Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9657
Peer-review started: May 17, 2022
First decision: June 16, 2022
Revised: June 30, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: September 26, 2022
Processing time: 122 Days and 3.9 Hours
Sex determining region Y-box 2 (SOX2) can promote squamous cell carcinoma (SSC) because it regulates the migration and invasion of several different types of squamous carcinoma cells. However, few studies have examined the prognostic value of SOX2 and its effect on the epithelial-mesenchymal transition (EMT) in esophageal SSC (ESCC), a cancer characterized by high invasion and rapid metastasis.
To verify the relationship of SOX2 and the EMT in ESCC and determine the prognostic value and significance of SOX2 and protein markers of the EMT in ESCC.
One hundred and eighty-five postsurgical ESCC patients were retrospectively examined. Immunohistochemistry was used to detect SOX2, E-cadherin, and vimentin in ESCC tissues. The chi-square test was used to determine the relationships of the expression of these proteins with clinical data. Kaplan-Meier survival curves were used to evaluate factors associated with overall survival (OS).
SOX2 and vimentin had high expression in ESCC tissues and correlated with the depth of local carcinoma invasion. SOX2 expression had positive correlations with tumor size, vimentin expression, and the EMT, and a negative correlation with E-cadherin expression. Expression of SOX2 and vimentin had negative correlations with OS. SOX2 expression was an independent prognostic risk factor for poor OS in patients with ESCC.
SOX2 expression was an independent risk factor for OS in patients with ESCC and its expression had a positive correlation with the expression of vimentin, a classic marker of the EMT. SOX2 promoted the migration and invasion of ESCC, and this may related to its effect on vimentin in promoting the EMT.
Core Tip: Sex determining region Y-box 2 (SOX2) functions in the pathogenesis of squamous cell carcinoma (SSC) by driving the increase of tumor size and invasion, and is also associated with the expression of β-catenin and the epithelial-mesenchymal transition (EMT). Our examination of the role of SOX2 in esophageal SSC (ESCC) indicated that its expression had a positive correlation with vimentin expression, an established marker of the EMT. SOX2 expression was also associated the migration and invasion of ESCC, and this may be related to the upregulation of vimentin and the desreased expression of E-cadherin, which promote the EMT. SOX2 expression was an independent risk factor for poor OS in patients with ESCC.
- Citation: Li C, Ma YQ. Prognostic significance of sex determining region Y-box 2, E-cadherin, and vimentin in esophageal squamous cell carcinoma. World J Clin Cases 2022; 10(27): 9657-9669
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9657.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9657
Cancer of the esophagus is the sixth most common cause of cancer-related deaths worldwide, and esophageal squamous cell carcinoma (ESCC) is the major pathological type of this cancer. In particular, ESCC accounts for 90% of esophageal cancers in Eastern Asia, East Africa, South America, and China, and is especially common in Xinjiang residents of Kazakh ethnicity[1]. ESCC originates from squamous epithelial cells of the inner lining of the esophagus, cells that have direct contact with carcinogenic substances[2]. However, multiple pathways, metabolic factors, and genes affect the onset and progression of ESCC, so prevention and treatment can be difficult. ESCC is also associated with a high rate of mortality, and the current 5-year survival rate is only about 20%[3].
The epithelial-mesenchymal transition (EMT) is one of the most important biological transformations during the progression of cancers[4,5]. The EMT is a key factor indicative of poor prognosis in patients with ESCC, because it indicates the presence of local invasion, lymph node metastasis, and distant metastasis[6-8]. E-cadherin and vimentin are two classical biomarkers of the EMT[9,10]. In particular, downregulation of E-cadherin and upregulation of vimentin in tumor cells correlate with tumor cell migration, a necessary step during local invasion and distant metastasis of tumor cells[11].
Sex determining region Y-box 2 (SOX2) regulates gene transcription and functions in the initiation and development of cancer stem cells. There is a close relationship between SOX2 and the EMT, but the nature of this relationship differs among different cancers. For example, SOX2 promotes the EMT in oral cancer[12]. Although the pathology of head and neck SCC is similar to that of ESCC, SOX inhibits the EMT and also inhibits the invasion and metastasis of head and neck SCC[13]. However, the relationships between SOX2 and the EMT in ESCC and between the expression of SOX2 and the prognosis of ESCC patients remain to be elucidated. We assessed the role of SOX2 in ESCC and its relationship with the EMT by performing immunohistochemical staining of SOX2, E-cadherin, and vimentin in ESCC tissues from patients of Han and Kazakh ethnicity, and evaluated their prognostic significance and relationships with the clinical features of these patients.
This retrospective study examined the records of 185 patients (96 of Han ethnicity and 89 of Kazakh ethnicity) who were diagnosed with ESCC and received radical primary resection of the cancer with lymph node dissection at the First Affiliated Hospital of Xinjiang Medical University between January 2010 and June 2019 (Table 1). None of the patients received neoadjuvant chemotherapy or radiation therapy. All diagnoses were reviewed by two expert pathologists (Ma YQ and Li MY). The available clinicopathological data included age (< 65 or ≥ 65 years-old), tumor location (upper, middle, or lower), tumor size (< 3 cm or ≥ 3 cm), tumor differentiation [uncertain (Gx), well-differentiated (G1), moderately differentiated (G2), or poorly differentiated (G3)], lymph node metastasis (yes or no), distant metastasis (yes or no), stage according to the eighth edition of the AJCC (I, II, III, or IV), and depth of invasion (mucosa, muscularis, or full thickness).
| Characteristic | n (%) |
| Age, yr | |
| < 65 | 100 (54.1) |
| ≥ 65 | 85 (45.9) |
| Gender | |
| Male | 129 (69.7) |
| Female | 56 (30.3) |
| Ethnicity | |
| Han | 96 (51.9) |
| Kazakh | 89 (48.1) |
| Tumor location | |
| Upper | 8 (4.3) |
| Middle | 97 (52.4) |
| Lower | 80 (43.2) |
| Tumor size | |
| < 3 cm | 121 (65.4) |
| ≥ 3 cm | 64 (34.6) |
| Degree of differentiation | |
| High | 23 (12.4) |
| Moderate | 117 (63.2) |
| Poor | 45 (24.3) |
| AJCC stage | |
| I | 15 (8.1) |
| II | 119 (64.3) |
| III | 35 (18.9) |
| IV | 16 (8.6) |
| T stage | |
| T1 | 7 (3.8) |
| T2 | 78 (42.2) |
| T3 | 91 (49.2) |
| T4 | 9 (4.9) |
| N stage | |
| N0 | 128 (69.2) |
| N1 | 33 (17.8) |
| N2 | 19 (10.3) |
| N3 | 5 (2.7) |
| M stage | |
| No | 176 (95.1) |
| Yes | 9 (4.9) |
| Depth of invasion | |
| Mucosa | 51 (27.6) |
| Muscularis | 49 (26.5) |
| Full thickness | 85 (45.9) |
| Lymph node metastasis | |
| No | 128 (69.2) |
| Yes | 57 (30.8) |
| Survival status | |
| Alive | 139 (75.1) |
| Dead | 46 (24.9) |
This study was approved by Ethical Committee of the First Affiliated Hospital of Xinjiang Medical University (20180223-08). Prior to the operation, each patient was clearly informed about the procedures, extraction of tissues, and pathological examination. The experimental purpose was explained verbally during the follow-up. The follow-up deadline was July 2020, based on review of the medical records and telephone calls. Overall survival (OS) was defined as the time from the date of surgery until death or the last follow-up.
From 2010 to 2017, the main surgical method was total incision (left posterolateral thoracotomy and total incision); from 2017 to 2019, the method of total chest and laparoscopic triple incision (right posterolateral thoracotomy and abdominal incision and left neck) was added. For early stage-cancer, the site was the upper esophagus, and if the esophageal tumor was easy to separate it was removed by separate endoscopic surgery. For intermediate-stage cancer, the lesion was behind the aortic arch, and right chest incision (right posterolateral thoracotomy and abdominal median incision) was used. For the middle and lower esophageal lesions (under the carina), a left posterolateral thoracotomy and incision was often used.
Immunohistochemical staining was performed using the following antibodies: Rabbit monoclonal anti-SOX2 antibody (ab97959, Abcam, 1:1000 dilution), rabbit monoclonal anti-E-cadherin antibody (ab227639, Abcam, 1:500 dilution), and rabbit monoclonal anti-vimentin antibody (ab92547, Abcam, 1:500 dilution).
Formalin-fixed samples were embedded in paraffin and then subjected to deparaffinization/ hydration and incubation at 58 to 65 °C for 1 h. On the day before the assays, sections were put into a 37 °C oven overnight to soften the wax layer. After dewaxing and dehydration, the sections were added to a boiling EDTA repair solution (pH 9.0) for 15 min, cooled at room temperature for 30 min, and then endogenous peroxidase was added for 20 min. The sections were then rinsed three times with phosphate-buffered saline, a goat anti-rabbit secondary antibody (PV-6001) was added, and the sections were then maintained at 37 °C for 1 h. Finally, DAB was added for staining. Each experiment included positive control sections and negative control sections, in which PBS replaced the primary antibody.
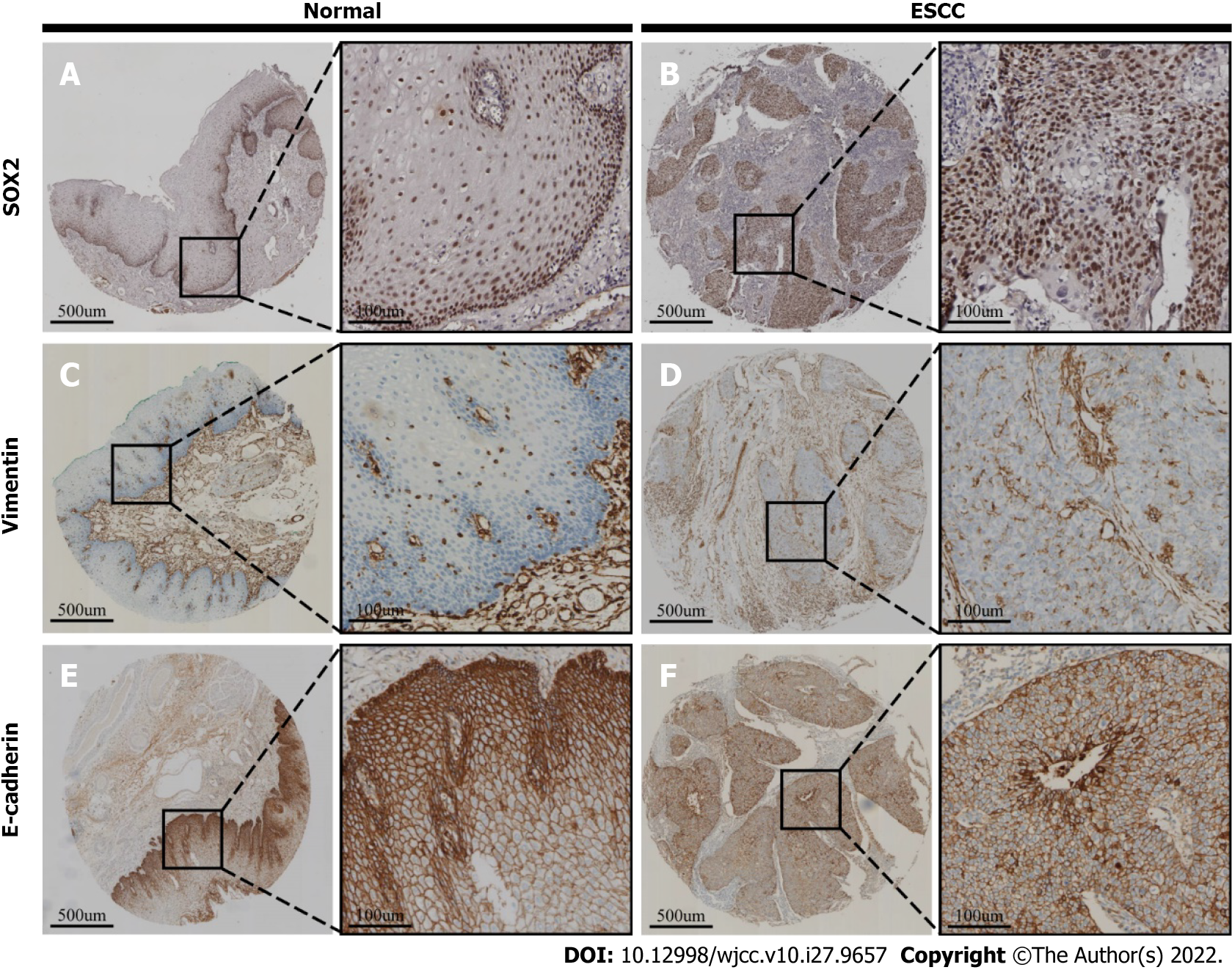
Samples of normal esophageal mucosal tissues that were adjacent to but separate from ESCC tissues were independently examined by two experienced pathologists. The microscopy of all slides was performed at the same incident light and compensation intensity, and five high-power fields (× 400) were randomly selected on each slide for analysis. SOX2 expression was assessed using a semi-quantitative staining index defined as the percentage of positive cells × staining intensity. For this calculation, staining intensity was scored 0 (negative), 1 (light brown), 2 (brown), or 3 (dark brown) and the percentage of positive cells was scored as 0 (0-10%), 1 (11%-25%), 2 (26%-50%), 3 (51%-75%), or 4 (76%-100%). Thus, the staining index ranged from 0 to 12. For analysis of SOX2 data, “positive expression” was defined by a staining index score of 4 or more and “negative expression” as a score of 3 or less[14].
E-cadherin expression was considered “negative” if stained cells accounted for 50% or more of continuous membrane staining and “positive” if this percentage was less than 50%, as described by Liu et al[15]. Vimentin expression was rated by determination of cytoplasmic staining in tumor cells, and “positive” expression was defined by staining of 3% or more of the cells. This threshold was used because nonspecific staining can occur in 1% or more of tumor cells[16].
In ESCC tissues (Figure 1), SOX2 is expressed in the nucleus (Figure 1B), E-cadherin is expressed in the plasma membrane (Figure 1F), and vimentin is expressed in the cytoplasm (Figure 1D). In normal esophageal epithelial tissues, SOX2 and vimentin are undetectable or only expressed in basal layer cells (Figure 1A and C), and E-cadherin is expressed in the plasma membrane of basal layer cells (Figure 1E).
Three different subtypes of the EMT were defined based on the expression of E-cadherin and vimentin: Epithelial cell group (negative for both E-cadherin and vimentin); mixed group (positive for E-cadherin and negative for vimentin, or negative for E-cadherin and positive for vimentin); and mesenchymal cell group (positive for both E-cadherin and vimentin)[16].
SPSS version 22.0 (SPSS Inc, Chicago, IL, United States) was used for all data management and statistical analyses. Categorical variables were expressed as numerals and continuous variables as means ± SD. Patient characteristics were compared using the χ2 test or Fisher’s exact test (when the expected frequency was less than 5 in a contingency table). OS was assessed using log-rank test with Kaplan-Meier survival curves. A Cox proportional hazard regression model was used to identify the significance and independence of the relationships of different factors with OS. A P value below 0.05 was considered significant.
We recorded the baseline clinicopathological characteristics of all 185 ESCC patients (Table 1). There were 129 males and 56 females and the mean age was 63.47 ± 8.568 years (range: 32–83, median: 64). Ninety-six patients (51.9%) had Han ethnicity and 89 (48.1%) had Kazakh ethnicity. We classified ESCC differentiation as high in 23 patients (12.4%), moderate in 117 patients (63.2%), and poor in 45 patients (24.3%). Analysis of AJCC staging indicated 15 patients (8.1%) with stage I, 119 (64.3%) with stage II, 35 (18.9%) with stage III, and 16 (8.6%) with stage IV. There were 57 patients (30.8%) with lymph node metastases and 9 patients (4.9%) with distant metastases.
Analysis of the immunohistochemical staining results indicated that 102 samples (55.1%) were positive for SOX2, 71 (38.4%) were positive for E-cadherin, and 116 (62.7%) were positive for vimentin (Table 2). Based on our EMT subtyping criteria, 38 patients (20.5%) were in the epithelial group, 107 (57.8%) were in the mixed group, and 40 (21.6%) were in the mesenchymal group (Supplementary Table 1).
| Characteristic | SOX2, n (%) | P value | E-cadherin, n (%) | P value | Vimentin, n (%) | P value | |||
| (-) | (+) | (-) | (+) | (-) | (+) | ||||
| Cases | 83 (44.9) | 102 (55.1) | 114 (61.6) | 71 (38.4) | 69 (37.3) | 116 (62.7) | |||
| Age, yr | |||||||||
| < 65 | 44 (53.0) | 56 (54.9) | 0.882 | 62 (54.4) | 38 (53.5) | 0.909 | 34 (49.3) | 66 (56.9) | 0.314 |
| ≥ 65 | 39 (47.0) | 46 (45.1) | 52 (45.6) | 33 (46.5) | 35 (50.7) | 50 (43.1) | |||
| Gender | |||||||||
| Male | 57 (68.7) | 72 (70.6) | 0.872 | 82 (71.9) | 47(66.2) | 0.409 | 53 (76.8) | 76 (65.5) | 0.106 |
| Female | 26 (31.3) | 30 (29.4) | 32 (28.1) | 24 (33.8) | 16 (23.2) | 40 (34.5) | |||
| Ethnicity | |||||||||
| Han | 49 (59.0) | 47 (46.1) | 0.103 | 62 (54.4) | 34 (47.9) | 0.390 | 38 (55.1) | 58 (50.0) | 0.504 |
| Kazakh | 34 (41.0) | 55 (53.9) | 52 (45.6) | 37 (52.1) | 31 (44.9) | 58 (50.0) | |||
| Tumor size | |||||||||
| < 3 cm | 71 (85.5) | 50 (49.0) | < 0.001a | 75 (65.8) | 46 (64.8) | 0.889 | 51 (73.9) | 70 (60.3) | 0.061 |
| ≥ 3 cm | 12 (14.5) | 52 (51.0) | 39 (34.2) | 25 (35.2) | 18 (26.1) | 46 (39.7) | |||
| Lymph node metastasis | |||||||||
| No | 60 (72.3) | 68 (66.7) | 0.428 | 76 (66.7) | 52 (73.2) | 0.346 | 53 (76.8) | 75 (64.7) | 0.083 |
| Yes | 23 (27.7) | 34 (33.3) | 38 (33.3) | 19 (26.8) | 16 (23.2) | 41 (35.3) | |||
| Tumor location | |||||||||
| Upper | 1 (1.2) | 7 (6.9) | 0.108 | 5 (4.4) | 3 (4.2) | 0.780 | 3 (4.3) | 5 (4.3) | 0.935 |
| Middle | 47 (56.6) | 50 (49.0) | 62 (54.4) | 35 (49.3) | 35 (50.7) | 62 (53.4) | |||
| Lower | 35 (42.2) | 45 (44.1) | 47 (41.2) | 33 (46.5) | 31 (44.9) | 49 (42.2) | |||
| Degree of differentiation | |||||||||
| High | 12 (14.5) | 11 (10.8) | 0.475 | 10(8.8) | 13 (18.3) | 0.035a | 9 (13.0) | 14 (12.1) | 0.953 |
| Moderate | 54 (65.1) | 63 (61.8) | 80 (70.2) | 37 (52.1) | 44 (63.8) | 73 (62.9) | |||
| Poor | 17 (20.5) | 28 (27.5) | 24 (21.1) | 21 (29.6) | 16 (23.2) | 29 (25.0) | |||
| AJCC stage | |||||||||
| I | 6 (7.2) | 9 (8.8) | 0.697 | 10(8.8) | 5(7.0) | 0.385 | 6 (8.7) | 9 (7.8) | 0.592 |
| II | 57 (68.7) | 62 (60.8) | 73 (64.0) | 46 (64.8) | 48 (69.6) | 71 (61.2) | |||
| III | 13 (15.7) | 22 (21.6) | 24(21.1) | 11 (15.5) | 10 (14.5) | 25 (21.6) | |||
| IV | 7 (8.4) | 9 (8.8) | 7 (6.1) | 9 (12.7) | 5 (7.2) | 11 (9.5) | |||
| T stage | |||||||||
| T1 | 3 (3.6) | 4 (3.9) | 0.333 | 5 (4.4) | 2 (2.8) | 0.887 | 2 (2.9) | 5 (4.3) | 0.059 |
| T2 | 41 (49.4) | 37 (36.3) | 49 (43.0) | 29 (40.8) | 37 (53.6) | 41 (35.3) | |||
| T3 | 36 (43.4) | 55 (53.9) | 54 (47.4 | 37 (52.1) | 29 (42.0) | 62 (53.4) | |||
| T4 | 3 (3.6) | 6 (5.9) | 6 (5.3) | 3 (4.2) | 1 (1.4) | 8 (6.9) | |||
| N stage | |||||||||
| N0 | 60 (72.3) | 68 (66.7) | 0.122 | 76 (66.7) | 52 (73.2) | 0.274 | 53 (76.8) | 75 (64.7) | 0.342 |
| N1 | 10 (12.0) | 23 (22.5) | 25 (21.9) | 8 (11.3) | 9 (13.0) | 24 (20.7) | |||
| N2 | 9 (10.8) | 10 (9.8) | 10(8.8) | 9(12.7) | 5 (7.2) | 14 (12.1) | |||
| N3 | 4 (4.8) | 1 (1.0) | 3 (2.6) | 2 (2.8) | 2 (2.9) | 3 (2.6) | |||
| M stage | |||||||||
| No | 81 (97.6) | 95 (93.1) | 0.190 | 111 (97.4) | 65(91.5) | 0.088 | 67 (97.1) | 109 (94.0) | 0.488 |
| Yes | 2 (2.4) | 7 (6.9) | 3 (2.6) | 6 (8.5) | 2 (2.9) | 7 (6.0) | |||
| Depth of invasion | |||||||||
| Mucosa | 35 (42.2) | 16(15.7) | < 0.001a | 29 (25.4) | 22 (31.0) | 0.412 | 27(39.1) | 24 (20.7) | 0.007a |
| Muscularis/Full thickness | 48(57.8) | 86 (84.3) | 85 (74.6) | 49 (69.0) | 42 (60.9) | 92 (79.3) | |||
We analyzed the relationships of the expression of SOX2, E-cadherin, and vimentin with the clinicopathological parameters of the 185 ESCC patients (Supplementary Table 2). The results indicated that SOX2 expression had positive correlations with tumor size (r = 0.382, P < 0.001) and full depth of tumor invasion (r = 0.295, P < 0.001), and vimentin expression had a positive correlation with full depth of tumor invasion (r = 0.266, P < 0.001). Analysis of the relationships of the different biomarkers indicated that expression of SOX2 and vimentin had a positive correlation, and expression of vimentin and E-cadherin had a negative correlation (all P < 0.05).
Further analysis (Table 2) indicated that SOX2 expression was greater in tumors that were 3 cm or larger, and in tumors with muscularis and full thickness invasion (all P < 0.001). Vimentin expression was also significantly greater in tumors with muscularis and full thickness invasion (P = 0.007). E-cadherin expression was lower in tumors with moderate differentiation (P = 0.035). However, pairwise comparisons, in which a difference was considered significant if the P value was less than 0.0125 (Bonferroni correction), indicated there were no significant differences in the expression of these proteins in the groups with high, moderate, and poor differentiation (all P > 0.0125).
Analysis of the different EMT subtypes (Supplementary Table 1) indicated that the mesenchymal subtype was more likely to be present in tumors with distant metastasis (12.5%, P = 0.040) than the epithelial subtype (2.6%) and the mixed subtype (2.8%).
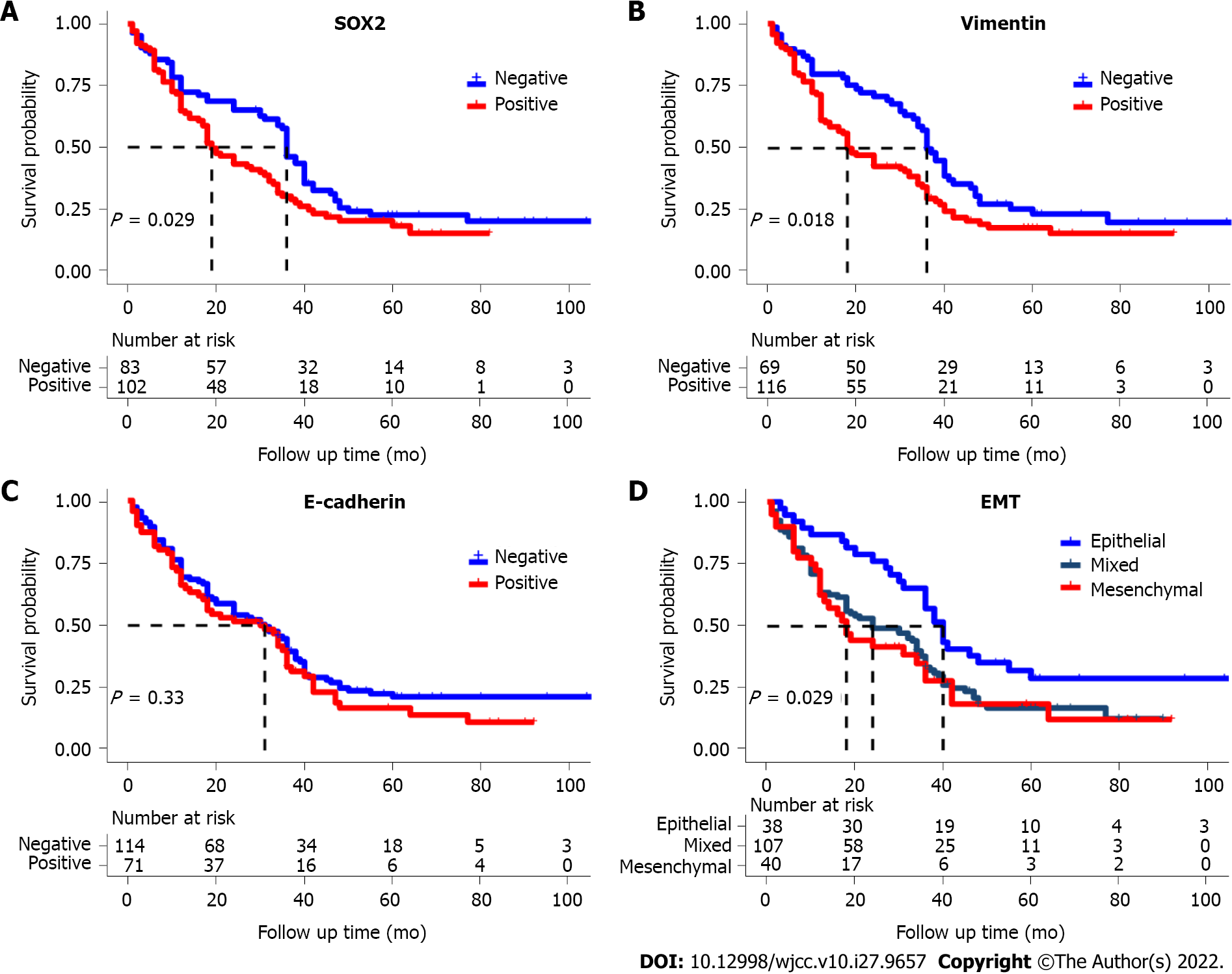
The Kaplan-Meier survival curves showed that OS was significantly and negatively associated with positivity for SOX2, positivity for vimentin, and the mesenchymal EMT subtype (Figure 2). However, E-cadherin positivity had no significant effect on OS (P = 0.12).
Univariate Cox proportional hazard regression analysis showed that vimentin expression, invasion, EMT subtype, and SOX2 expression were associated with poor OS (all P < 0.05; Table 3). However, multivariate analysis showed that SOX2 expression (HR: 1.53, 95%CI: 1.06–2.20, P = 0.022) and invasion (HR: 0.49, 95%CI: 0.331–0.771, P < 0.001) were the only factors associated with OS.
| Characteristic | Univariate analysis | Multivariate analysis | ||||
| Hazard ratio | 95%CI | P value | Hazard ratio | 95%CI | P value | |
| Age, yr (≥ 65 vs < 65) | 1.23 | 0.88-1.71 | 0.233 | |||
| Gender (Female vs Male) | 0.94 | 0.66-1.36 | 0.753 | |||
| Ethnicity (Han vs Kazakh) | 1.05 | 0.75-1.46 | 0.782 | |||
| Tumor size (≥ 3 cm vs < 3 cm) | 0.92 | 0.64-1.33 | 0.658 | |||
| Tumor location (Upper vs Middle and Lower) | 1.08 | 0.80-1.47 | 0.605 | |||
| AJCC stage (I vs II vs III vs IV) | 1.21 | 0.96-1.51 | 0.100 | |||
| Lymph node metastasis (No vs Yes) | 1.41 | 0.99-2.00 | 0.058 | |||
| Metastasis (No vs Yes) | 1.70 | 0.83-3.48 | 0.147 | |||
| Degree of differentiation (High vs Moderate vs Poor) | 0.76 | 0.56-1.02 | 0.069 | |||
| E-cadherin expression (Positive vs Negative) | 1.18 | 0.84-1.66 | 0.337 | |||
| Invasion (Mucosa vs Muscularis vs Full) | 0.65 | 0.45-0.92 | 0.015a | 0.49 | 0.331-0.711 | < 0.001a |
| EMT (Epithelial vs Mixed vs Mesenchymal) | 1.36 | 1.06-1.74 | 0.016a | 1.22 | 0.867-1.719 | 0.253 |
| SOX2 expression (Positive vs Negative) | 1.44 | 1.03- 2.03 | 0.034a | 1.53 | 1.06-2.20 | 0.022a |
| Vimentin expression (Positive vs Negative) | 1.51 | 1.06- 2.13 | 0.022a | 1.37 | 0.83-2.26 | 0.224 |
In the present study, we performed immunohistochemical staining to assess the relationship of SOX2 expression with EMT-associated markers and the clinical characteristics of patients with ESCC. Our results indicated that SOX2 positivity was associated with tumors that had full depth invasion, greater tumor cell proliferation, poor patient prognosis, and the mesenchymal EMT subtype. The transition of epithelial to stromal cells increases the aggressiveness and malignancy of ESCC. SOX2 is a biomarker of ESCC severity, so therapeutic targeting of this protein may potentially improve the prognosis of patients with these tumors.
SOX2, a well-known pluripotent transcription factor and tumor stem cell marker, plays a key role in the EMT during the normal physiological processes of embryogenesis[17] and development of the esophagus[18,19], but it also promotes the proliferation and metastasis of cancer cells[4,20]. In particular, SOX2 has high expression in tongue SCC, oral SCC, and ESCC[21]. Some researchers found that SOX2 can promote the proliferation of SCC. In this study, we found that high expression of SOX2 was related to tumor volume, so we speculate that SOX2 also promotes ESCC[22]. However, other research indicated that overexpression of SOX2 inhibited cell proliferation by activating cyclin-dependent kinase inhibitor 1B (p27Kip1)[23]. In addition, a critical range of SOX2 expression is needed to promote the proliferation of pancreatic tumor cells; these tumor cells do not proliferate when the SOX2 Level is too high or too low.
The role of SOX2 in promoting the proliferation of ESCC cells needs verification by further in vivo and in vitro studies. Some of our results are consistent with those of Takahashi et al[21]. For example, we both found that high expression of SOX2 in ESCC positively correlated with tumor size and the EMT. SOX2 can also promote the EMT in laryngeal cancer[24], esophageal cancer[25], and gastric cancer[26]. Conversely, EMT can promote SOX2 expression and the development of cells with stem cell-like properties in bladder cancer[27]. There is also evidence that SOX2 promoted the EMT via the STAT3 signaling pathway in ESCC[25].
Our results indicated ESCC patients with the mesenchymal EMT subtype had greater tissue metastases and shorter OS time than patients with the epithelial EMT subtype. We also identified correlations in the expression of SOX2 with EMT subtype. Therefore, SOX2 expression may promote distant metastasis of ESCC via the EMT. The EMT is a dynamic biological process in which epithelial cells transform into mesenchymal cells[28], and E-cadherin and vimentin are key markers of the initiation of the EMT in tumor cells[29]. At the molecular level, E-cadherin is a calcium-dependent adhesion protein that connects epithelial cells and forms adherens junctions between adjacent cells[30]. The loss of E-cadherin expression stimulates the EMT in tumors and increases tumor invasion and metastasis[31,32]. Twist, Snail and Zeb1 inhibit the transcription of E-cadherin and induce the EMT[33,34]. However, Padmanaban et al[35] found that although a reduced level of E-cadherin promoted tumor cell invasion, it also reduced cell survival, proliferation, distant metastatic growth, and the number of tumor cells in circulation.
We found no correlation of the E-cadherin level with the clinical characteristics or prognosis of patients with ESCC. This may be because of the complex dynamic processes of the EMT, so that E-cadherin is a suitable marker of the EMT for in vitro studies but not in clinical settings. Nevertheless vimentin, a type III intermediate filament protein, affects the migration of tumor cells, and plays a key role in allowing deformed tumor cells to pass through the basement membrane, manifesting as invasive growth and distant metastases[36-38]. Multiple transcription factors regulate vimentin and promote the EMT, such as Twist, Slug, and Zeb1, but vimentin itself can enhance the expression of other transcription factors, such as Snail[39]. The microRNA miR-515-3P also silences vimentin by directly binding to the coding region in its mRNA, and this inhibits expression of this protein and the metastasis of ESCC[40]. In our study, vimentin expression was associated with local invasion of ESCC, and there was a close positive correlation between the expression of vimentin and SOX2. In contrast[41], studies of head and neck SCC reported that down-regulation of SOX2 led to up-regulation of vimentin and promotion of tumor cell invasion and metastasis. However, the signal pathway responsible for the interaction between SOX2 and vimentin in ESCC is still unclear. Whether the EMT promotes tumor stem cell formation because of vimentin acting on SOX2 needs further experimental verification. Nonetheless, vimentin has prognostic value in ESCC, gastric cancer, and colorectal cancer[42], in that higher expression correlates with worse prognosis. Vimentin, a critical prognostic factor for OS in patients with ESCC, may affect patient outcome by directly or indirectly interacting with SOX2.
There were some shortcomings in this study. First, because this was a retrospective study, we can only identify associations and cannot infer causal relationships. Second, because ESCC patients with advanced disease or distant metastasis do not routinely undergo surgical treatment, we only examined 16 patients (8.6%) with stage IV cancer and 9 patients (4.9%) with distant metastasis. This may partly explain why we did not identify a significant association between AJCC stage and OS.
It is exciting to note that a recent single-cell transcriptomics study of ESCC reported that SOX2 was highly expressed in these cells and was an effective marker gene for identification of tumor cells and other cell types[43]. The results of this single-cell transcriptomics study, combined with our immunohistochemical results, further confirmed the key role of SOX2 in ESCC.
Thus, our immunohistochemistry results support the use of SOX2 as prognostic biomarker for ESCC. Importantly, vimentin may mediate the effects of SOX2 in promoting the EMT during ESCC progression. Further in-depth studies of these two prognostic biomarkers may help to elucidate the mechanism of cell invasion and metastasis during ESCC and also provide insights needed to develop targeted therapies for this cancer.
Sex determining region Y-box 2 (SOX2) is a promoter of squamous cell carcinoma (SCC), and high expression of SOX2 is related to the proliferation, migration, and invasion of SCC. However, there is limited knowledge of the relationship between SOX2 and the epithelial-mesenchymal transition (EMT) in esophageal SCC (ESCC).
Single cell sequencing proteomics studies can characterize the heterogeneity of cells within a tissue. For example, studies using this method reported that SOX2 was only expressed in epithelial cells, and was highly expressed in the epithelial cells of ESCC. Our previous bioinformatics research using TCGA database found that SOX2 expression was closely related to the EMT and the Wnt/β-catenin signaling pathway in ESCC. The present study was performed to verify the role of SOX2 during the EMT in ESCC and to determine its value as a prognostic indicator in these patients.
Perform tissue-level studies to determine if SOX2 is related to the EMT and clinicopathological characteristics in ESCC patients, and its possible role as a prognostic indicator in these patients.
The expression of SOX2, vimentin, and E-cadherin were determined by immunohistochemical staining and scoring, and the relationship between SOX2 expression and two classical marker proteins of the EMT was analyzed.
SOX2 had higher expression in ESCC than normal tissue, and its expression had positive correlations with the tumor invasion and tumor size. There was a negative correlation between SOX2 and overall survival, and SOX2 expression was an independent risk factor for prognosis of patients with ESCC. There was also a positive correlation between the expression of SOX2 and vimentin. SOX2 may promote the EMT in ESCC due to its direct or indirect interaction with vimentin.
SOX2 expression is an important prognostic indicator in patients with ESCC, and it appears to promote the migration, invasion, and infiltration of ESCC via vimentin.
Our clinical experiments indicated a correlation of SOX2 expression with the EMT and with activation of the Wnt/β-catenin signaling pathway. It is possible that inhibition of SOX2 expression in ESCC will inhibit the EMT, reduce tumor invasiveness, and improve patient prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lim SC, South Korea; Okamoto H, Japan S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology. 2018;154:360-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1155] [Article Influence: 165.0] [Reference Citation Analysis (1)] |
| 2. | Prabhu A, Obi KO, Rubenstein JH. The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: a meta-analysis. Am J Gastroenterol. 2014;109:822-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 3. | Hesari A, Azizian M, Sheikhi A, Nesaei A, Sanaei S, Mahinparvar N, Derakhshani M, Hedayt P, Ghasemi F, Mirzaei H. Chemopreventive and therapeutic potential of curcumin in esophageal cancer: Current and future status. Int J Cancer. 2019;144:1215-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 4. | Malinee M, Kumar A, Hidaka T, Horie M, Hasegawa K, Pandian GN, Sugiyama H. Targeted suppression of metastasis regulatory transcription factor SOX2 in various cancer cell lines using a sequence-specific designer pyrrole-imidazole polyamide. Bioorg Med Chem. 2020;28:115248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Tsunedomi R, Yoshimura K, Suzuki N, Hazama S, Nagano H. Clinical implications of cancer stem cells in digestive cancers: acquisition of stemness and prognostic impact. Surg Today. 2020;50:1560-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, Cunningham D. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 736] [Article Influence: 92.0] [Reference Citation Analysis (2)] |
| 7. | Tachimori Y, Ozawa S, Numasaki H, Matsubara H, Shinoda M, Toh Y, Udagawa H; Registration Committee for Esophageal Cancer of the Japan Esophageal Society. Supraclavicular node metastasis from thoracic esophageal carcinoma: A surgical series from a Japanese multi-institutional nationwide registry of esophageal cancer. J Thorac Cardiovasc Surg. 2014;148:1224-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Sleeman J, Steeg PS. Cancer metastasis as a therapeutic target. Eur J Cancer. 2010;46:1177-1180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 815] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 10. | Chung BM, Rotty JD, Coulombe PA. Networking galore: intermediate filaments and cell migration. Curr Opin Cell Biol. 2013;25:600-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Tripathi S, Levine H, Jolly MK. The Physics of Cellular Decision Making During Epithelial-Mesenchymal Transition. Annu Rev Biophys. 2020;49:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 12. | Akimoto N, Nakamura K, Hijioka H, Kume K, Matsumura Y, Sugiura T. Transfection of T-Box Transcription Factor BRACHYURY and SOX2 Synergistically Promote Self-Renewal and Invasive Phenotype in Oral Cancer Cells. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390:2383-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 847] [Article Influence: 105.9] [Reference Citation Analysis (0)] |
| 14. | Zhou YX, Liu Q, Wang H, Ding F, Ma YQ. The expression and prognostic value of SOX2, β-catenin and survivin in esophageal squamous cell carcinoma. Future Oncol. 2019;15:4181-4195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Liu Y, Feng X, Lai J, Yi W, Yang J, Du T, Long X, Zhang Y, Xiao Y. A novel role of kynureninase in the growth control of breast cancer cells and its relationships with breast cancer. J Cell Mol Med. 2019;23:6700-6707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Matsubara T, Toyokawa G, Takada K, Kinoshita F, Kozuma Y, Akamine T, Shimokawa M, Haro A, Osoegawa A, Tagawa T, Mori M. The association and prognostic impact of enhancer of zeste homologue 2 expression and epithelial-mesenchymal transition in resected lung adenocarcinoma. PLoS One. 2019;14:e0215103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Kinney BA, Al Anber A, Row RH, Tseng YJ, Weidmann MD, Knaut H, Martin BL. Sox2 and Canonical Wnt Signaling Interact to Activate a Developmental Checkpoint Coordinating Morphogenesis with Mesoderm Fate Acquisition. Cell Rep. 2020;33:108311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Trisno SL, Philo KED, McCracken KW, Catá EM, Ruiz-Torres S, Rankin SA, Han L, Nasr T, Chaturvedi P, Rothenberg ME, Mandegar MA, Wells SI, Zorn AM, Wells JM. Esophageal Organoids from Human Pluripotent Stem Cells Delineate Sox2 Functions during Esophageal Specification. Cell Stem Cell. 2018;23:501-515.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 19. | Williamson KA, Hever AM, Rainger J, Rogers RC, Magee A, Fiedler Z, Keng WT, Sharkey FH, McGill N, Hill CJ, Schneider A, Messina M, Turnpenny PD, Fantes JA, van Heyningen V, FitzPatrick DR. Mutations in SOX2 cause anophthalmia-esophageal-genital (AEG) syndrome. Hum Mol Genet. 2006;15:1413-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Chaudhary S, Islam Z, Mishra V, Rawat S, Ashraf GM, Kolatkar PR. Sox2: A Regulatory Factor in Tumorigenesis and Metastasis. Curr Protein Pept Sci. 2019;20:495-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Takahashi K, Asano N, Imatani A, Kondo Y, Saito M, Takeuchi A, Jin X, Hatta W, Asanuma K, Uno K, Koike T, Masamune A. Sox2 induces tumorigenesis and angiogenesis of early-stage esophageal squamous cell carcinoma through secretion of Suprabasin. Carcinogenesis. 2020;41:1543-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E, Brohée S, Salmon I, Dubois C, del Marmol V, Fuks F, Beck B, Blanpain C. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 513] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 23. | Liu Z, Walters BJ, Owen T, Brimble MA, Steigelman KA, Zhang L, Mellado Lagarde MM, Valentine MB, Yu Y, Cox BC, Zuo J. Regulation of p27Kip1 by Sox2 maintains quiescence of inner pillar cells in the murine auditory sensory epithelium. J Neurosci. 2012;32:10530-10540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Yang N, Hui L, Wang Y, Yang H, Jiang X. Overexpression of SOX2 promotes migration, invasion, and epithelial-mesenchymal transition through the Wnt/β-catenin pathway in laryngeal cancer Hep-2 cells. Tumour Biol. 2014;35:7965-7973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Gao H, Teng C, Huang W, Peng J, Wang C. SOX2 Promotes the Epithelial to Mesenchymal Transition of Esophageal Squamous Cells by Modulating Slug Expression through the Activation of STAT3/HIF-α Signaling. Int J Mol Sci. 2015;16:21643-21657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Guo J, Wang B, Fu Z, Wei J, Lu W. Hypoxic Microenvironment Induces EMT and Upgrades Stem-Like Properties of Gastric Cancer Cells. Technol Cancer Res Treat. 2016;15:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Migita T, Ueda A, Ohishi T, Hatano M, Seimiya H, Horiguchi SI, Koga F, Shibasaki F. Epithelial-mesenchymal transition promotes SOX2 and NANOG expression in bladder cancer. Lab Invest. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori G, Dedhar S, Derynck R, Ford HL, Fuxe J, García de Herreros A, Goodall GJ, Hadjantonakis AK, Huang RYJ, Kalcheim C, Kalluri R, Kang Y, Khew-Goodall Y, Levine H, Liu J, Longmore GD, Mani SA, Massagué J, Mayor R, McClay D, Mostov KE, Newgreen DF, Nieto MA, Puisieux A, Runyan R, Savagner P, Stanger B, Stemmler MP, Takahashi Y, Takeichi M, Theveneau E, Thiery JP, Thompson EW, Weinberg RA, Williams ED, Xing J, Zhou BP, Sheng G; EMT International Association (TEMTIA). Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2020;21:341-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 624] [Cited by in RCA: 1386] [Article Influence: 277.2] [Reference Citation Analysis (0)] |
| 29. | Chen T, You Y, Jiang H, Wang ZZ. Epithelial-mesenchymal transition (EMT): A biological process in the development, stem cell differentiation, and tumorigenesis. J Cell Physiol. 2017;232:3261-3272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 410] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 30. | Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6575] [Cited by in RCA: 7908] [Article Influence: 494.3] [Reference Citation Analysis (0)] |
| 31. | Watanabe Y, Imanishi Y, Ozawa H, Sakamoto K, Fujii R, Shigetomi S, Habu N, Otsuka K, Sato Y, Sekimizu M, Ito F, Ikari Y, Saito S, Kameyama K, Ogawa K. Selective EP2 and Cox-2 inhibition suppresses cell migration by reversing epithelial-to-mesenchymal transition and Cox-2 overexpression and E-cadherin downregulation are implicated in neck metastasis of hypopharyngeal cancer. Am J Transl Res. 2020;12:1096-1113. [PubMed] |
| 32. | Wijshake T, Zou Z, Chen B, Zhong L, Xiao G, Xie Y, Doench JG, Bennett L, Levine B. Tumor-suppressor function of Beclin 1 in breast cancer cells requires E-cadherin. Proc Natl Acad Sci U S A. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 33. | Kang Y, Massagué J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 1182] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 34. | Mikami S, Katsube K, Oya M, Ishida M, Kosaka T, Mizuno R, Mukai M, Okada Y. Expression of Snail and Slug in renal cell carcinoma: E-cadherin repressor Snail is associated with cancer invasion and prognosis. Lab Invest. 2011;91:1443-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Padmanaban V, Krol I, Suhail Y, Szczerba BM, Aceto N, Bader JS, Ewald AJ. E-cadherin is required for metastasis in multiple models of breast cancer. Nature. 2019;573:439-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 552] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 36. | Jiu Y, Peränen J, Schaible N, Cheng F, Eriksson JE, Krishnan R, Lappalainen P. Vimentin intermediate filaments control actin stress fiber assembly through GEF-H1 and RhoA. J Cell Sci. 2017;130:892-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 37. | Gregor M, Osmanagic-Myers S, Burgstaller G, Wolfram M, Fischer I, Walko G, Resch GP, Jörgl A, Herrmann H, Wiche G. Mechanosensing through focal adhesion-anchored intermediate filaments. FASEB J. 2014;28:715-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 38. | Tsuruta D, Jones JC. The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J Cell Sci. 2003;116:4977-4984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 189] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 39. | Strouhalova K, Přechová M, Gandalovičová A, Brábek J, Gregor M, Rosel D. Vimentin Intermediate Filaments as Potential Target for Cancer Treatment. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 167] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 40. | Hu HF, Xu WW, Zhang WX, Yan X, Li YJ, Li B, He QY. Identification of miR-515-3p and its targets, vimentin and MMP3, as a key regulatory mechanism in esophageal cancer metastasis: functional and clinical significance. Signal Transduct Target Ther. 2020;5:271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Bayo P, Jou A, Stenzinger A, Shao C, Gross M, Jensen A, Grabe N, Mende CH, Rados PV, Debus J, Weichert W, Plinkert PK, Lichter P, Freier K, Hess J. Loss of SOX2 expression induces cell motility via vimentin up-regulation and is an unfavorable risk factor for survival of head and neck squamous cell carcinoma. Mol Oncol. 2015;9:1704-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 42. | Al-Maghrabi J. Vimentin immunoexpression is associated with higher tumor grade, metastasis, and shorter survival in colorectal cancer. Int J Clin Exp Pathol. 2020;13:493-500. [PubMed] |
| 43. | Chen Z, Zhao M, Liang J, Hu Z, Huang Y, Li M, Pang Y, Lu T, Sui Q, Zhan C, Lin M, Guo W, Wang Q, Tan L. Dissecting the single-cell transcriptome network underlying esophagus non-malignant tissues and esophageal squamous cell carcinoma. EBioMedicine. 2021;69:103459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |