Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9619
Peer-review started: December 17, 2021
First decision: January 26, 2022
Revised: January 27, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: September 26, 2022
Processing time: 272 Days and 13.8 Hours
There have been increased reports of dry eyes in the coronavirus disease 2019 (COVID-19) pandemic era.
To analyze the differences in tear film properties from pre- and post-pandemic of the COVID-19 era.
It was a retrospective comparative study. Patients were divided into three groups according to the data of multimodal ocular surface evaluation: (1) Group 1 if it was before Portugal lockdown decision (from August 2019 to March 2020); (2) Group 2 if it was after Portugal lockdown decision but without mask mandate (from April 2020 to October 2020); and (3) Group 3 if it was after Portugal lockdown but with mask mandate in health public highway (from November 2020 to April 2021). The following variables were analyzed: Lipid layer thickness, blink rate, Schirmer test, tear meniscus height, tear osmolarity, non-invasive break-up time, and loss area of the meibomian glands.
The study included 548 eyes of 274 patients, aged 18 years to 89 years, with a mean age of 66.15 ± 13.40 years at the time of multimodal ocular surface evaluation. Compared to group 1: (1) Mean lipid layer thickness was better in group 2 (P = 0.001) and group 3 (P < 0.001); (2) Schirmer test was similar in group 2 (P = 0.576) and better in group 3 (P = 0.002); (3) Tear osmolarity and loss area of the meibomian glands were worse in group 2 (P = 0.031 and P < 0.001, respectively) and in group 3 (both with P < 0.001); (4) Blink rate and tear meniscus height were similar in group 2 (P = 0.821 and P = 0.370, respectively) and worse in group 3 (P < 0.001 and P = 0.038, respectively); and (5) Non-invasive break-up time was worse in group 2 (P = 0.030) and similar in group 3 (P = 0.263).
Our study demonstrated that differences existed in tear film properties comparing data from the pre- and post-pandemic of the COVID-19 era.
Core Tip: There were differences in tear film properties comparing the pre- and post-pandemic of the coronavirus disease 2019 era data. Over time, there was an increase in the lipid layer thickness, a decrease in the area of the meibomian glands, and a decrease in the blink rate. These changes seemed related to face masks and screen time. Therefore, the ophthalmologist must be aware of these changes and educate patients according to the most likely potential causal factor.
- Citation: Marta A, Marques JH, Almeida D, José D, Sousa P, Barbosa I. Impact of COVID-19 pandemic on the ocular surface. World J Clin Cases 2022; 10(27): 9619-9627
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9619.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9619
On March 11, 2020, a new global pandemic was declared by the World Health Organization: Severe acute respiratory syndrome caused by the coronavirus 2019-nCOV[1]. The spread was primarily via respiratory droplets. With widespread transmissibility and no vaccine or validated therapies, some public health measures had been adopted to control the pandemic situation[2]. Critical strategies included the use of personal protective equipment such as face masks[3], physical distancing, and hand hygiene. There was an increase for the demand placed on healthcare workers including ophthalmologists, and many suffered burnout[4]. To avoid social contact, some countries like Portugal declared lockdowns and other measures like mandatory telework and face masks in the public highway and closed spaces. Thereby, screen time increased considerable because most of the work and meetings were performed through devices[3].
Screen time is a well-known risk factor for dry eye disease[5-7]. The main causative factor is thought to be the increase of evaporation of tear fluid attributable to prolonged time without blinking while gazing. On the other hand, it is known that the prolonged use of face masks, besides pain and pressure on the nose and ears, can also lead to increased respiratory resistance, temporomandibular joint changes, and ocular discomfort[8-12]. The voluntary or involuntary displacement of the mask could direct the breathing air around the eyes leading to rapid evaporation of tears[3,6]. Due to increased reports of dry eyes[6,13], the term mask-associated dry eye was created by scientists from Canada to describe dry eye after use of a face mask[14-16]. According to the authors’ knowledge, there is no published study in the literature about the differences in tear film properties comparing data from the pre- and post-pandemic of the coronavirus disease 2019 (COVID-19) era. Our study aimed to evaluate the tendencies in this area after the new pandemic reality.
A retrospective comparative study included those who underwent multimodal ocular surface evaluation in the Ophthalmology Department of Centro Hospitalar Universitário do Porto between August 2019 and April 2021. This study was conducted following the tenets of the Declaration of Helsinki (1964). The authors ensured that all patients’ anonymity was carefully protected. Study approval was obtained from the “Departamento de Ensino, Formação e Investigação”.
We included all adult patients with complete multimodal ocular surface evaluation in the past (as mentioned in the parameters section). Patients who performed any ocular surgery (except cataract surgery if performed more than 6 mo before ocular surface evaluation) or meibomian gland dysfunction treatments with laser devices such as intense pulsed light were excluded. Contact lens wearers were also excluded.
After collection, patients were divided into 3 groups according to the date of multimodal ocular surface evaluation: (1) Group 1 if it was before Portugal lockdown (until March 2020); (2) Group 2 if it was after Portugal lockdown decision but without mask mandate (from April to October 2020); and (3) Group 3 if it was after Portugal lockdown but with mask mandate in health public highway (from November 2020 to April 2021).
The following variables were analyzed: Demographic characteristics and multimodal ocular surface evaluation. The last, as recommended by international consensus[17], included: (1) Tear film stability [tear osmolarity (OSM), non-invasive break-up time (NIBUT)]; (2) Tear volume [Schirmer test (ST), tear meniscus height (TMH)]; (3) Tear film composition [tear OSM, lipid layer thickness (LLT)]; and (4) Eyelid aspects [loss area of the meibomian glands (LAMG) and blink rate (BR) analysis].
ST I was chosen to evaluate tear production or basic secretion. The ST was performed after the instillation of a topical anesthetic. To minimize irritation of the cornea during the test, a thin filter-paper strip (5 mm wide, 30 mm long) was placed at the junction of the middle and lateral thirds of the lower eyelid, with 5 mm of the paper’s length folded into the inferior cul-de-sac and the remaining 25 mm projecting over the lower eyelid. The test was performed with the patient’s eyes closed to eliminate blinking. We used TearLab® Osmolarity System (Tearlab, San Diego, CA, United States) to evaluate tear OSM. We used IDRA® Ocular Surface Analyser (SBM SISTEMI, Italy) to assess the NIBUT and BR, LLT through auto-interferometry, LAMG through meibography, and TMH. The BR determined the quality of blinking. It measured blink frequency, count of full blinks, of partial blinks, and duration between blinks.
Statistical analysis was performed using the SPSS program (SPSS Statistics, version 22.0 for Windows, SPSS Inc., IBM, Somers, NY, United States). We used the Kolmogorov-Smirnov test to evaluate the normality of the variables and the Student’s t test to compare between independent continuous variables. The Fisher exact test was used for nominal scaled data. Statistically significant P values were less than 0.05.
The study included 548 eyes of 274 patients, 43.4% male and 56.6% female, aged 18 years to 89 years. The mean age was 66.15 ± 13.40 years at the time of multimodal ocular surface evaluation. Group 1 included 290 eyes of 145 patients, 44.8% male and 55.2% female, aged 18 years to 89 years with a mean age of 65.01 ± 15.05. Group 2 included 40 eyes of 20 patients, 40% male and 60% female, aged 21 years to 87 years with a mean age of 64.15 ± 15.58. Group 3 included 216 eyes of 108 patients, 42.2% male and 57.8% female, aged 29 years to 85 years with a mean age of 68.06 ± 10.01.
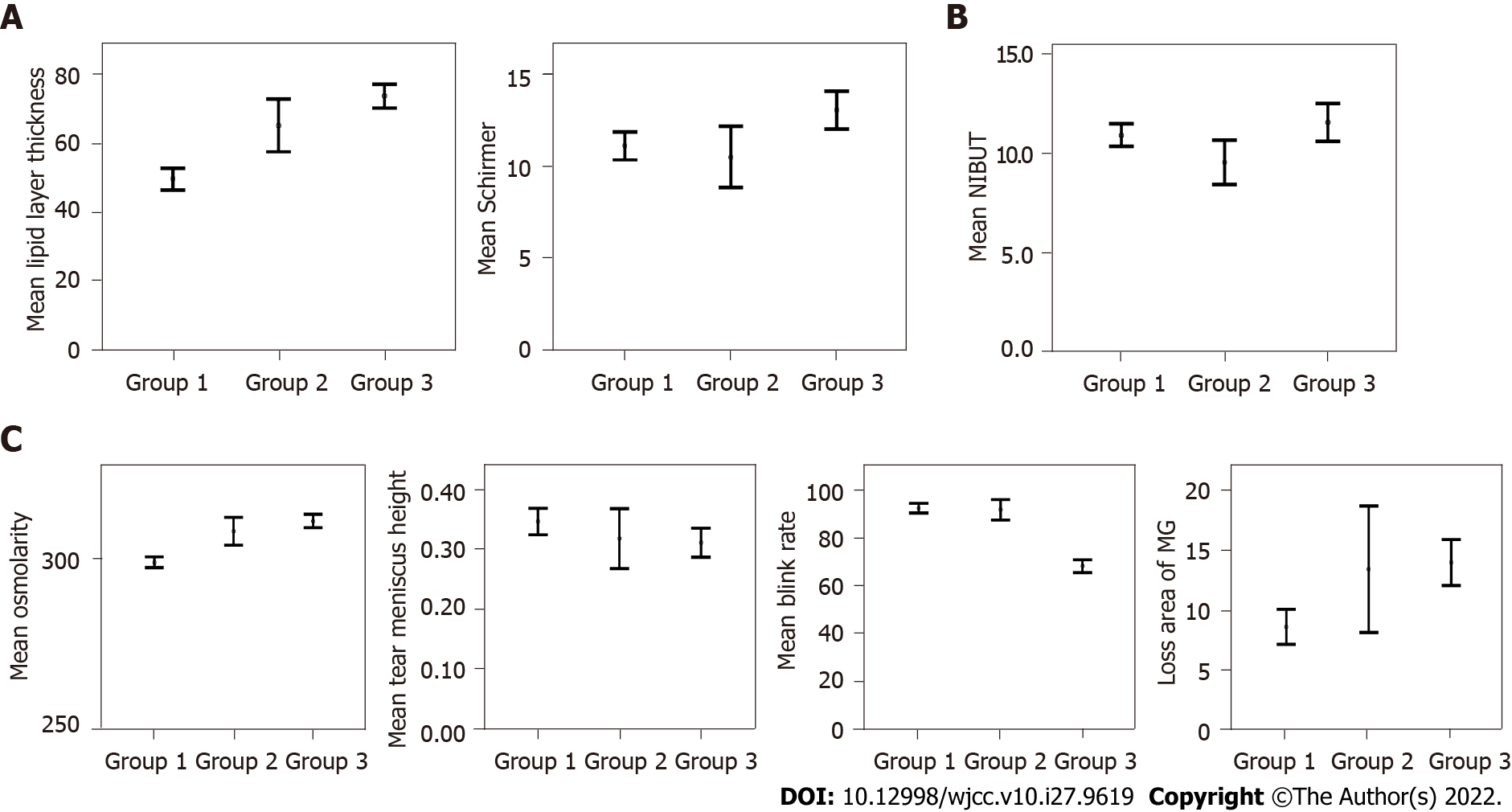
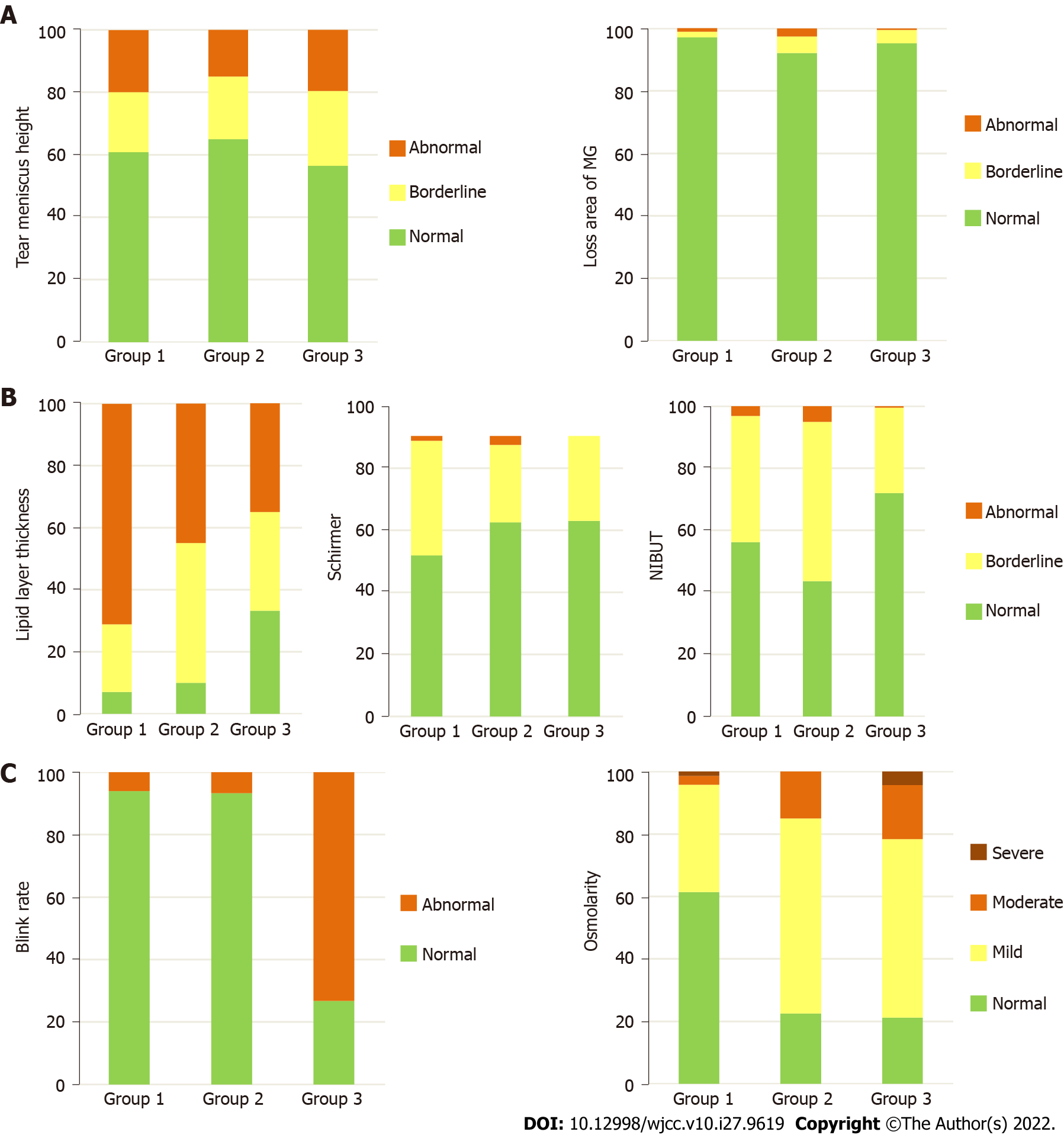
The mean values and grade frequencies of the quality of tear film parameters according to the three groups are represented in Table 1 and Figures 1 and 2. The mean LLT was better in group 2 (P = 0.001) and group 3 (P < 0.001) compared to group 1. These differences were also expressed in grade frequencies in Figure 2 (P < 0.001). The mean ST value was similar in group 2 (P = 0.576) and better in group 3 (P = 0.002) compared to group 1. These differences were also expressed in grade frequencies in Figure 2 (P = 0.013).
| Variables1 | Group 1: Before lockdown (August 2019 to March 2020) | Group 2: After lockdown without mask mandate (April 2020 to October 2020) | P value: Group 1 vs group 2 | Group 3: After lockdown with mask mandate (November 2020 to April 2021) | P value: Group 1 vs group 3 | P value: Group 2 vs group 3 |
| NIBUT, s | 10.92 ± 4.87 | 9.53 ± 3.45 | 0.0303 | 11.56 ± 7.08 | 0.263 | 0.007 |
| Blink rate, % | 92.43 ± 17.52 | 91.78 ± 13.29 | 0.821 | 68.24 ± 19.67 | < 0.0013 | < 0.001 |
| Lipid layer thickness, nm | 49.44 ± 27.07 | 65 ± 23.91 | 0.0012 | 73.53 ± 25.60 | < 0.0012 | 0.052 |
| Loss area of the MG, % | 8.68 ± 12.45 | 13.50 ± 16.06 | 0.0313 | 14.06 ± 14.23 | < 0.0013 | 0.828 |
| Tear meniscus height, mm | 0.35 ± 0.19 | 0.32 ± 0.16 | 0.370 | 0.31 ± 0.18 | 0.0383 | 0.820 |
| Tear osmolarity, mOsm/L | 299.14 ± 13.23 | 308.25 ± 12.96 | < 0.0013 | 311.30 ± 14.54 | < 0.0013 | 0.218 |
| Schirmer, mm | 11.08 ± 6.55 | 10.48 ± 5.19 | 0.576 | 13.02 ± 7.54 | 0.0022 | 0.011 |
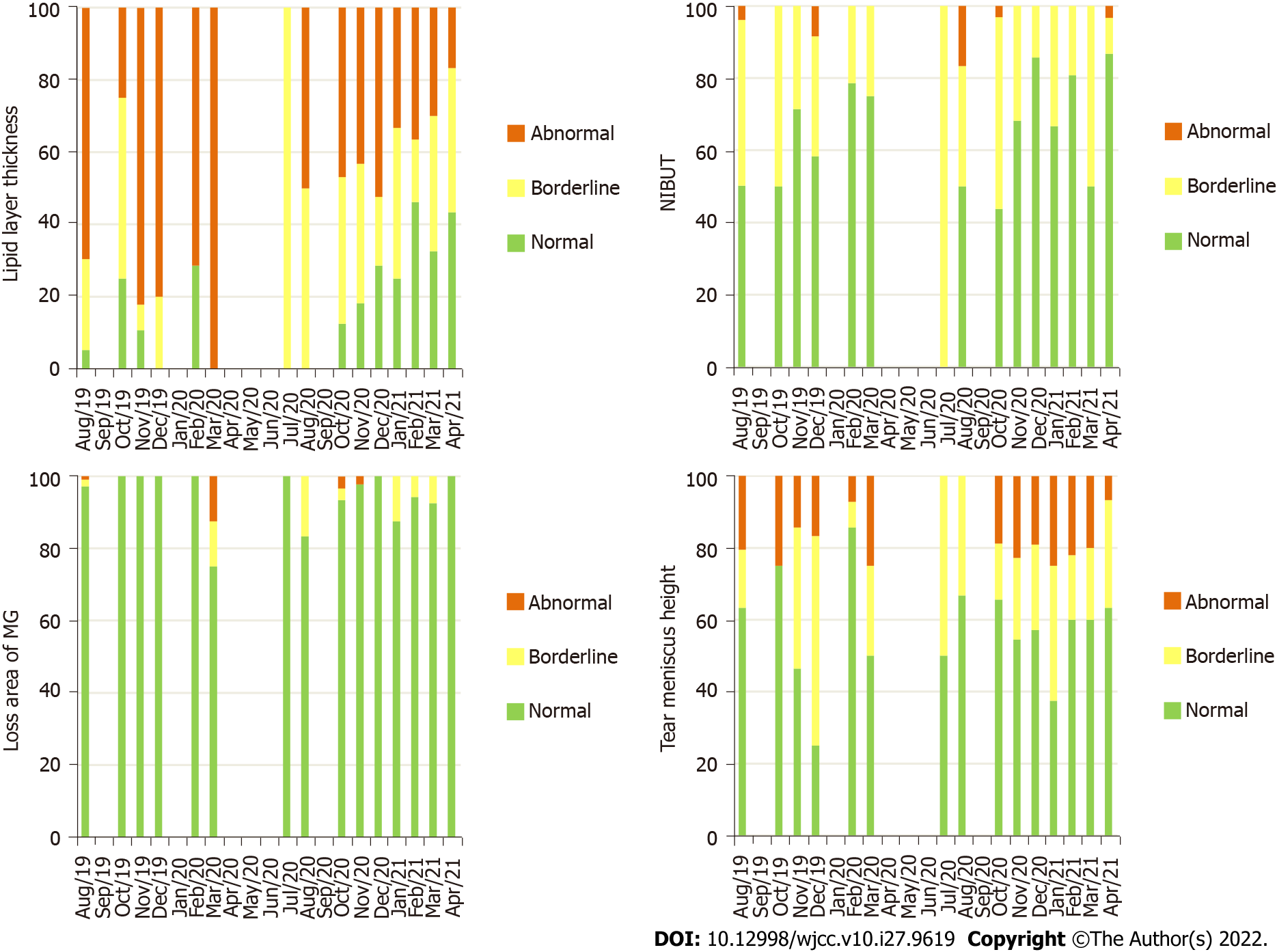
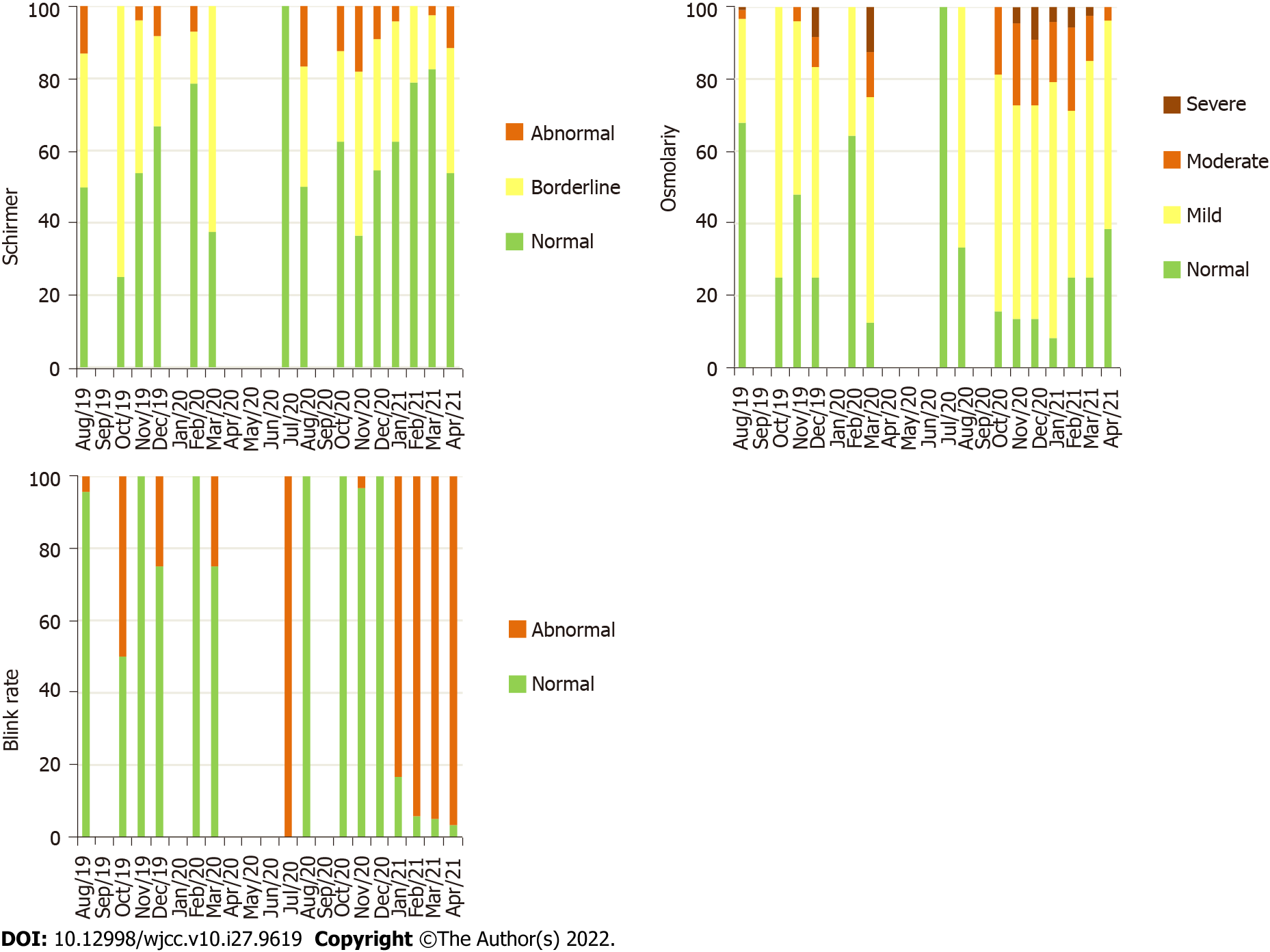
The mean OSM and LAMG were worse in group 2 (P = 0.031 and P < 0.001, respectively) and in group 3 (both with P < 0.001) compared to group 1. Although these differences were also expressed in grade frequencies for OSM (P < 0.001), it was not enough to change the grade frequencies for the LAMG (P = 0.529). The mean BR and TMH were similar in group 2 (P = 0.821 and P = 0.370, respectively) and worse in group 3 (P < 0.001 and P = 0.038, respectively) compared to group 1. These differences were also expressed in grade frequencies for BR (P < 0.001), but it was not enough to change the grade frequencies for TMH (P = 0.598). The mean NIBUT was worse in group 2 (P = 0.030) and similar in group 3 (P = 0.263) compared to group 1. These differences were also expressed in grade frequencies (P < 0.001). The grade frequencies of quality of tear film parameters according to the months included in the analysis are represented in Figures 3 and 4.
The ocular manifestations of severe acute respiratory syndrome coronavirus 2 infection are known not to pose particular treatment problems, being self-limiting and healing spontaneously[18]. The results of our study suggest differences in film properties before and after the COVID-19 era independently of infection, probably related to different behaviors (screen time, face mask, etc).
After the lockdown period without face mask mandate, the NIBUT significantly decreased, LLT and OSM increased, and there was a significantly higher LAMG. In this period, although there was no face mask mandate for public highways in Portugal, people used it frequently since it was needed for public services. The trigger factor initiating the cascade of structural changes of the tear film is likely the air directed at the ocular surface during expiration[6,13]. The hypothesis that high air velocity causes evaporation of water from the precorneal tear film was confirmed by some studies[19,20]. This can explain the decrease of NIBUT, also shown in a recent study[21]. The LLT increase can be an adaptive response to increased NIBUT, an overestimation since there was a significant decrease of tear film water, or as a result of traumatic secretion of the meibomian gland by face mask. Our study showed a higher LAMG, which may mean that face masks can have a mechanical effect, leading to the trauma of meibomian glands in the lower eyelid. The increase of OSM can be explained by the higher proportion of lipid layer (solute) compared to the aqueous layer (solvent) or by the increase of inflammatory markers as already been shown with face mask use in another study[21].
After the lockdown period with face mask mandate, the mean LLT, OSM, and LAMG were kept higher than before the lockdown group. This is supported by the longer usage time of face masks that occurred consequently to the mandate. However, the mean NIBUT improved. This can be caused by the higher lipid layer together with Shirmer value in this group of patients. The worsened BR could be a long-term consequence of screen time, due to prolonged blinking intervals while gazing[6].
Considering all this, the ophthalmologist must be aware of these changes and educate patients according to the greatest potential causal factor. We recommend an appropriate fitting of the face mask and if necessary with taping the top edge on the nose, the frequent use of lubricant eye drops, a limited stay in air-conditioned rooms, and following the 20:20:20 rule regularly to provide breaks from digital devices. Even if face masks are no longer mandatory in this context, we can use this evidence for other professional situations where screen time is prolonged (e.g., administrative) or a face mask is in the routine (e.g., surgeons and nurses in the operating room).
To our knowledge, this is the first study documenting the differences in tear film properties between the pre- and post-pandemic of the COVID-19 era in a real-world study. Two of the strengths of this study are the sample size, including broad age groups, and the multimodal assessment, allowing us to better understand which factors contribute to the loss of the tear film homeostasis. Although it is not a prospective study, we have had an ocular surface evaluation protocol in our department since August 2019, which allowed us to have patients with a complete multimodal assessment of the ocular surface. IDRA® assessment was performed by only three technicians (Almeida D, José D, and Sousa P) since August 2019 when the department received the device. Therefore, we did not consider operator dependent variability as an important factor. The major limitation of this study is not including the evaluation of patient-reported symptoms. Different scales were used along the period analyzed, and comparison is not possible. Additionally, this study did not include a control group with patients not using face masks or not exposed to screen time because it was against recommendations. Prospective studies with a longer follow-up including a time of face mask discontinuation may reveal the possibility of recovery from these changes at the suspension of face mask use and how long the ocular surface will be able to recover the baseline state.
Overall, our study demonstrated that differences existed in tear film properties when comparing data from the pre- and post-pandemic of the COVID-19 era. Over time, there was an increase in the LLT to compensate for the initial decrease of NIBUT (probably due to evaporation of precorneal tear film by the airflow reaching the ocular surface during expiration with a face mask), a decrease in the area of the meibomian glands (probably due to the mechanical effect of the face mask on meibomian glands in the lower eyelid), and a decrease in the BR (probably a long-term consequence of screen time). Therefore, the ophthalmologist must be aware of these changes and educate patients according to the most likely potential causal factor.
There have been increased reports of dry eyes in the coronavirus disease 2019 (COVID-19) pandemic era.
To study the ocular surface to better understand the reason for exacerbated dry eye symptoms in the COVID-19 pandemic era.
The purpose was to analyze the differences in tear film properties from the pre- and post-pandemic COVID-19 era.
It was a retrospective comparative study. Patients were divided into 3 groups according to the date of multimodal ocular surface evaluation: (1) Group 1 if it was before Portugal lockdown decision (from August 2019 to March 2020); (2) Group 2 if it was after Portugal lockdown decision but without mask mandate (from April 2020 to October 2020); and (3) Group 3 if it was after Portugal lockdown but with mask mandate in health public highway (from November 2020 to April 2021).
Over time, there was an increase in the lipid layer thickness, a decrease in the area of the meibomian glands, and a decrease in the blink rate. These changes seemed related to face masks and screen time.
There were differences in tear film properties comparing data from the pre- and post-pandemic of the COVID-19 era.
The ophthalmologist must be aware of these changes and educate patients according to the most likely potential causal factor.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Portugal
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Laddha UD, India; Socea B, Romania S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 - 3 March 2020. Mar 3, 2020. [cited 22 November 2021]. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---3-march-2020. |
| 2. | Marinova E, Dabov D, Zdravkov Y. Ophthalmic complaints in face-mask wearing: prevalence, treatment, and prevention with a potential protective effect against SARS-CoV-2. Biotechnol Biotechnol Equip. 2020;34:1323-1335. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Pandey SK, Sharma V. Mask-associated dry eye disease and dry eye due to prolonged screen time: Are we heading towards a new dry eye epidemic during the COVID-19 era? Indian J Ophthalmol. 2021;69:448-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Dimitriu MCT, Pantea-Stoian A, Smaranda AC, Nica AA, Carap AC, Constantin VD, Davitoiu AM, Cirstoveanu C, Bacalbasa N, Bratu OG, Jacota-Alexe F, Badiu CD, Smarandache CG, Socea B. Burnout syndrome in Romanian medical residents in time of the COVID-19 pandemic. Med Hypotheses. 2020;144:109972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 5. | Uchino M, Yokoi N, Uchino Y, Dogru M, Kawashima M, Komuro A, Sonomura Y, Kato H, Kinoshita S, Schaumberg DA, Tsubota K. Prevalence of dry eye disease and its risk factors in visual display terminal users: the Osaka study. Am J Ophthalmol. 2013;156:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 279] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 6. | Giannaccare G, Vaccaro S, Mancini A, Scorcia V. Dry eye in the COVID-19 era: how the measures for controlling pandemic might harm ocular surface. Graefes Arch Clin Exp Ophthalmol. 2020;258:2567-2568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 7. | Donthineni PR, Kammari P, Shanbhag SS, Singh V, Das AV, Basu S. Incidence, demographics, types and risk factors of dry eye disease in India: Electronic medical records driven big data analytics report I. Ocul Surf. 2019;17:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 8. | Greenhalgh T, Schmid MB, Czypionka T, Bassler D, Gruer L. Face masks for the public during the covid-19 crisis. BMJ. 2020;369:m1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 356] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 9. | Kyung SY, Kim Y, Hwang H, Park JW, Jeong SH. Risks of N95 Face Mask Use in Subjects With COPD. Respir Care. 2020;65:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 10. | Kainth GS. Novel tip to prevent ear irritation with surgical face masks (FRSM) during the coronavirus (COVID-19) pandemic. Ann R Coll Surg Engl. 2020;102:470-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Szepietowski JC, Matusiak Ł, Szepietowska M, Krajewski PK, Białynicki-Birula R. Face Mask-induced Itch: A Self-questionnaire Study of 2,315 Responders During the COVID-19 Pandemic. Acta Derm Venereol. 2020;100:adv00152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 12. | Huang X, Cen X, Liu J. Effect of protraction facemask on the temporomandibular joint: a systematic review. BMC Oral Health. 2018;18:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Moshirfar M, West WB Jr, Marx DP. Face Mask-Associated Ocular Irritation and Dryness. Ophthalmol Ther. 2020;9:397-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 14. | Centre for Ocular Research & Education. CORE Alerts Practitioners to Mask-Associated Dry Eye (MADE). [cited 22 November 2021]. Available from: https://corestudies.ca/news/waterloo-study-finds-kids-eyesight-worsening-earlier-and-largely-uncorrected-copy-copy/. |
| 15. | Centre for Ocular Research & Education. Practitioners Should Be Aware of Mask-Associated Dry Eye (MADE). [cited 22 November 2021]. Available from: https://eyewire.news/articles/core-alerts-practitioners-to-mask-associated-dry-eye-made/?c4src=article:infinite-scroll. |
| 16. | Chadwick O, Lockington D. Addressing post-operative Mask-Associated Dry Eye (MADE). Eye (Lond). 2021;35:1543-1544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, Gupta PK, Karpecki P, Lazreg S, Pult H, Sullivan BD, Tomlinson A, Tong L, Villani E, Yoon KC, Jones L, Craig JP. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15:539-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 1365] [Article Influence: 170.6] [Reference Citation Analysis (0)] |
| 18. | Dascalu A, Tudosie M, Smarandache C, Serban D. Impact of the COVID-19 pandemic upon the ophthalmological clinical practice. Rom J Leg Med. 2020;28:96-100. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Wyon NM, Wyon DP. Measurement of acute response to draught in the eye. Acta Ophthalmol (Copenh). 1987;65:385-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Nakamori K, Odawara M, Nakajima T, Mizutani T, Tsubota K. Blinking is controlled primarily by ocular surface conditions. Am J Ophthalmol. 1997;124:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 190] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Mastropasqua L, Lanzini M, Brescia L, D'Aloisio R, Nubile M, Ciancaglini M, D'Amario C, Agnifili L, Mastropasqua R. Face Mask-Related Ocular Surface Modifications During COVID-19 Pandemic: A Clinical, In Vivo Confocal Microscopy, and Immune-Cytology Study. Transl Vis Sci Technol. 2021;10:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |