occur in diverse bodily locations and present a vast array of clinical and radiological features[4]. Orbital SFT as a type of extrapleural SFT, is considered a rare type of orbital tumor with ill-defined clinical characteristics. Recent understanding of the orbital SFT demonstrates that it is more aggressive than a pleural form with a substantially unpredictable prognosis[3]. As such, exploring the clinical characteristics and pathogenesis of this tumor is of profound clinical significance.
There is no evidence on whether orbital SFT is linked to sex and side of affected eye predilection. Based on the findings from previous studies and the present results, adult cases seem to account for the majority. Meanwhile, children patients have been reported previously[5]. The clinical manifestations are diverse and do not significantly differ from those of other orbital tumors. The common manifestations of orbital SFT include proptosis and eyeball dislocation, and some patients may present with eyelid swelling, visual disturbances, a palpable painless mass, epiphora, ptosis, and spontaneous periorbital pain or tenderness[6].
Orbital SFT mostly presents as a soft-tissue mass on CT, being round or oval; the density of the lesions is uneven. In most cases, the lesions occur outside the muscular cone and are localized at the superomedial quadrant and inferomedial quadrant of the orbit. Tumors mostly develop on the outside of the muscular cone, and in partial cases, the lesions may be associated with or within the orbital muscle cone. In the present study, the mean CT values ranged between 22.8 Hu and 66.4 Hu, demonstrating a variability in the mean CT values of the tumors on CT. Remodeling of the adjacent bones is associated with long-standing orbital SFT[6], due to the compression of the lesions. In addition, mounting evidence shows that the disease can spread to the nasal cavity or the brain in recurrent or long-standing cases.
Although not pathognomonic, CT and MRI reports show that homogeneous or heterogeneous-attenuated enhancement is the most prominent feature of SFT. And a markedly enhancing mass showing similar characteristics to those of the internal carotid artery on postcontrast CT or MRI can be seen[7]. Most tumors could be significantly enhanced and the majority of cases showed heterogeneous enhancement, though unenhanced lamellar regions were also revealed. In some recurrent cases, most lesions may be not enhanced, except for cystic enhancement around the lesion. The present study found that the MRI signals of orbital SFT were relatively complex in practice, mostly medium or low signal on T1WI, but it could show arbitrary signal on T2WI, which may be related to the tumor composition. Orbital SFT showed a variable intensity on T2WI. Kurtosis in the histogram analysis on T2WI had a strong correlation with the amount of collagenous tissue[8].
Due to the uncertainty of lesion signal on MRI, it is necessary to distinguish between orbital hemorrhage and schwannoma. Moreover, the lesions in hemorrhage are not enhanced on MRI, and the composition of orbital schwannoma is complex. Secondary degenerative changes of the tumor, including cyst formation, hyalinization, and hemorrhage, are relatively common and are suggested to contribute to extremely complex MRI findings of schwannomas. However, the schwannoma boundary is very clear. Other orbital tumors for differential consideration include histiocytomas, giant cell angiofibromas, and hemangiopericytomas[9].
SFT is originally confused for other lesions such as fibrous histiocytoma and giant angiofibroma. It is thought to be a separate entity from hemangiopericytoma. Currently, the clinical diagnosis of SFT is getting more accurate with much greater frequency. The typical SFT phenotype is spindle-shaped tumor cells and the alternately arranged sparse and dense cells separated by eosinophilic collagen fiber bands and staghorn hemangiopericytoma-like vessels[10]. IHC markers are fundamental for successful diagnosis. The diagnosis of SFT depends on the diffuse and intense positivity of CD34 staining through IHC. SFT is highly sensitive to CD34 staining but shows a weak specificity. Although Bcl-2 expression is extremely high in SFT, it is negative in most malignant mesotheliomas. In this view, integrating these two IHC markers would increase the diagnostic accuracy[11,12]. In the present study, the IHC staining pattern (CD34+, Bcl-2+) was consistent with most cases of orbital SFT in previous reports. But we found that Bcl-2 was negatively expressed in two cases. Furthermore, the IHC staining for the STAT6 protein is key in the rapid detection of the NAB2-STAT6 gene fusion status in tumor cells, and it is highly sensitive and specific for SFT diagnosis. Mounting evidence indicates that the fusion of NAB2 and STAT6 genes can trigger the entry of cytoplasmic STAT6 protein into the nucleus such that the nucleus strongly expresses the STAT6 protein. STAT6 also has a great auxiliary effect for the accurate distinction of SFT in morphologically similar tumors[13]. Therefore, the combined application of vimentin, STAT6, CD34, and other markers may guide the accurate SFT diagnosis.
The Ki-67 labelling index is very low since orbital SFTs routinely exhibit a benign course. But malignant forms with an increased propensity for local recurrence have also been reported[12]. There is evidence on the correlation of Ki67 protein expression with the proliferative activity of intrinsic cell populations in malignant tumors, permitting its application as a marker of tumor aggressiveness[14]. Owing to the high expression rate in orbital SFT and contribution to the development and epithelial-mesenchymal transition and metastasis in cancer, attention should be paid to vimentin to explore the recurrence or metastasis of this tumor. Vimentin, a key component of the cytoskeleton, plays critical biological functions at the cellular and organismal levels[15].
Owing to the rarity of orbital SFT, limited studies have investigated the various treatment modalities. But as with many soft tissue tumors, the mainstay of treatment for orbital SFT is en bloc surgical resection with negative margins. The choice of surgical approach is related to the lesion location. Orbital division based on the anatomical location of the coordinate system of four quadrants is valuable. Usually, a lateral orbitotomy is administered for lesions located in the superomedial or inferomedial quadrant, behind the globe, or within the muscle cone. In patients with an ill-defined boundary of lesions, with incomplete or no capsule, non-contact excision and thorough rinsing of the operation area after extensive excision may reduce the recurrence rate of the lesion. If the initial excision is incomplete, the recurrent tumor tends to spread into surrounding tissues and bone, rendering a second excision much more difficult[9]. For recurrent cases, more extensive excision is required, and the margin should be examined if necessary.
Significantly, our examination of flow void-like intensity on preoperative T1WI demonstrated that four patients exhibited vessel-emptied signals corresponding to the abnormal arterial components within the tumor tissue. It was deduced that surgical resection in orbital SFT may be very dangerous. Nevertheless, to avoid accidental intraoperative hemorrhage, surgical resection should be performed carefully. If necessary, surgeons should consider embolization before commencing surgical resection.
Currently, there is little evidence on whether adjuvant chemotherapy and radiation therapy following complete surgical resection are beneficial, which limits their routine application[16,17]. In our cohort, there was no evidence of malignant transformation in recurrent cases, as such the subjects were not managed using radiotherapy or chemotherapy.
In the present investigation, the lesions grew more rapidly and invasively after recurrence. For the two patients with tumor recurrence, it is possible that the tumors had no capsule and was located above the orbit. The surgery scope is limited by factors such as the levator palpebral muscle and the trochlear nerve. Recently, a study showed the recurrence rate after transorbital approach operations was 83.3 %, and the recurrence rate after transfronto-orbital approach operations was 17.6 %[18]. The fairly high local recurrence rate underscores their aggressive potential and uncovers the importance of prospective recognition[19]. In the cohort of recurrent orbital hemangiopericytoma/SFT, the median time to recurrence was 4 years, underscoring the importance of careful continued follow-up[20].
The clinical manifestations of orbital SFT are diverse and not specific. The lesions also show uneven density. The radiological features are variable, with few features being consistent. Complete gross resection or more aggressive wide excision is preferred in most cases. In patients with an ill-defined boundary of lesions, with incomplete or no capsule, non-contact excision and thorough rinsing of the operation area after extensive excision may reduce the recurrence rate. Delineating SFT from histologic mimics requires nuclear staining for STAT6 as a diagnostic adjunct in conjunction with CD34 positivity. Although the Ki-67 labelling index may be extremely low, malignant forms with an increased propensity for local recurrence have been reported.
Solitary fibrous tumor (SFT) is predominant within the pleura but very rare in the orbit, which is why the diagnosis of orbital SFT poses challenges in clinical practice.
Unspecific symptoms and a wide range of clinical manifestations of SFT can significantly hamper the establishment of a definitive diagnosis and treatment.
To retrospectively explore the clinical and imaging characteristics, treatment, outcomes of a series of patients with orbital SFT.
This is a retrospective analysis of patients diagnosed with a histopathologic orbital SFT treated at a single institution. All data on demographics, clinical characteristics, imaging, treatment, postoperative histopathological and immunohistochemical (IHC) examinations, and prognosis were collected.
In total, 13 patients were enrolled, 7 (53.8%) of whom had the tumors located in the superomedial quadrant of the orbit. Notably, 12 (92.3%) patients showed significant enhancement, whereas there were patchy slightly enhanced areas in the tumor. IHC analysis demonstrated that the tumor cells were immunoreactive for CD34, CD99, STAT-6, and vimentin in all patients. The lesions showed Ki-67 positivity < 5% in 1 (7.7) patient, 5%-10% in 10 (76.9%), and > 10% in 2 (15.4%). Two (15.4%) patients exhibited tumor recurrence.
The clinical manifestations and radiologic characteristics of orbital SFT are diverse and not specific. Accurate diagnosis and treatment require detailed radiological and histopathological/IHC evaluation.
A more comprehensive analysis of the clinical symptoms, therapeutic efficacy, and prognosis of orbital SFT should be performed in the future.
Thanks a lot to the related doctors and patients who took part in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Mohapatra SSD, India; Nazari N, Iran S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Gupta S, Verma R, Sen R, Singh I, Marwah N, Kohli R. Solitary fibrous tumor of the orbit. Asian J Neurosurg. 2016;11:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Koylu MT, Ozge G, Uysal Y, Deveci MS. Solitary fibrous tumor of the orbit: Case report and review of the literature. J Fr Ophtalmol. 2017;40:e85-e87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Ronchi A, Cozzolino I, Zito Marino F, Accardo M, Montella M, Panarese I, Roccuzzo G, Toni G, Franco R, De Chiara A. Extrapleural solitary fibrous tumor: A distinct entity from pleural solitary fibrous tumor. An update on clinical, molecular and diagnostic features. Ann Diagn Pathol. 2018;34:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 4. | Musyoki FN, Nahal A, Powell TI. Solitary fibrous tumor: an update on the spectrum of extrapleural manifestations. Skeletal Radiol. 2012;41:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Vu AF, Chundury RV, Blandford AD, Perry JD. Recurrent Orbital Solitary Fibrous Tumor in a 12-Year-Old. Ocul Oncol Pathol. 2017;3:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Sayit AT, Elmali M, Gul A, Sullu Y. Solitary fibrous tumor of the orbit: Computed tomography and histopathological findings. J Cancer Res Ther. 2019;15:719-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Kim HJ, Kim HJ, Kim YD, Yim YJ, Kim ST, Jeon P, Kim KH, Byun HS, Song HJ. Solitary fibrous tumor of the orbit: CT and MR imaging findings. AJNR Am J Neuroradiol. 2008;29:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Masuno R, Yunaiyama D, Shishido-Hara Y, Yoshimaru D, Maruyama C, Araki Y, Goto H, Nagao T, Saito K. Magnetic Resonance Imaging of Orbital Solitary Fibrous Tumors: Radiological-Pathological Correlation Analysis. J Belg Soc Radiol. 2021;105:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Shen J, Li H, Feng S, Cui H. Orbital solitary fibrous tumor: a clinicopathologic study from a Chinese tertiary hospital with a literature review. Cancer Manag Res. 2018;10:1069-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Yang EJ, Howitt BE, Fletcher CDM, Nucci MR. Solitary fibrous tumour of the female genital tract: a clinicopathological analysis of 25 cases. Histopathology. 2018;72:749-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Araújo M, Borges T, Mahia Y, Lages V, Pereira A. Orbital solitary fibrous tumor: A painless mass after a dacryochystorhinostomy. Saudi J Ophthalmol. 2019;33:316-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Genc A, Toktas Z, Azman C, Bozkurt SU, Kilic T. Solitary Fibrous Tumor of the Orbit: A Case Report and Review of the Literature. Turk Neurosurg. 2015;25:984-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Huang SC, Li CF, Kao YC, Chuang IC, Tai HC, Tsai JW, Yu SC, Huang HY, Lan J, Yen SL, Lin PC, Chen TC. The clinicopathological significance of NAB2-STAT6 gene fusions in 52 cases of intrathoracic solitary fibrous tumors. Cancer Med. 2016;5:159-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Li LT, Jiang G, Chen Q, Zheng JN. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol Med Rep. 2015;11:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 550] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 15. | Battaglia RA, Delic S, Herrmann H, Snider NT. Vimentin on the move: new developments in cell migration. F1000Res. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 16. | Olson NJ, Linos K. Dedifferentiated Solitary Fibrous Tumor: A Concise Review. Arch Pathol Lab Med. 2018;142:761-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Tanaka K, Yano H, Hayashi H, Hirano A. Total resection combined with osteotomy is more effective for orbital solitary fibrous tumor excision: a report of three cases. Int Ophthalmol. 2018;38:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Yang P, Liu HC, Qiu E, Wang W, Zhang JL, Jiang LB, Liu HG, Kang J. Factors for postoperative recurrence of orbital solitary fibrous tumor: an analysis of long-term clinical follow-up results from a Chinese tertiary hospital. BMC Ophthalmol. 2021;21:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Smith SC, Gooding WE, Elkins M, Patel RM, Harms PW, McDaniel AS, Palanisamy N, Uram-Tuculescu C, Balzer BB, Lucas DR, Seethala RR, McHugh JB. Solitary Fibrous Tumors of the Head and Neck: A Multi-Institutional Clinicopathologic Stud. Am J Surg Pathol. 2017;41:1642-1656. [RCA] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Sagiv O, Bell D, Guo Y, Su S, Wester ST, Jiang K, Yin VT, Shinder R, Hayek B, Kim HJ, Tetzlaff MT, Esmaeli B. Pathological Features and Clinical Course in Patients With Recurrent or Malignant Orbital Solitary Fibrous Tumor/Hemangiopericytoma. Ophthalmic Plast Reconstr Surg. 2019;35:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |