Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9628
Peer-review started: April 6, 2022
First decision: June 16, 2022
Revised: June 30, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: September 26, 2022
Processing time: 162 Days and 23.3 Hours
Loss of motor function in the trapezius muscle is one complication of radical neck dissection after cutting the accessory nerve (AN) during surgery. Nerve repair is an effective method to restore trapezius muscle function, and includes neurolysis, direct suture, and nerve grafting. The suprascapular nerve (SCN) and AN are next to each other in position. The function of the AN and SCN in shoulder elevation and abduction movement is synergistic. SCN might be considered by surgeons for AN reanimation.
To obtain anatomical and clinical data for partial suprascapular nerve-to-AN transfer.
Ten sides of cadavers perfused with formalin were obtained from the Department of Human Anatomy, Histology and Embryology, Peking University Health Science Center. The SCN (n = 10) and AN (n = 10) were carefully dissected in the posterior triangle of the neck, and the trapezius muscle was dissected to fully display the accessory nerve. The length of the SCN from the origin of the brachial plexus (a point) to the scapular notch (b point) and the distance of the SCN from the origin point (a point) to the point (c point) where the AN entered the border of the trapezius muscle were measured. The length and branches of the AN in the trapezius muscle were measured. A female patient aged 55 years underwent surgery for partial SCN to AN transfer at Department of Oral and Maxillofacial Surgery, Peking University School and Hospital of Stomatology. The patient suffered from recurrent upper gingival cancer. Radical neck dissection was performed on the right side, and the right AN was removed at the intersection between the nerve and the posterior border of the SCM muscle. One-third of the diameter of the SCN was cut off, and combined epineurial and perineurial sutures were applied between the distal end of the cut-off fascicles of the SCN and the proximal end of the AN without tension. Both subjective and objective evaluations were performed before, three months after, and nine months after surgery. For the subjective evaluation, the questionnaire included the Neck Dissection Impairment Index (NDII) and the Constant Shoulder Scale. Electromyography was used for the objective examination. Data were analyzed using t tests with SPSS 19.0 software to determine the relationship between the length of the SCN and the linear distance. A P value of < 0.05 was considered as statistically significant.
The whole length of the AN in the trapezius muscle was 16.89 cm. The average numbers of branches distributed in the descending, horizontal and ascending portions were 3.8, 2.6 and 2.2, respectively. The diameter of the AN was 1.94 mm at the anterior border of the trapezius. The length of the suprascapular nerve from the origin of the brachial plexus to the scapular notch was longer than the distance of the suprascapular nerve from the origin point to the point where the accessory nerve entered the upper edge of the trapezius muscle. The amplitude of trapezius muscle electromyography indicated that both the horizontal and ascending portions of the trapezius muscle on the right side had better function than the left side nine months after surgery. The results showed that the right-sided supraspinatus and infraspinatus muscles did not lose more function than the left side.
Based on anatomical data and clinical application, partial suprascapular nerve-to-AN transfer could be achieved and may improve innervation of the affected trapezius muscle after radical neck dissection.
Core Tip: We performed the dissection and measurement of ten sides of cadavers to obtain the data of the suprascapular nerve and accessory nerve. In the posterior cervical triangle, we found that the suprascapular nerve could obtain enough nerve length, from its origin to the suprascapular notch, to perform suprascapular nerve-accessory nerve partial nerve transplantation and achieve tension-free suture, suggesting the feasibility of transplantation of the suprascapular nerve as a donor. Nerve transfer from the partial suprascapular nerve to the accessory nerve was performed on one patient, and electromyography examinations were performed three months and nine months after surgery. Our research showed that suprascapular nerve transfer may improve trapezius muscle function and reduce loss of function in the supraspinatus and infraspinatus muscles after suprascapular nerve transfer.
- Citation: Wang JW, Zhang WB, Li F, Fang X, Yi ZQ, Xu XL, Peng X, Zhang WG. Anatomy and clinical application of suprascapular nerve to accessory nerve transfer. World J Clin Cases 2022; 10(27): 9628-9640
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9628.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9628
Iatrogenic injury is one of the major causes of human nerve injury. Approximately 94% of accessory nerve (AN) injuries are iatrogenic[1,2]. Injury to the AN can cause weakness or paralysis in the sternocleidomastoid (SCM) and trapezius muscles[3]. Loss of motor function in the trapezius muscle leads to a painful and disabling condition[2], and this syndrome is frequently observed after radical neck dissection. Pain and dysfunction associated with a loss of innervation by the AN have been reported[4,5].
Anatomic information and the precise position of the AN are very important for the protection of the AN during surgery. AN is generally accepted as being a motor nerve. It mainly innervates the sternocleidomastoid and trapezius muscles. AN is classically described as having a spinal root and a cranial root. The spinal root originates from the spinal nucleus of cervical vertebrae C1 to C5. The cranial root originates from the dorsolateral surface of the medulla oblongata. The spinal and cranial roots merge into a total stem and exit the skull. Then, it passes through the posterior cervical triangle, lateral to the vagus nerve, ahead of the internal jugular vein (IJV), while sending out muscle branches to the SCM and the trapezius muscle. In the posterior cervical triangle region, its position is relatively superficial and vulnerable to injury[6,7]. Previous studies have shown that the AN passes through the SCM in three ways[8]. In addition to AN, one study showed that the branches of the cervical plexus that join the main AN or separate branches innervated the trapezius muscle[9], which are conducive to the protection of the AN or other branch nerves and the function of the trapezius muscle during surgery.
Current surgical treatment for trapezius paralysis mainly includes two strategies. First, fascial muscle transplantation mainly includes static and dynamic transplantation. A static procedure involves fixing the scapula to the spinous process by fascia transplantation, but it could lead to weakened fascia due to prolonged stretching. The dynamic approach is used to restore the function of the scapula through muscle transfer[2]. Another surgical strategy is nerve repair[10,11], which includes neurolysis, direct suture, and nerve grafting. A key point in nerve transplantation for the treatment of trapezius paralysis is finding the appropriate donor nerves. It has been reported that trapezius muscle function can be restored by directly transplanting a nerve in the upper trunk of the brachial plexus and suturing the distal end of the AN[12]. According to Tubbs's anatomical study, the suprascapular nerve (SCN) could be used as a donor nerve for nerve repair[13]. SCN mainly originates from the C5, C6, and occasionally C4 nerve roots. It contains motor and sensory nerve fibers. It walks in the posterior triangle of the neck, passes through the scapular notch and walks on the back of the scapula. Its main function is motor innervation of the supraspinatus and infraspinatus muscles. In addition, it also sends out sensory branches to the acromioclavicular and glenohumeral joints and the coracoacromial ligament[14]. The accessory nerve and suprascapular nerve are adjacent to each other and have similar functions. To further explore the feasibility of transplanting the suprascapular nerve as a donor nerve to the accessory nerve to restore trapezius muscle function, we conducted exploratory studies of both human anatomy and clinical application.
Ten sides of cadavers perfused with formalin through the femoral artery were obtained from the Department of Human Anatomy, Histology and Embryology, Peking University Health Science Center. This study was approved by the Peking University Institutional Review Board (IRB00001052-21011-Exempt). The age range of cadavers was 60-80 years. All cadavers had no visible scars, deformities, or obvious trauma to the face, neck, or shoulders.
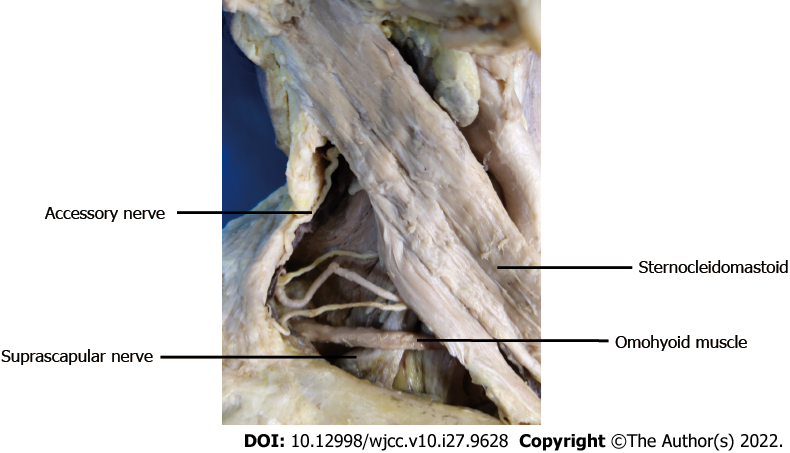
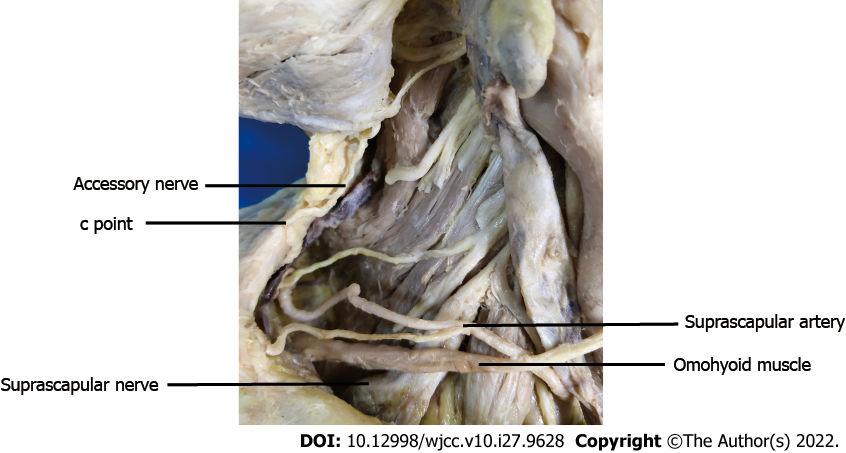
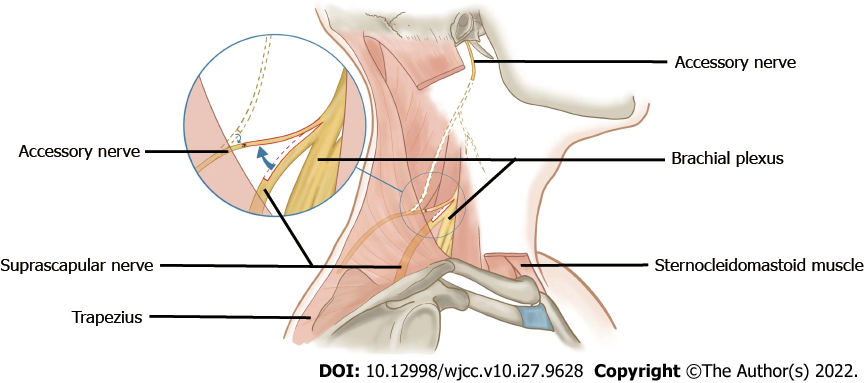
Cadavers were dissected, and the SCN (n = 10) and AN (n = 10) at the lateral cervical region were exposed. The SCN and AN were carefully dissected in the posterior triangle of the neck as follows. First, the SCM was exposed after removing the superficial tissue. Second, the SCN from C5 of the brachial plexus could be found beneath and close to the lower belly of the omohyoid muscle, and AN emerges in the posterior triangle at the anterior margin of the trapezius (Figure 1). Third, the SCM was elevated, beneath which we found the accessory nerve at the anterior edge of the trapezius muscle and the suprascapular nerve from the beginning to the scapular notch segment (Figures 2 and 3). Fourth, the trapezius muscle was dissected to fully display the accessory nerve (Figure 4).
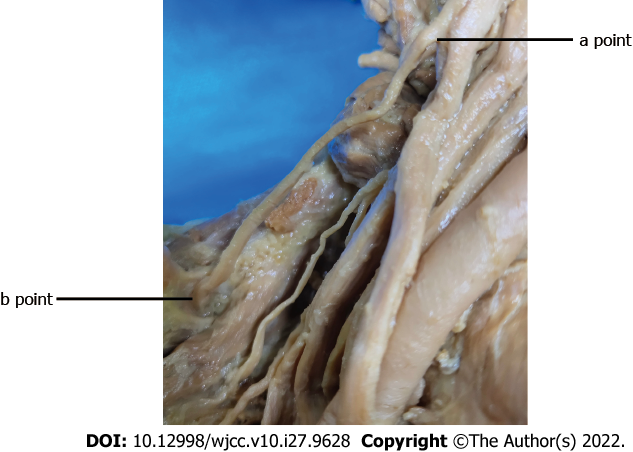
The length and branches of the AN in the trapezius muscle, the length of the SCN from the origin of the brachial plexus (a point, Figure 2) to the scapular notch (b point, Figure 2), and the distance of the SCN from the origin point (a point, Figure 2) to the point (c point, Figure 3) where the AN entered the border of the trapezius muscle were measured.
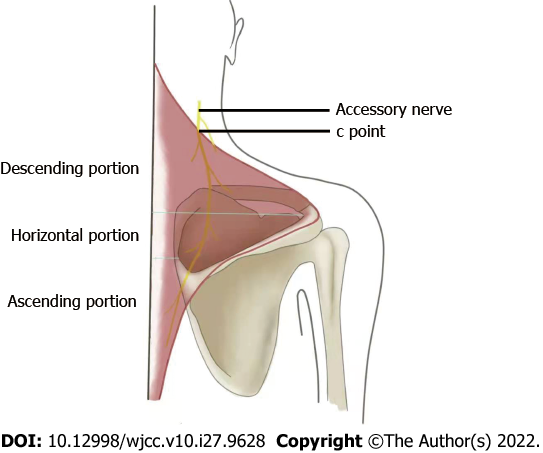
The length and branches of the AN in the trapezius muscle were measured. The trapezius muscle was divided into three portions: The descending portion, horizontal portion, and ascending portion. The division was based on two horizontal lines: one was through the vertex of the angle between the acromiom and the spine of the scapula at the lateral dorsal edge of the scapula, and the other was through the uppermost edge of the plane of the deltoid tubercle at the medial edge of the scapula (Figure 4). We measured the number of branches of the accessory nerve in three portions. A branch whose diameter was less than 0.5 mm was considered as the end branch and was no longer dissected.
Data were analyzed using t tests with SPSS 19.0 software to determine the relationship between the length of the SCN and the linear distance. A P value of < 0.05 was considered as statistically significant.
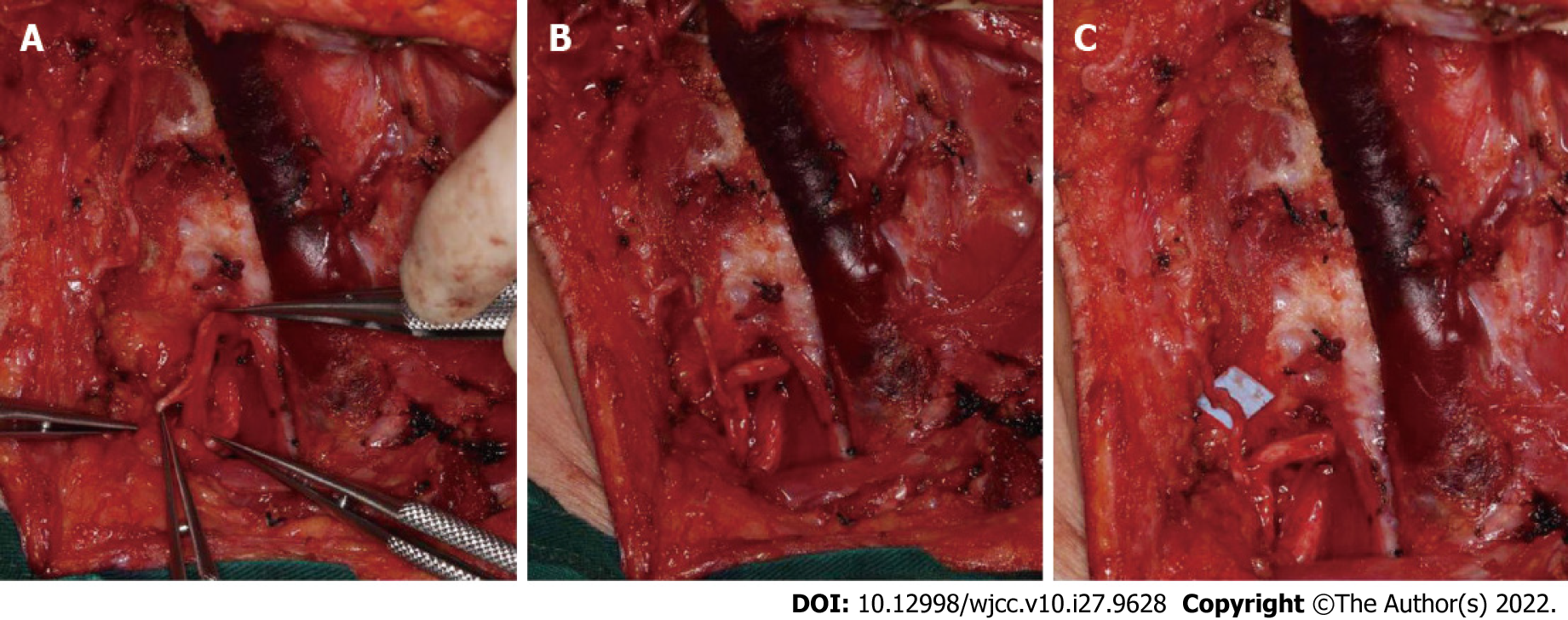
A female patient aged 55 years underwent surgery for partial SCN to AN transfer at Department of Oral and Maxillofacial Surgery, Peking University School, and Hospital of Stomatology. All procedures were approved by the Biomedical Ethics Committee of Peking University School and Hospital of Stomatology (PKUSSIRB-2013040). The patient suffered from recurrent upper gingival cancer, which demonstrated lymphatic metastasis in the right cervical region. Radical neck dissection in cervical regions I, II, III, IV, and V was performed on the right side, and the SCM muscle was removed. On the left side, selective neck dissection in cervical regions I, II, and III was performed without AN injury. The right AN was removed at the intersection between the nerve and the posterior border of the SCM muscle. The SCN was dissected along the branch of the brachial plexus. Groups of fascicles of the SCN were identified. One-third of the diameter of the SCN was cut off, and combined epineurial and perineurial sutures were applied between the distal end of the cut-off fascicles of the SCN and the proximal end of the AN without tension (Figures 5 and 6). Nylon suture (8-0) was used for nerve transfer.
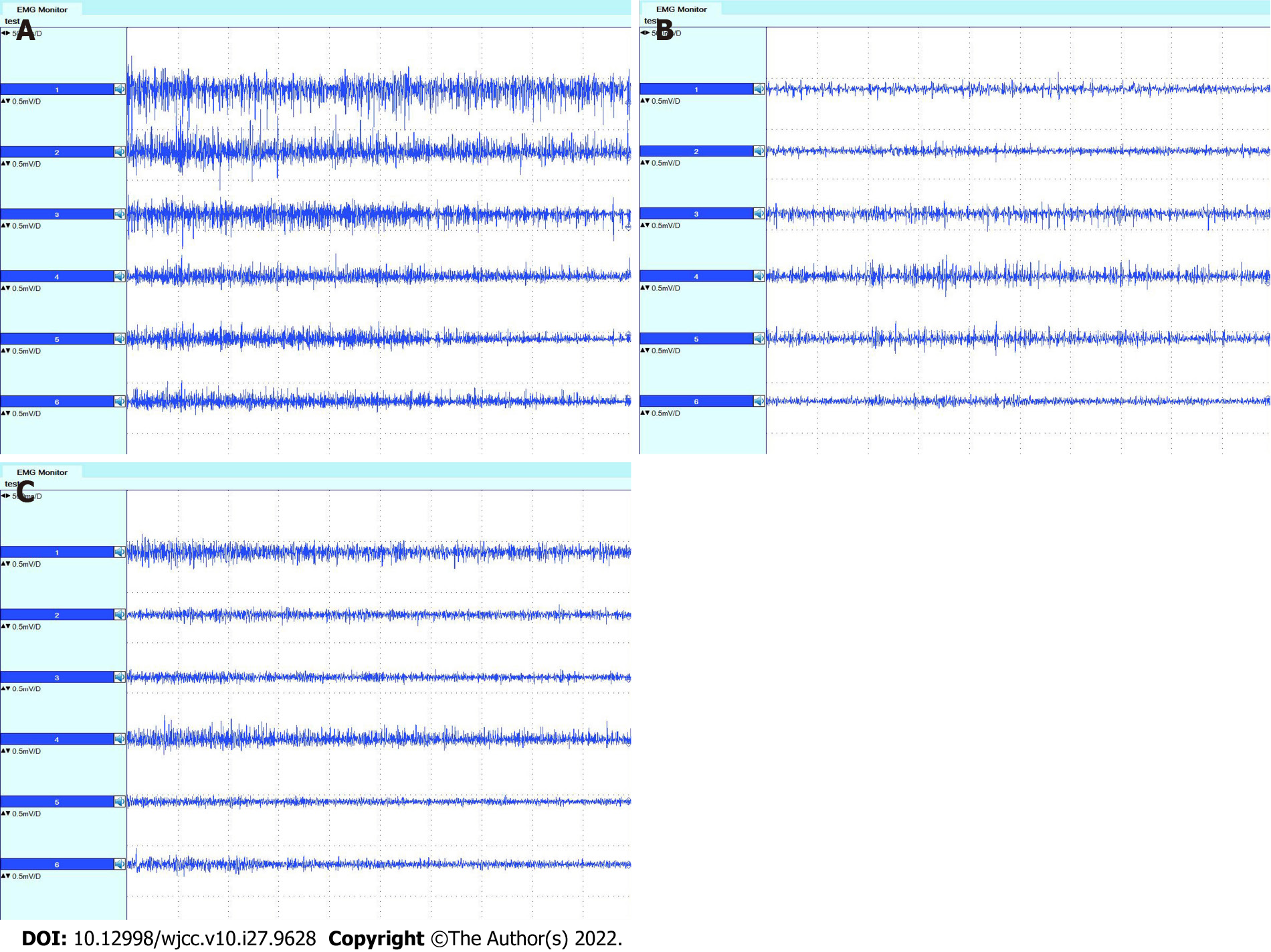
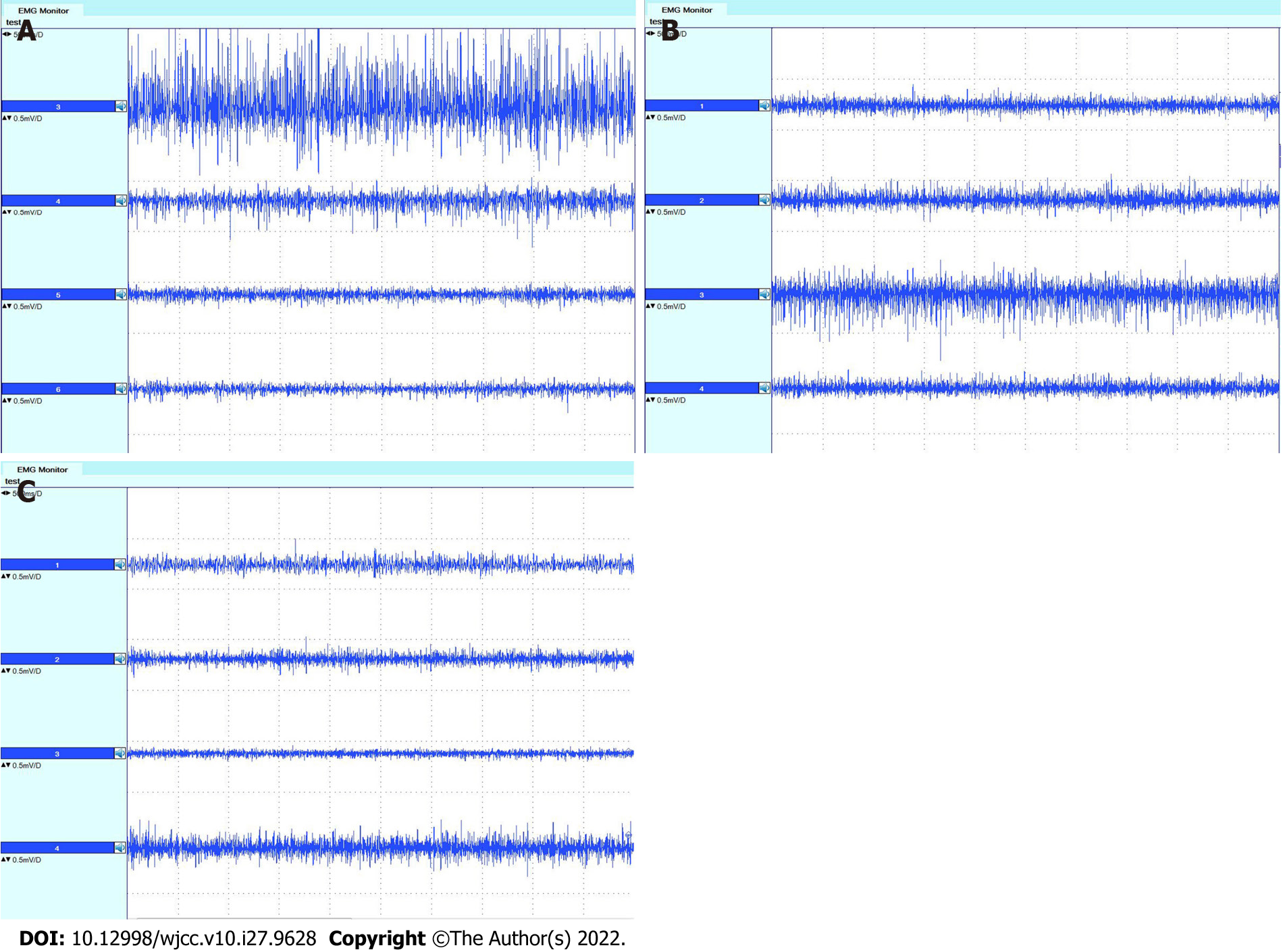
Both subjective and objective evaluations were performed before, three months after, and nine months after surgery. For the subjective evaluation, the questionnaire included the Neck Dissection Impairment Index (NDII) and the Constant Shoulder Scale[15,16]. Thirty-five points on the Constant Shoulder Scale were recorded in this section. For the objective evaluation, two methods were used. One was to examine the movement of the shoulder and upper arm and record the 65 points of objective evaluation using the Constant Shoulder Scale before, three months after, and nine months after surgery. Another was to carry out electromyography of the trapezius, supraspinatus, and infraspinatus muscles before, three months after, and nine months after surgery. Electromyography (Dentec Keypoint, Natus Medical Incorporated, Denmark) was used for the examination. The descending, horizontal, and ascending parts of the trapezius muscle were detected in this study according to the method used by Krause et al[17]. The middle was placed at the line from the level of the second thoracic vertebra to the suprascapular angle, 3 cm from the longitudinal axis of the spine. The ascending portion was placed at the horizontal line of the inferior corner of the shoulder, 3 cm from the longitudinal axis of the spine. During examination of the trapezius muscle, electromyography was performed when the patient shrugged shoulders and closed shoulder blades with maximum strength and abducted the upper arm to 90° for two seconds. For supraspinatus and infraspinatus electromyography, external rotation of the upper arm around the humerus bone with maximum strength and abduction of the upper arm to 90° for two seconds were performed. Surface electrodes were placed on the supraspinatus and infraspinatus just above and below the scapular spine and at the middle of the supraspinous and infraspinous fossa of the scapula, respectively[18,19]. The ratio, R, of the amplitude of electromyography before and after surgery was calculated as follows:
R = (amplitude of EMG before surgery-amplitude of EMG after surgery)/amplitude of EMG before surgery.
The range of R was 0–1. The lower the value, or the closer it was to 0, the smaller the degree of loss of muscle function.
The length of the SCN from the origin of the brachial plexus (a point, Figure 3) to the scapular notch (b point, Figure 3) was approximately 5.64 ± 0.78 cm (n = 10). The linear distance from the SCN origin point (a point, Figure 3) to the point (c point, Figure 2) where the AN entered the border of the trapezius muscle was approximately 4.30 ± 0.79 cm (n = 10). The length of the SCN from the origin of the brachial plexus to the scapular notch (a-b) was longer than the linear distance (a-c) between two points (P < 0.01) (Table 1).
| (mean ± SD, cm) | P (95%CI) | |
| a-b | 5.64 ± 0.78 | < 0.01 |
| a-c | 4.30 ± 0.79 |
The whole length of the AN in the trapezius muscle was 16.89 cm, with a length range of 12.70-20.50 cm. The average number of branches distributed in the descending portion was 2.6 with a number range of 0-5; the average number of branches distributed in the horizontal portion was 2.6 with a number range of 1-4; and the average number of branches distributed in the ascending portion was 2.2 with a number range of 1-4. There were still 1.2 branches with a number range of 0-3 distributed in the descending portion before the AN entered into the trapezius muscle. Therefore, there were 3.8 branches distributed in the descending portion of the trapezius muscle.
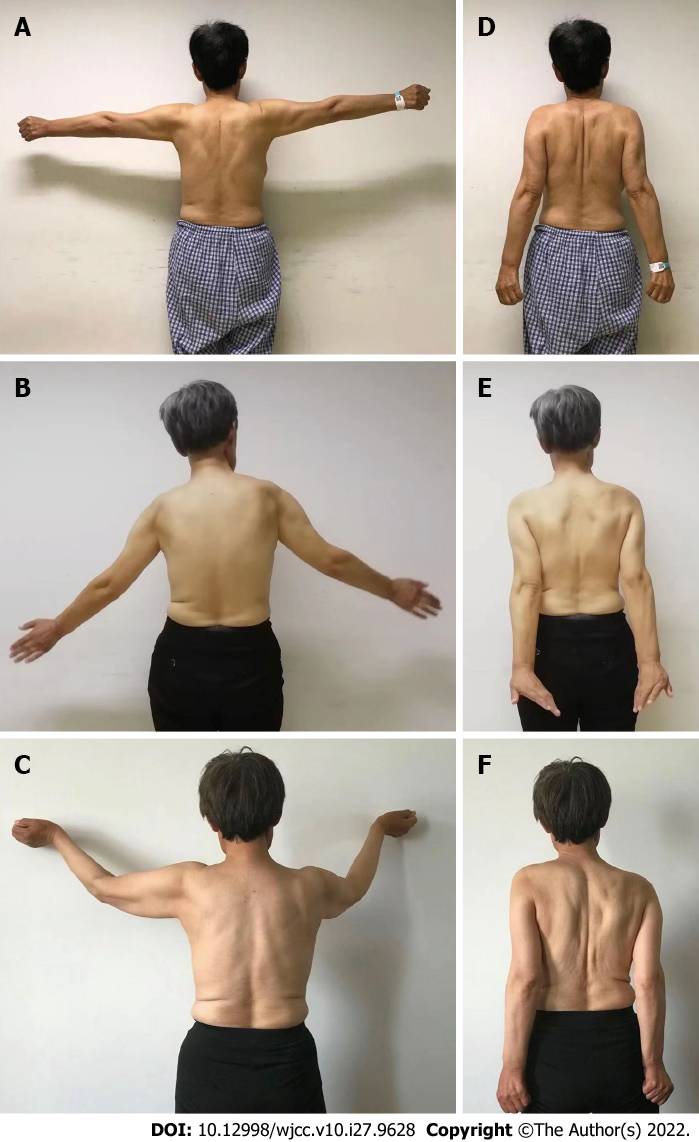
The NDII of the patient was 37.5 points three months and 47.5 points nine months after surgery. The results of the CMS are shown in Table 2 and suggested that shoulder function recovered better at nine months after surgery than at three months. The amplitudes of electromyography of the trapezius, supraspinatus and infraspinatus muscles are shown in Tables 3 and 4. Even though muscle function did not recover completely three months and nine months after surgery, more function was regained within nine months than in three months. Excluding the descending portion, both the horizontal and ascending portions of the right-sided trapezius muscle had larger amplitudes than the left side nine months after surgery, which demonstrates the effect of SCN transfer in this patient. The amplitude of right-sided supraspinatus and infraspinatus electromyography revealed that the right side did not lose more function than the left side three months and nine months after surgery, which indicates less loss of function in the supraspinatus and infraspinatus muscles after SCN transfer. Electromyographs of the trapezius, supraspinatus, and infraspinatus muscles are shown in Figures 7 and 8. The results are similar to the amplitude of the muscles demonstrated in Tables 3 and 4. The patient’s clinical ma
| Subjective evaluation | Objective evaluation | |||
| Left | Right | Left | Right | |
| Before surgery | 27 | 27 | 63 | 63 |
| Three months after surgery | 22 | 21 | 25 | 23 |
| Nine months after surgery | 26 | 23 | 48 | 46 |
| Left side | Right side | |||||
| Descending portion | Horizontal portion | Ascending portion | Descending portion | Horizontal portion | Ascending portion | |
| Before surgery | 288.25 | 260.67 | 187.33 | 278.00 | 186.00 | 182.67 |
| Three months after surgery | 130.00 | 135.25 | 132.50 | 125.25 | 141.25 | 124.50 |
| R3m | 0.55 | 0.48 | 0.29 | 0.55 | 0.24 | 0.32 |
| Nine months after surgery | 182.35 | 138.16 | 129.36 | 139.15 | 179.38 | 140.57 |
| R9m | 0.37 | 0.47 | 0.31 | 0.50 | 0.04 | 0.23 |
| Left side | Right side | |||
| Supraspinatus | Infraspinatus | Supraspinatus | Infraspinatus | |
| Before surgery | 423.75 | 267.33 | 183.00 | 181.33 |
| Three months after surgery | 189.50 | 245.75 | 181.25 | 170.00 |
| R3m | 0.55 | 0.08 | 0.01 | 0.06 |
| Nine months after surgery | 170.28 | 253.46 | 172.33 | 175.30 |
| R9m | 0.60 | 0.05 | 0.06 | 0.03 |
The spinal AN would usually be sacrificed during radical neck dissection. Therefore, trapezius muscle paralysis often results in destabilization and dropping of the shoulder girdle[20]. Several patients experience severe upper extremity impairment with deficits in functional movement and continuous shoulder pain[21].
Understanding the anatomical information of the accessory nerve is very important in protecting the accessory nerve during neck dissection. Some studies have disclosed the anatomy of the accessory nerve in detail. Shiozaki et al[8] found that the AN passed through the SCM in three ways. Another study showed that the branches of the cervical plexus that join the main AN or separate branches innervated the trapezius muscle[9]. There have also been some studies revealing the relationship between AN and the IJV[7,22]. The results of these studies[7-9,22] were conducive to the protection of the AN or other branch nerves and the function of the trapezius muscle during surgery.
There are several approaches to repair the injured AN, including nerve transfer from the upper trunk[12], which is much thicker than the SCN. Taking a fascicle from the upper trunk might cause more side effects than the SCN. Considering the number of myelinated axons, with 6004 for the SCN and 1500-3000 for the AN, partially splitting the SCN could be used to transfer to the distal stump of the AN[23,24]. The SCN could be an attractive substitute donor for some nerve graft procedures. Lanisnik et al[25] found that the AN could give off the branches before entering the SCM muscle and joining the cervical plexus at the posterior part of the SCM muscle in some cases. The function of the AN and SCN in shoulder elevation and abduction movement is synergistic. This synergistic function implied that SCN could be an alternative donor nerve to restore AN function. Tubbs et al[13] reported a cadaveric study related to the SCN connection with the facial nerve. They measured the length and diameter of the SCN in 10 human cadavers. They believed that using the SCN might be considered by surgeons for facial nerve reanimation. In addition, parts of the SCN and AN are located in the same region of the neck[26]. They are next to each other in position, which is conducive to nerve transplantation to achieve no tension suture. We found that the length of the a-b segment of SCN was significantly greater than the distance length of the a-c segment. This result indicated that a sufficient suprascapular nerve length could be achieved to realize a tension-free suture during the SCN-AN partial nerve transplantation procedure. The a-b segment of SCN was chosen because the other part of the SCN was covered by the supraspinatus and infraspinatus muscles, which would increase the difficulty of surgical exposure. In the present study, the SCN was split longitudinally, and partial nerve transfer was performed in one patient. The results of clinical outcomes showed that supraspinatus and infraspinatus muscle function were not affected much three and nine months after surgery.
AN to SCN transfer has been proven to be a valid strategy to repair nerve injury[27-30]. The AN could be used as a donor nerve in brachial plexus injury, especially injury to the SCN[31]. One advantage of transfer between these two nerves is the greater proximity to the muscle to be reinnervated[30]. Although transfer from the AN to the SCN does not completely recover the strength of the shoulder, it still improves pain control and shoulder stability[32]. Bertelli and Ghizoni described a unique approach to identify and harvest the AN for nerve transfer in brachial plexus injuries[33]. These studies indicated that the spinal AN had a similar histological compatibility with the SCN[27-33]. Therefore, the SCN could be a suitable donor to recover AN function when iatrogenic injury or resection occurs during surgery.
Although the results showed that the descending portion of the right trapezius muscle did not recover better than the left side, the electromyography results indicated that the horizontal and ascending portions on the right side had larger amplitudes than those on the left side nine months after surgery. The possible reason for this result is that SCN transfer improved trapezius muscle innervation. Meanwhile, partial nerve transfer maintained the original function of the supraspinatus and infraspinatus muscles in the patient. Therefore, shoulder function was enhanced. The trapezius muscle is the main dominant nerve of the AN. There are also C2-C4 nerve branches into the AN trunk or directly into the trapezius muscle. However, the location of the branches from the cervical nerve is often not constant. Some branches may contain sensory nerves, and even play a role in the innervation of the trapezius muscle[9]. Therefore, partial SCN transfer was not the only factor that improved the amplitude of the trapezius muscle. The feature of this SCN transfer is “partial”, which does not completely sacrifice the SCN and maintains the function of the nerve. In addition, neck dissection can expose the SCN well, which is one benefit of this technique. However, the evidence for clinical promotion was insufficient in our study due to the limited number of clinical cases performed.
Based on anatomical data and clinical application, partial suprascapular nerve-to-accessory nerve transfer could be achieved and may improve shoulder function after radical neck dissection. Further basic research and clinical evidence are necessary to elaborate on the findings presented in this study.
Injury to the accessory nerve (AN) can cause weakness or paralysis in the sternocleidomastoid (SCM) and trapezius muscles. Loss of motor function in the trapezius muscle leads to a painful and disabling condition. This syndrome is frequently observed after radical neck dissection. Current surgical treatment for trapezius paralysis mainly includes two strategies. The first is fascial muscle transplantation, which mainly includes static and dynamic transplantation. Another surgical strategy is nerve repair, which includes neurolysis, direct suture, and nerve grafting.
A key point in nerve transplantation for the treatment of trapezius paralysis is finding the appropriate donor nerves. It has been reported that trapezius muscle function can be restored by directly transplanting a nerve in the upper trunk of the brachial plexus and suturing the distal end of the AN. According to Tubbs's anatomical study, the suprascapular nerve (SCN) could be used as a donor nerve for nerve repair.
To further explore the feasibility of transplanting the suprascapular nerve as a donor nerve to the accessory nerve to restore trapezius muscle function, we conducted exploratory studies of both human anatomy and clinical application.
Based on the dissection of ten sides of cadavers, the length of the SCN (n = 10) from the origin of the brachial plexus (a point) to the scapular notch (b point) and the distance of the SCN from the origin point (a point) to the point (c point) where the AN entered the border of the trapezius muscle were measured. The length and branches of the AN (n = 10) in the trapezius muscle were measured. A female patient aged 55 years underwent surgery for partial SCN to AN transfer. One-third of the diameter of the SCN was cut off, and combined epineurial and perineurial sutures were applied between the distal end of the cut-off fascicles of the SCN and the proximal end of the AN without tension. Both subjective and objective evaluations were performed before, three months after, and nine months after surgery.
The whole length of the AN in the trapezius muscle was 16.89 cm. The average numbers of branches distributed in the descending, horizontal and ascending portions were 3.8, 2.6 and 2.2, respectively. The diameter of the AN was 1.94 mm at the anterior border of the trapezius. The length of the suprascapular nerve from the origin of the brachial plexus to the scapular notch was longer than the distance of the suprascapular nerve from the origin point to the point where the accessory nerve entered the upper edge of the trapezius muscle. The amplitude of trapezius muscle electromyography indicated that both the horizontal and ascending portions of the trapezius muscle on the right side had better function than the left side nine months after surgery. The results showed that the right-sided supraspinatus and infraspinatus muscles did not lose more function than the left side.
Based on anatomical data and clinical application, partial suprascapular nerve-to-AN transfer could be achieved and may improve innervation of the affected trapezius muscle after radical neck dissection.
The suprascapular nerve could be used as a donor nerve for nerve repair according to the anatomical measurements and clinical findings in our study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anatomy and morphology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ishigaki T, Japan; Liu J, United States S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Kim DH, Cho YJ, Tiel RL, Kline DG. Surgical outcomes of 111 spinal accessory nerve injuries. Neurosurgery. 2003;53:1106-1112; discussion 1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Wiater JM, Bigliani LU. Spinal accessory nerve injury. Clin Orthop Relat Res. 1999;5-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Lloyd S. Accessory nerve: anatomy and surgical identification. J Laryngol Otol. 2007;121:1118-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Nahum AM, Mullally W, Marmor L. A syndrome resulting from radical neck dissection. Arch Otolaryngol. 1961;74:424-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 157] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Leipzig B, Suen JY, English JL, Barnes J, Hooper M. Functional evaluation of the spinal accessory nerve after neck dissection. Am J Surg. 1983;146:526-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 123] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Overland J, Hodge JC, Breik O, Krishnan S. Surgical anatomy of the spinal accessory nerve: review of the literature and case report of a rare anatomical variant. J Laryngol Otol. 2016;130:969-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Anehosur V, Kulkarni K, Kumar N. Variations in the Anatomy of Spinal Accessory Nerve and its Landmarks for Identification in Neck Dissection: A Clinical Study. J Maxillofac Oral Surg. 2021;20:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Shiozaki K, Abe S, Agematsu H, Mitarashi S, Sakiyama K, Hashimoto M, Ide Y. Anatomical study of accessory nerve innervation relating to functional neck dissection. J Oral Maxillofac Surg. 2007;65:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Pu YM, Tang EY, Yang XD. Trapezius muscle innervation from the spinal accessory nerve and branches of the cervical plexus. Int J Oral Maxillofac Surg. 2008;37:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Anderson R, Flowers RS. Free grafts of the spinal accessory nerve during radical neck dissection. Am J Surg. 1969;118:796-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Vastamäki M, Solonen KA. Accessory nerve injury. Acta Orthop Scand. 1984;55:296-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Cambon-Binder A, Preure L, Dubert-Khalifa H, Marcheix PS, Belkheyar Z. Spinal accessory nerve repair using a direct nerve transfer from the upper trunk: results with 2 years follow-up. J Hand Surg Eur Vol. 2018;43:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Tubbs RS, Louis RG Jr, Wartmann CT, Loukas M, Shoja MM, Ardalan MR, Oakes WJ. Suprascapular nerve as a donor for extracranial facial nerve reanimation procedures: a cadaveric feasibility study. J Neurosurg. 2008;108:145-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Michael C, MHS Bhargavi Maheshwer, Nikhil N Verma. Suprascapular Neuropathy. Operative Techniques in Sports Medicine 2021; 29: 1-6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Taylor RJ, Chepeha JC, Teknos TN, Bradford CR, Sharma PK, Terrell JE, Hogikyan ND, Wolf GT, Chepeha DB. Development and validation of the neck dissection impairment index: a quality of life measure. Arch Otolaryngol Head Neck Surg. 2002;128:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1732] [Cited by in RCA: 1761] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 17. | Krause HR, Bremerich A, Herrmann M. The innervation of the trapezius muscle in connection with radical neck-dissection. An anatomical study. J Craniomaxillofac Surg. 1991;19:87-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Tsuruike M, Ellenbecker TS. Serratus anterior and lower trapezius muscle activities during multi-joint isotonic scapular exercises and isometric contractions. J Athl Train. 2015;50:199-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | de Witte PB, Werner S, ter Braak LM, Veeger HE, Nelissen RG, de Groot JH. The Supraspinatus and the Deltoid - not just two arm elevators. Hum Mov Sci. 2014;33:273-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Weisberger EC. The efferent supply of the trapezius muscle: a neuroanatomic basis for the preservation of shoulder function during neck dissection. Laryngoscope. 1987;97:435-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Brown H, Burns S, Kaiser CW. The spinal accessory nerve plexus, the trapezius muscle, and shoulder stabilization after radical neck cancer surgery. Ann Surg. 1988;208:654-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Mills S, Aristotelous C, Touil LL, James RCW. Rare anatomical variant of the spinal accessory nerve: case report and comprehensive review. Int J Oral Maxillofac Surg. 2022;51:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 23. | Merrell GA, Barrie KA, Katz DL, Wolfe SW. Results of nerve transfer techniques for restoration of shoulder and elbow function in the context of a meta-analysis of the English literature. J Hand Surg Am. 2001;26:303-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 261] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 24. | Pruksakorn D, Sananpanich K, Khunamornpong S, Phudhichareonrat S, Chalidapong P. Posterior approach technique for accessory-suprascapular nerve transfer: a cadaveric study of the anatomical landmarks and number of myelinated axons. Clin Anat. 2007;20:140-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Lanisnik B, Zargi M, Rodi Z. Identification of three anatomical patterns of the spinal accessory nerve in the neck by neurophysiological mapping. Radiol Oncol. 2014;48:387-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Harold Ellis, Vishy Mahadevan. The posterior triangle of the neck. Surgery. 2014;32 suppl 2:e17-e27. [DOI] [Full Text] |
| 27. | Bertelli JA, Ghizoni MF. Reconstruction of C5 and C6 brachial plexus avulsion injury by multiple nerve transfers: spinal accessory to suprascapular, ulnar fascicles to biceps branch, and triceps long or lateral head branch to axillary nerve. J Hand Surg Am. 2004;29:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 201] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Waikakul S, Wongtragul S, Vanadurongwan V. Restoration of elbow flexion in brachial plexus avulsion injury: comparing spinal accessory nerve transfer with intercostal nerve transfer. J Hand Surg Am. 1999;24:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Lu J, Xu J, Xu W, Xu L, Fang Y, Chen L, Gu Y. Combined nerve transfers for repair of the upper brachial plexus injuries through a posterior approach. Microsurgery. 2012;32:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Souza FH, Bernardino SN, Filho HC, Gobbato PL, Martins RS, Martins HA, Silva-Néto RP. Comparison between the anterior and posterior approach for transfer of the spinal accessory nerve to the suprascapular nerve in late traumatic brachial plexus injuries. Acta Neurochir (Wien). 2014;156:2345-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Rezzadeh K, Donnelly M, Vieira D, Daar D, Shah A, Hacquebord J. The extent of brachial plexus injury: an important factor in spinal accessory nerve to suprascapular nerve transfer outcomes. Br J Neurosurg. 2020;34:591-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 32. | Abdouni YA, Checoli GF, Salles HC, da Costa AC, Chakkour I, Fucs PMMB. Assessment of the results of accessory to suprascapular nerve transfer. Acta Ortop Bras. 2018;26:332-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Bertelli JA, Ghizoni MF. Improved technique for harvesting the accessory nerve for transfer in brachial plexus injuries. Neurosurgery. 2006;58:ONS-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |