Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9588
Peer-review started: May 4, 2022
First decision: June 11, 2022
Revised: June 22, 2022
Accepted: August 16, 2022
Article in press: August 16, 2022
Published online: September 26, 2022
Processing time: 134 Days and 22.8 Hours
Patient-specific human-induced pluripotent stem cell-derived atrial cardio
Core Tip: New medications to treat atrial fibrillation (AF) without causing ventricular arrhythmias are urgently needed. However, access to atrial human tissue is restricted, a problem that may be largely addressed by the general availability of human induced pluripotent stem cell derived atrial cardiomyocytes (hiPSC-aCMs), which provide an excellent opportunity to investigate the pathophysiology of AF and the efficacy and toxicity of treatment options. The primary drawback of using hiPSC-aCMs is their immature phenotype. Several laboratories are researching CM maturation techniques, including culture conditions, electrical stimulation, and biophysical and biochemical features. The current strategies being investigated for use in the maturation of patient-specific hiPSC-aCMs and their application towards a tailored strategy for the pharmacologic management of AF are covered in this review.
- Citation: Leowattana W, Leowattana T, Leowattana P. Human-induced pluripotent stem cell-atrial-specific cardiomyocytes and atrial fibrillation. World J Clin Cases 2022; 10(27): 9588-9601
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9588.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9588
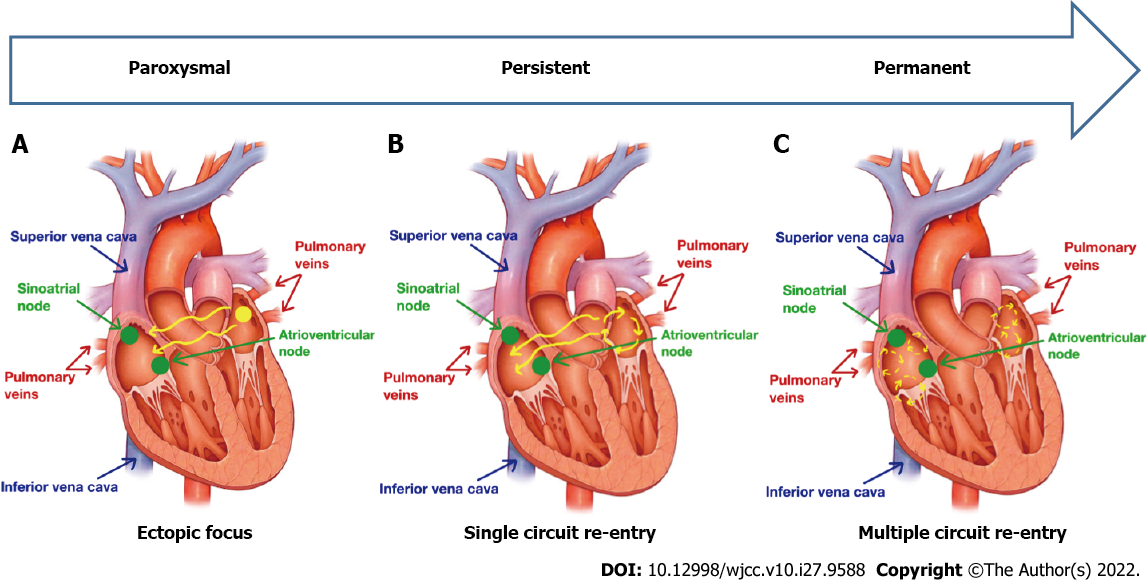
Atrial fibrillation (AF) is the most common cardiac arrhythmia in clinical practice. More than 33 million people worldwide are affected by AF, which is still escalating. Over the last 50 years, the prevalence of AF has increased threefold. AF generates a substantial burden in terms of costs, morbidity, and mortality. There are many limitations in terms of its management due to high rates of recurrence, multiple medical side effects, and high variability in pathophysiological mechanisms among in
Recent decades have seen the emergence of SC technologies that have brought about great promises for the management of various human ailments, particularly non-communicable diseases. Certainly, cardiovascular diseases have been one of the most popular fields for SC-based therapeutic approaches. Previous research in cardiovascular medicine has put much effort into the derivation of human embryonic SCs (hESCs) due to their pluripotency. Unfortunately, the ethical issues surrounding the destruction of embryos to obtain hESCs, and the possibility of ESCs forming teratomas when transplanted undifferentiated, have resulted in imposed bans on their use and research funding in many countries[7].
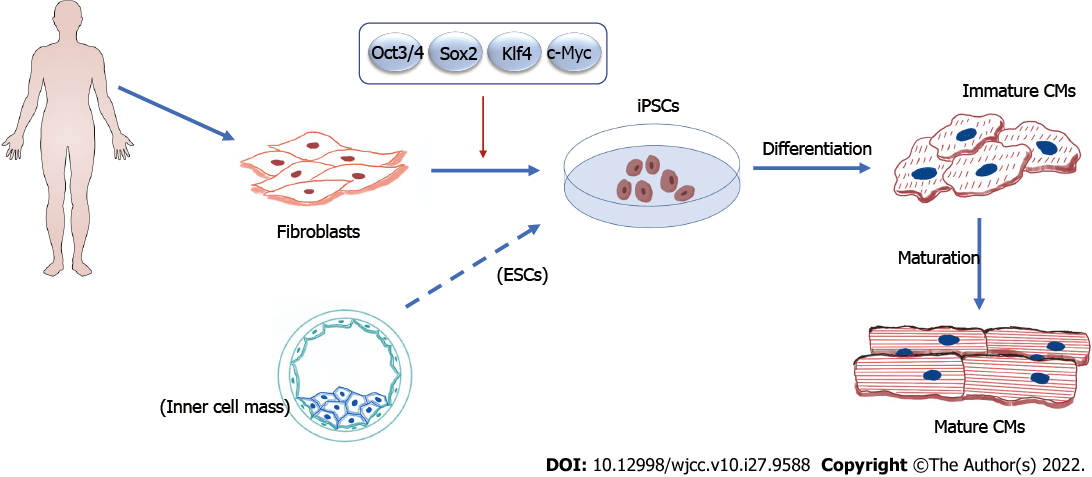
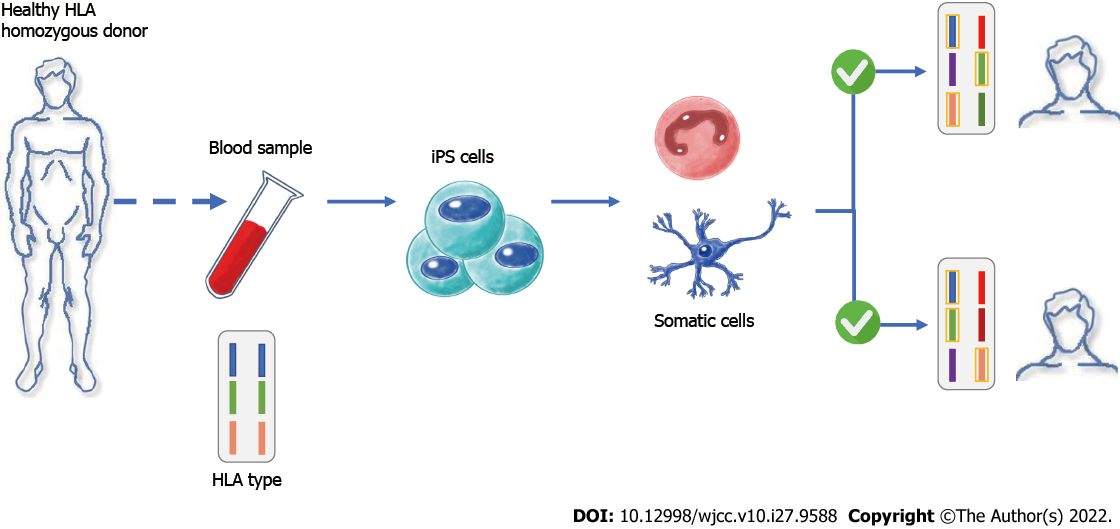
However, things took a turn when Nobel prize recipient Shinya Yamanaka and colleagues discovered a way to generate PSCs from mouse somatic cells such as skin fibroblasts, T cells, renal tubular cells, keratinocytes, and oral mucosal cells by expressing four crucial transcription factors, namely octamer-binding transcription factor 3/4, SRY-box transcription factor 2, c-Myc, and Kruppel-like factor 4, which resulted in cellular reprogramming to ESC-like inner mass cells. The reprogrammed cells were named iPSCs[8]. Subsequently, hiPSCs were generated just 1 year later (Figure 2). Because the manufacture of autologous hiPSCs from individual people is a slow, tedious, and expensive technique that hinders acute therapy and is prohibitive for wider patient care, Yamanaka and colleagues overcome this difficulty by encouraging the use of allogeneic iPSCs. They developed hiPSC banks where blood cells are taken from “super donors” and reprogrammed into clinical-grade hiPSCs. With time and cost savings, these allogeneic hiPSCs can be given to a larger patient population (Figure 3)[9].
In the last decade, advances in SC biology and CM development have been made. Several laboratories have made major contributions to the development of low-cost, easy-to-use procedures for efficiently obtaining CMs from hiPSCs. For efficient CM formation from SCs, differentiation approaches have generally tried to mimic, alter, and adopt embryonic development signals. Early research utilizing embryonic SCs has indicated that by manipulating growth factors and hormones involved in the formation of the heart, SCs could be steered towards a cardiac lineage. Early endoderm expresses transforming growth factor superfamily members, the wingless/integrated (Wnt) protein signaling pathway, and fibroblast growth factors have all been discovered to be important in the development of the mammalian heart[10-13]. Mummery et al[14] proposed three techniques for transforming hiPSCs into CMs: floating embryoid body, monolayer culture, and inductive co-culture. Various strategies have been used to establish disease models for therapeutic drug testing and drug toxicity in AF using hiPSC-CMs. Almost all of these methods rely on single-cell models, which do not effectively mimic the cardiovascular environment in vivo. Major sarcomeric myofilament organization features are absent in single-cell CMs. As a result, the assessment of contractile force in single cells was ineffective[15,16]. Because cells grown in culture flasks (two-dimensional [2D] culture) behave differently than their in vivo counterparts, the novel concept of cultivating cells in 3D was born. Furthermore, 2D cultures do not sufficiently replicate the physiological tissue milieu or the intricacy of in vivo tissue dynamics. 3D organoid cultures have recently advanced to the point that it is now possible to produce human tissues in vitro that mimic human physiology and pathology[17-20].
HiPSC-CMs are a mixed population of ventricular CMs (vCMs), aCMs, and pacemaker cells. The separation of each subtype is critical for a variety of applications. vCMs are the most common subtypes created from hiPSCs in general differentiation procedures, but aCMs are also obtained[21,22]. Several organizations have developed techniques for enhancing the differentiation of each subtype of human PSCs[23-25]. These approaches simulate in vivo heart growth by manipulating many signaling pathways such as Wnt, retinoic acid (RA), bone morphogenetic proteins (BMPs), and activin/nodal signaling. Wnt pathway inhibition at the mesoderm stage is required for heart development, while RA signaling is required for atrial chamber formation[26-33]. Furthermore, hiPSC-vCMs and hiPSC-aCMs are enriched by manipulating BMP4 and activin/nodal signaling levels in addition to RA signaling. The subtypes can be distinguished by their distinct gene expression patterns and electrophysiological features. Retinoic signaling is important for controlling the specification of atrial and ventricular CMs. Inhibiting RA signaling in mouse and chicken embryos led to bigger ventricles and smaller or non-existent atria. Conversely, adding RA reversed the phenotypes, resulting in bigger atria. The effects of RA on CM differentiation and subtype specification were investigated, and it was observed that RA injection during the early embryonic stage caused CM to develop into an atrial phenotype[34].
Chirikian et al[35] employed the chamber-specific reporter gene, sarcolipin (SLN), to separate homogeneous populations of hiPSC-aCMs from a single hiPSC line using clustered regularly interspaced short palindromic repeats associated protein 9 (CRISPR/Cas9) to create fluorescent reporter lines for aCMs with SLN cyan fluorescent protein. They confirmed chamber-specific isolation of hiPSC-aCMs using genetic and electrophysiological characteristics. The studies by Josowitz et al[36] and Gharanei et al[5] found that SLN expression was an indicator of atrial selectivity. They developed a bacterial artificial chromosome reporter design in which fluorescence was triggered by expression of the atrial-specific gene SLN. Cells with strong fluorescence express atrial genes and have functional calcium (Ca2+) handling and electrophysiological features similar to atrial CMs and were isolated by a flow cytometer.
Even though interest in SC biology and CM development has increased recently, maturation of hiPSC-aCMs has been challenging. Modeling and drug screening for cardiovascular disease are limited by their immature characteristics. HiPSC-aCMs' mature and immature phenotypes significantly differ from one another, which may have important ramifications for how well they may simulate adult disorders and be used in regenerative medicine. Several laboratories worldwide have examined ways for hiPSC-CM maturation by aiming to imitate events and components involved in cardiac development. Although ventricular cells have been the focus of the majority of hiPSC-CM maturation research, atrial cells may also benefit from the techniques and results. A variety of tactics have been employed, including electrical stimulation, microenvironment alteration, and various cultural contexts. CM development in vivo might take months or years. As a result, lengthening the hiPSC-aCM culture time was one of the first strategies to accelerate maturation to be examined. Long-term cell culture causes structural alterations in the form of increased cell length, area, and length-to-width ratios as well as modifications to sarcomere structure and decreased proliferation[37]. Long-term culture resulted in a tenfold increase in the population of multinucleated aCMs, small improvements in contractility, Ca2+ handling, and electrophysiological properties, as well as higher myofibril density and alignment, better sarcomere organization, and smaller improvements in cell size and anisotropy[38]. The majority of maturation changes usually occur within the first 4 wk of culture. Although extended culture increased maturation, it did not result in the formation of t-tubules or other characteristics of a completely developed aCM.
Biophysical stressors have been discovered to be another critical factor in the development of aCMs in the heart. By using biophysical signals during the stages of development and maturation, these settings have been attempted to be recreated in order to facilitate the maturation of aCMs in culture. Adult aCMs' rod form is important for myofibril alignment and contractility. By culturing the cells in micropatterns or by printing nanogrooves on the culture substrates, it is possible to promote the elon
Electrical impulses continually activate CMs in the heart, causing it to contract synchronously. The consequences of excitation-contraction coupling are crucial for the growth and function of the heart. In vitro investigations have shown that electrical stimulation has an important function in CM differentiation and maturation. Ma et al[42] evaluated how electrical stimulation affected the growth and function of the designed heart. They discovered that just 7 d of in vitro electrical field stimulation caused cell alignment and coupling, enhanced contractility, and resulted in structural organization. They concluded that significantly improved aCM maturation can be achieved by culturing iPSC-aCMs as spheroids and exposing them to cyclic, uniaxial stretch, and electrical stimulation. Later studies sought to understand how electrical stimulation affects the development of aCMs by adjusting stimulation parameters including frequency, duration, and timing. They showed that ultrastructural maturation happens as stimulus frequency is gradually increased (via intensity training)[43,44]. Additional research on the impact of electrical stimulation on hiPSC-aCM growth and maturity was conducted and found that aCM contractility was increased by a combination of electrical stimulation and mechanical stress, which also improved collagen fiber arrangement, Ca2+ handling, and mitochondrial alignment[45,46].
It is crucial to stimulate metabolism in the hiPSC-aCMs maturation process. Recent studies discovered that changes in hormone signaling led to significant differences in maturation in cell cultures. Triiodothyronine (T3), a thyroid hormone, is an essential regulator of heart growth, deve
A number of non-CMs surround aCMs in vivo, which help to create a milieu where the cardiac tissue may develop and mature. According to a study that examined electrophysiological maturation and developmental changes in embryoid bodies, the formation of aCM depends on the existence of non-CMs[49]. Tulloch et al[50] discovered that including endothelial and stromal cells in the constructed human cardiac tissue increased CM proliferation and the formation of vessel-like structures in 2011. These tissues were inserted into the hearts of rats with athymia, where they flourished and united with the host myocardium to produce grafts. Combining hiPSC-CMs with cardiac fibroblasts (CFs) and cardiac endothelial cells improved maturation in scaffold-free 3D microtissues, as evidenced by improved sarcomeric structures with t-tubules, increased contractility, mitochondrial respiration, and a mature electrophysiological profile, according to Giacomelli et al[51]. Connexin 43 gap junctions were used in the interaction between hiPSC-CM and CFs to increase intracellular cyclic AMP. While the precise mechanisms underlying co-culture enhanced maturation are unknown, it is believed that non-CMs play a significant role in CM maturation by promoting direct physical adhesion and secreting cytokines like granulocyte-macrophage colony-stimulating factor, vascular endothelial growth factors, stromal cell-derived factor 1, and basic fibroblast growth factor[52].
Several investigators have examined the influence of generating 3D tissue conditions to improve CM maturation. Neonatal rat CMs were developed in a 3D hydrogel environment utilizing microfabricated elastomeric modules with hexagonal posts to mimic the orientation of the epicardial fibers in the adult heart. After 3 wk of culturing, the 3D tissue demonstrated improved structural and functional maturation as compared to 2D monolayers, as proven by the presence of dense, aligned, and electromechanically active cells. The 3D structures also showed co-localization of L-type Ca2+ channels and mature action potential (AP) propagation, conduction velocity, and robust development of t-tubules aligned with Z-disks[53]. Compared to ventricular engineered heart tissue (EHT), Lemme et al[54] atrial EHT (aEHT) had higher levels of atrial selective marker expression, faster contraction dynamics, lower force output, and shorter AP duration (APD).
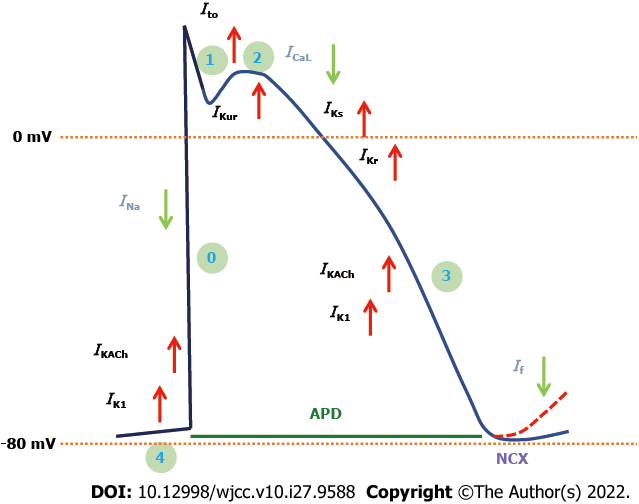
The aCMs are distinct from other types of CMs in that they are more responsive than vCMs to certain disease states and drug treatments. Atrial cells are smaller, thinner, and have fewer t-tubules than ventricular cells, which allows them to handle Ca2+ differently from ventricular and nodal cells. To distinguish between ventricular and nodal-like CMs, aCMs have a distinct behavioral and transcriptome expression profile that can be exploited in subtype specification studies[55-57]. The Ca2+ propagation pattern is delayed and shorter in aCMs, and sarcoendoplasmic reticulum Ca2+ ATPase 2a and SLN, a phospholamban paralog, are expressed more abundantly. Ryanodine receptor expression is also lower in aCMs[58]. Because aCMs express the ultra-rapid delayed-rectifier K+ (potassium) channel (IKUR) and an acetylcholine-activated inward-rectifying K+ current (IKACh), which are mainly lacking in vCMs, there are differences in channels and currents between aCMs and vCMs that affect electrophysiology. Thus, the resting membrane potential and AP amplitude of the atrial AP are less negative. Additionally, the atrial AP is more triangular in shape, with a smaller plateau phase and quicker repolarization phase, which is regulated by a K+ current that is expressed at lower levels and that is internally rectifying K+ current (IK1), slowly activating delayed rectifier K+ channels (IKs), rapidly activating delayed rectifier K+ channels (IKr), sodium current (INa), IKACh, T-type Ca2+ channel, and Ca2+-activated K+ channels expression in the atrium[59-61] (Figure 4).
The effective differentiation of aCMs from hiPSC-CMs can be easily assessed by gene expression, protein quantification, and cell characterization methods. From the standpoint of gene expression, defining the atrial lineage might require a few consensus cell lineage-specific genes. These hiPSC-aCMs should increase SLN, natriuretic peptide A, myosin light chain 7, and T-Box transcription factor 5, with a decrease in myosin light chain-2 and iroquois homeobox 4 genes[23,62]. To assess the aCMs and vCMs of hiPSC-CMs, Gharanei et al[5] used simple two-parameter flow cytometry using cardiac troponin T (cTnT) and myosin light chain 2v (MLC-2v), respectively. As a marker of hiPSC-aCMs, they used the MLC-2v negative/cTNT-positive population since MLC-2v has high selectivity for ventricular cells. The multiparameter flow cytometry panel might be enhanced to include additional atrial-specific markers such as T-box transcription factor 5 to more precisely select the aCMs in addition to the specificity of commercially available antibodies and the accessibility of a multicolor flow cytometry device. When these approaches are combined, they may be used to quickly determine the specificity of aCMs.
Benzoni et al[63] investigated the clinical cases of three siblings with untreatable persistent AF whose whole-exome sequence analysis revealed several mutated genes using three iPSC clones from two of these patients and differentiated these cells towards the cardiac lineage. They discovered that the electrophysiological characterization of patient-derived aCMs (AF-aCMs) beat at a greater rate than control-aCMs. The pacemaker current (If) and ICaL currents were found to be more important in the analysis. There were no variations in the repolarizing current IKr or Ca2+ handling in the sarcoplasmic reticulum. Paced AF-aCMs had much longer APs, and under stress, both delayed after-depolarizations of greater amplitude and more ectopic beats than control-aCM cells. They concluded that the patients' common genetic background causes functional changes in the If and ICaL currents, resulting in a cardiac substrate that is more prone to arrhythmias under stressful situations. They proposed that using patient-derived aCMs grown from iPSC might reveal a plausible cellular mechanism underlying this complex familial variation of AF (Table 1).
| Ref. | Specimens | Experiment | Results |
| AF disease modeling | |||
| Benzoni et al[63] | -2 untreatable persistent AF siblings (3 hiPSC clones) | -Differentiated 3 hiPSC clones’ cells towards the atrial cardiomyocytes (AF-aCMs) | -AF-aCMs had much longer action potentials, beat at a greater rate, and more ectopic beats than control-aCM cells. The patients' common genetic background causes functional changes in the If and ICa,L currents, resulting in a cardiac substrate that is more prone to arrhythmias under stressful situations |
| Argenziano et al[64] | -RA-derived hiPSC-aCMs | -Molecular, transcriptomic, and electrophysiological analysis of RA-derived hiPSC-aCMs | -RA causes differential expression of Ca2+ homeostasis genes that directly interact with the RA receptor via COUP-TFII |
| Nakanishi et al[65] | -2D monolayer of hiPSC-aCMs and atrial fibroblasts (aFbs) | -Conduction disruption influenced geometrical patterning and constituent cell heterogeneity under high frequency stimulation | -A higher frequency electrical stimulus preferentially caused poorer electrical conduction in hiPSC-aCMs monolayer preparations with an abrupt geometrical transition rather than those with uniform geometry. The addition of human aFbs tended to worsen the integrity of electrical conduction |
| Lemoine et al[66] | -hiPSC-aCMs cultured into atrial engineered heart tissue (aEHT) | - Optogenetic activation by blue light pulses after aEHTs were transduced with a lentiviral expression channel expressing rhodopsin-2 | -The spontaneous beating rhythm of tachypaced aEHTs was more irregular; NT-proBNP and RNA levels were greater in the targeted group. Intermittent tachypacing in aEHTs causes some of the electrical changes seen in AF as well as an arrhythmic spontaneous beating pattern |
| Hong et al[67] | -hiPSC-aCMs from 2 relatives who carried SCN5A mutations (E428K and N470K) | -Characterize the pathogenesis of AF-linked SCN5A mutations compared with isogenic controls | - Mutant AF iPSC-aCMs demonstrated spontaneous arrhythmogenic activity with beat-to-beat irregularity, longer APD, and triggered-like beats. Single-cell recordings demonstrated that AF iPSC-aCMs had increased INa,L |
| Soepriatna et al[68] | -3D atrial microtissue from hiPSC-aCMs and hiPSC-vCMs | - AP responses to the atrial-specific potassium repolarizing current IKur-blocker 4-Aminopyridine and the funny current If-blocker Ivabradine were characterized in vitro | -An atrial microtissues having a quicker spontaneous beating rate, a slower AP rise time, and a shorter APD than ventricular microtissues |
| Drug screening platform for AF | |||
| Honda et al[69] | -hiPSCs with and without RA | -Gene expression and membrane potential analyses | -Pulse width duration 30cF lengthening was verified exclusively in hiPSC-aCMs using IKur channel inhibitor unique to aCMs. While hiPSC-vCMs displayed an early following depolarization by treatment with IKr channel inhibitor, which generates ventricular arrhythmia in clinical settings |
| Schmid et al[70] | -Nodal hiPSC-CMs, hiPSC-aCMs, and hiPSC-vCMs | -Assess the potential of drugs that cause chronotropic effects, AF, and ventricular arrhythmias | -Electrophysiological characteristics and ion channel expression differed across the three commercially available hiPSC-CM cultures. Whereas atrial/ventricular pluricytes demonstrate a tendency toward chamber specificity |
| Personalized regenerative medicine for AF | |||
| Wang et al[71] | - hiPSC-aCMs from patients with paroxysmal AF and healthy controls. A miR-155 transgenic (Tg) and knock-out mouse | -Expression of miR-155 and CACNA1C on the ICa,L | -The expression of miR-155 was elevated while the expression of CACNA1C was decreased in the hiPSC-aCMs of patients with AF. MiR-155/Tg mice exhibited a shorter action potential duration and increased susceptibility to AF, which was related to reduced ICa,L and was inhibited by a miR-155 inhibitor |
Argenziano et al[64] conducted comprehensive molecular, transcriptomic, and electrophysiological analyses of RA-derived hiPSC-aCMs and showed that RA causes differential expression of Ca2+ homeostasis genes that directly interact with the RA receptor via chicken ovalbumin upstream promoter-transcription factor 2 (COUP-TFII). They described a mechanism through which RA may induce an atrial-like electrophysiological signature via COUP-TFII-mediated downstream control of Ca2+ channel gene expression and Ca2+ handling modulation. They concluded that the findings provided critical insight into the underlying molecular pathways that drive hiPSC-aCMs electrophysiology and justified the use of hiPSC-aCMs as an AF disease model. In 2019, Nakanishi et al[65] used an in vitro 2D monolayer preparation of hiPSC-aCMs and atrial fibroblasts (aFbs) to see if conduction disruption influenced geometrical patterning and constituent cell heterogeneity under high frequency stimulation. They performed a directed cardiac differentiation strategy using all-trans RA to generate hiPSC-aCMs. The hiPSC-aCMs and aFbs were transplanted in predetermined ratios (aCMs/aFbs: 100%/0% or 70%/30%) on manually produced plates with or without geometrical patterning, simulating the pulmonary veins (PV)/left atrial junction. After that, high frequency field stimulation imitating recurrent ectopic foci originating in PVs was administered, and optical mapping was used to determine electrical propagation. They discovered that a higher frequency electrical stimulus preferentially caused poorer electrical conduction in hiPSC-aCMs monolayer preparations with an abrupt geometrical transition rather than those with uniform geometry. Furthermore, the addition of human aFbs to the geometrically patterned hiPSC-aCMs tended to worsen the integrity of electrical conduction as compared to preparations employing the hiPSC-aCMs alone. Thus, electrical conduction inside in vitro hiPSC-aCMs monolayers was selectively endangered by geometrical narrow-to-wide patterning in response to high frequency stimuli. The aFbs, which indicate constituent cell heterogeneity, also contributed to a further decrease in conduction stability.
In 2020, Lemoine et al[66] studied whether optogenetic tachypacing of hiPSC-aCMs cultured into aEHT may produce AF-remodeling. The aEHTs were created from 1 million hiPSC-aCMs after RA differentiation. To enable optogenetic activation by blue light pulses, aEHTs were transduced with a lentiviral expression channel expressing rhodopsin-2. Over a 3-wk period, aEHTs were subjected to optical tachypacing at 5 Hz for 15 s twice a minute and compared to transducing spontaneously beating isogenic aEHTs (1.95 ± 0.07 Hz). The force and length of APs did not differ between spontaneously beating and tachypaced aEHTs. The upstroke velocity in tachypaced aEHTs was greater (138 ± 15 vs 87 ± 11 V/s; P = 0.018), potentially reflecting a predisposition for more negative diastolic pressure. The spontaneous beating rhythm of tachypaced aEHTs was more irregular; N-terminal pro B-type natriuretic peptide and RNA levels were greater in the targeted group. Intermittent tachypacing in aEHTs causes some of the electrical changes seen in AF as well as an arrhythmic spontaneous beating pattern, but has no effect on resting force. They proposed that further research using longer, continuous, or more intense stimulation may provide insight on the function of different rate patterns in the changes in aEHT that reflect the remodeling process from paroxysmal to permanent AF.
Recently, Hong et al[67] conducted a study to elucidate the pathogenesis of AF-linked sodium voltage-gated channel alpha subunit 5 (SCN5A) mutations using iPSC-aCMs from two relatives who carried SCN5A mutations (E428K and N470K) compared with isogenic controls. They found that mutant AF iPSC-aCMs demonstrated spontaneous arrhythmogenic activity with beat-to-beat irregularity, longer APD, and triggered-like beats. Single-cell recordings demonstrated that AF iPSC-aCMs had increased late sodium currents (INaL) that were lacking in a heterologous expression model. AF iPSC-aCMs gene expression analysis revealed different expressions of the nitric oxide (NO)-mediated signaling pathway driving increased INaL. They also demonstrated that patient-specific AF iPSC-aCMs exhibited a dramatic in vitro electrophysiological pattern of AF-linked SCN5A mutations, and transcriptomic analysis showed the NO signaling pathway modulating the INa,L and triggering AF.
In 2021, Soepriatna et al[68] created an in vitro model of 3D atrial microtissue from hiPSC-aCMs and hiPSC-vCMs and tested chamber-specific chemical responses both experimentally using fluorescence imaging and computationally. For high resolution AP optical mapping, lactate purified aCMs, vCMs, and 5% human cardiac fibroblasts were used to produce self-assembling 3D microtissues, which were then electrically stimulated for 1 wk before high resolution AP optical mapping. Within their therapeutic window, AP responses to the atrial-specific K+ repolarizing current IKur-blocker 4-aminopyridine and the funny current If-blocker Ivabradine were characterized. They found that high purity CMs (> 75% cTnT+) exhibited subtype specification via MLC2v expression. Spontaneous beating rates were dramatically reduced after 3D microtissue development, with atrial microtissues having a quicker spontaneous beating rate, a slower AP rise time, and a shorter APD than ventricular microtissues. They found that the in vitro platform for screening atrial-specific responses is both robust and sensitive, with high throughput, enabling research into the mechanisms underlying atrial arrhythmias.
The differential expression of unique sets of ion channels and other proteins that maximize their specialized functions determines the varied features of atrial and ventricular CMs. Drugs that preferentially target atrial ion channels can thereby induce disparities in pharmacological action between the two chambers. This atrial-selective pharmacology is crucial in the investigation and treatment of atrial-specific illnesses like AF, the most prevalent heart rhythm condition. Investigating atrial-selective pharmacology can help and guide the development of new cardiac drugs while also enhancing safety and efficacy by avoiding potentially damaging electrophysiological effects on the ventricular chambers. In 2021, Gunawan et al[6] conducted a study to differentiate hiPSC-CMs into a monolayer of CMs with an atrial phenotype (hiPSC-aCMs) by modifying the GiWi protocol. To demonstrate a clear and distinct atrial phenotype, they used multiple phenotypic approaches such as quantitative PCR, digital multiplexed gene expression analysis with NanoString technology, flow cytometry, enzyme-linked immunoassay, voltage measurements with current clamp electrophysiology, and simultaneous voltage and Ca2+ transient measurements with optical mapping. They performed an in-depth pharmacological analysis with simultaneous voltage and Ca2+ measurements to demonstrate the differential responses of these chamber-specific CMs, as well as their utility as a translational model for screening the safety and efficacy of novel atrial-specific compounds for the treatment of AF. They demonstrated the role of atrial-specific ionic currents in their model system using a variety of drugs such as 4-aminopyridine, dofetilide, vernakalant, AVE0118, UCL1684, and nifedipine and were able to reveal the predicted chamber specific distinctions between hiPSC-aCMs and hiPSC-vCMs. They concluded that a model system comprised of hiPSC-aCMs and optical mapping is well-suited for preclinical drug screening of novel and targeted atrial selective medicines.
Honda et al[69] studied the possibility of atrial-like CMs produced from hiPSCs for the assessment of drug-induced atrial arrhythmia. During the process of myocardial differentiation, RA was added to create atrial-like CMs, and their features were compared to those of RA-free CMs. Using gene expression and membrane potential analyses, it was demonstrated that cells with or without RA therapy have atrial or ventricular-like CMs, respectively. Pulse width duration 30cF lengthening was verified exclusively in hiPSC-aCMs using an ultra-rapid activating delayed rectifier K+ current (IKur) channel inhibitor unique to aCMs. Furthermore, vCMs displayed an early following depolarization by treatment with a rapidly activating IKr channel inhibitor, which generates ventricular arrhythmia in clinical settings. They concluded that RA therapy produced a platform for human hiPSC-CMs with atrial and nodal characteristics. By comparing ventricular and atrial drug responses, membrane potential-based drug testing on these platforms might uncover propensities for drug-induced tachyarrhythmias. Furthermore, atrial platforms are more susceptible to bradyarrhythmia.
Schmid et al[70] conducted a study to assess the potential of drugs that cause chronotropic effects (nodal hiPSC-CMs), AF (hiPSC-aCMs), or ventricular arrhythmias (hiPSC-vCMs) using single-cell patch-clamp RT-PCR to clarify the composition of the iCell CMs population and to compare it with atrial and ventricular pluricytes and primary human aCMs and vCMs. They found that the comparison of beating and non-beating iCell CMs did not support the presence of true nodal, atrial, and ventricular cells in this hiPSC-CM population. On the other hand, the comparison of atrial and ventricular pluricytes with primary human CMs showed trends, indicating the potential to derive more subtype-specific hiPSC-CM models using appropriate differentiation protocols. They concluded that electrophysiological characteristics and ion channel expression differed across the three commercially available hiPSC-CM cultures. Whereas atrial/ventricular pluricytes demonstrate a tendency toward chamber specificity, the majority of individual cells from all three hiPSC-CM groups studied do not resemble chamber-specific cell populations found in the adult human heart due to unusual combinations of the analyzed characteristics.
Wang et al[71] used the expression of microRNA-155 (miR-155) and Ca2+ voltage-gated channel subunit alpha1 C (CACNA1C) in hiPSC-aCMs from patients with paroxysmal AF and healthy controls to examine the influence of miR-155 on the expression of L-type Ca2+ current (ICaL) and how it contributes to electrical remodeling in AF. After miR-155 transfection, ICaL characteristics were identified in hiPSC-aCMs. In addition, a miR-155 transgenic (Tg) and knockout (KO) mouse model was created to clarify whether miR-155 was engaged in ICa-L-related electrical remodeling in AF via targeting CACNA1C. They discovered that the expression of miR-155 was elevated while the expression of CACNA1C was decreased in the hiPSC-aCMs of patients with AF. Transfection of hiPSC-aCMs with miR-155 resulted in alterations in ICa-L characteristics that were qualitatively comparable to those caused by AF. MiR-155/Tg mice exhibited shorter APD and increased susceptibility to AF, which was related to reduced ICaL and was inhibited by a miR-155 inhibitor. Moreover, genetic suppression of miR-155 blocked AF induction in miR-155/KO mice while leaving ICaL characteristics unchanged. They concluded that miR-155 had a crucial role in the control of Cav1.2, which was responsible for electrical remodeling in AF. The electrical remodeling in AF caused by large decreases in ICaL density was mitigated in miR-155-KO hearts. Although genetic deletion of miR-155 prevented the development of AF, overexpression of miR-155 in Tg mice dramatically aggravated AF, showing that miR-155 suppression may be a favorable therapeutic strategy in preventing electrical remodeling and AF.
The glutamatergic transmitter system, as an excitatory transmitter system, regulates the excitability and conductivity of neurons. Since CMs and neurons are both excitable cells, CMs may be controlled by a similar mechanism. Xie et al[72] found that aCMs have an intrinsic glutamatergic transmitter system that governs AP production and propagation. There are many glutamate-containing vesicles beneath the plasma membrane of rat atrial CMs. Moreover, important components of the glutamatergic transmitter system, such as the glutamate metabolic enzyme, ionotropic glutamate receptors (iGluRs), and glutamate transporters, are expressed in rat aCMs, and iGluR agonists elicit iGluR-gated currents and lower the electrical excitability threshold in those cells. In addition, both in vitro and in vivo, iGluR antagonists significantly reduce the conduction velocity of electrical impulses in the rat aCMs. In cultured hiPSC-aCMs monolayers, knockdown of glutamate ionotropic receptor AMPA type subunit 3 or glutamate ionotropic receptor NMDA type subunit 1, two highly expressed iGluR subtypes in atria, significantly reduced excitatory firing rate and slowed electrical conduction velocity. Finally, in a rat isolated heart model, iGluR antagonists efficiently prevent and terminate AF. They concluded that an intrinsic glutamatergic transmitter system directly modulates aCMs’ excitability and conductivity by influencing iGluR-gated currents. Manipulation of this system may offer new paths for the treatment of cardiac arrhythmias.
Recently, Benzoni et al[63] reported the first human AF iPSC-derived cells, which were created from two of three siblings who developed a drug-resistant type of AF at a young age (55 years). They investigated the molecular and electrophysiological features of hiPSC-CMs from AF patients (AF-CMs) and controls (CTRL-CMs) using these cells, indicating changes in ionic currents that might reflect one of the cellular pathways that contribute to AF start. In the near future, this approach might serve as a foundation for personalized regenerative medicine for AF.
With these positive prospects in sight, it is highly probable that the future management of AF can be personalized with the use of AF disease models, which are constructed from patient-derived hiPSC-aCMs to test for individually-tailored drugs that illicit specific responses in different patients. Through the use of these models, different mutations in various AF patients can be explored, and their corresponding responses to treatment can be evaluated. This may lead to the development of new medications that are specific to distinct mutation subtypes, enabling a more precise treatment regimen. Moreover, AF models can provide a platform for further studies in regenerative medicine.
In light of the various issues encountered in current treatments of AF, it becomes clear that more personalized therapeutic approaches need to be adopted in order to enhance the safety and efficacy of AF therapy. With the use of hiPSC-aCMs, AF disease models can be constructed and they can play a major role in future developments in precision medicine. The AF models can serve as a novel platform for drug discovery and development, and eventually personalized therapies for AF. Furthermore, recent study discoveries predict future success in regenerative medicine, and AF models can help pave the way for the development of regenerative therapy for AF patients, potentially leading to the discovery of an absolute cure for AF.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mishra AK, United States; Yoshikawa M, Japan S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ Res. 2020;127:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 907] [Article Influence: 181.4] [Reference Citation Analysis (0)] |
| 2. | Aronis KN, Ali RL, Liang JA, Zhou S, Trayanova NA. Understanding AF Mechanisms Through Computational Modelling and Simulations. Arrhythm Electrophysiol Rev. 2019;8:210-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Samak M, Hinkel R. Stem Cells in Cardiovascular Medicine: Historical Overview and Future Prospects. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Musunuru K, Sheikh F, Gupta RM, Houser SR, Maher KO, Milan DJ, Terzic A, Wu JC; American Heart Association Council on Functional Genomics and Translational Biology; Council on Cardiovascular Disease in the Young; and Council on Cardiovascular and Stroke Nursing. Induced Pluripotent Stem Cells for Cardiovascular Disease Modeling and Precision Medicine: A Scientific Statement From the American Heart Association. Circ Genom Precis Med. 2018;11:e000043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 5. | Gharanei M, Shafaattalab S, Sangha S, Gunawan M, Laksman Z, Hove-Madsen L, Tibbits GF. Atrial-specific hiPSC-derived cardiomyocytes in drug discovery and disease modeling. Methods. 2021;203:364-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Gunawan MG, Sangha SS, Shafaattalab S, Lin E, Heims-Waldron DA, Bezzerides VJ, Laksman Z, Tibbits GF. Drug screening platform using human induced pluripotent stem cell-derived atrial cardiomyocytes and optical mapping. Stem Cells Transl Med. 2021;10:68-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Rikhtegar R, Pezeshkian M, Dolati S, Safaie N, Afrasiabi Rad A, Mahdipour M, Nouri M, Jodati AR, Yousefi M. Stem cells as therapy for heart disease: iPSCs, ESCs, CSCs, and skeletal myoblasts. Biomed Pharmacother. 2019;109:304-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14315] [Article Influence: 842.1] [Reference Citation Analysis (0)] |
| 9. | Yoshida Y, Yamanaka S. iPS cells: a source of cardiac regeneration. J Mol Cell Cardiol. 2011;50:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Devalla HD, Passier R. Cardiac differentiation of pluripotent stem cells and implications for modeling the heart in health and disease. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Filipczyk AA, Passier R, Rochat A, Mummery CL. Regulation of cardiomyocyte differentiation of embryonic stem cells by extracellular signalling. Cell Mol Life Sci. 2007;64:704-718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Ban K, Bae S, Yoon YS. Current Strategies and Challenges for Purification of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Theranostics. 2017;7:2067-2077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Sacchetto C, Vitiello L, de Windt LJ, Rampazzo A, Calore M. Modeling Cardiovascular Diseases with hiPSC-Derived Cardiomyocytes in 2D and 3D Cultures. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 14. | Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res. 2012;111:344-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 552] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 15. | Goedel A, My I, Sinnecker D, Moretti A. Perspectives and Challenges of Pluripotent Stem Cells in Cardiac Arrhythmia Research. Curr Cardiol Rep. 2017;19:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Abi-Gerges N, Miller PE, Ghetti A. Human Heart Cardiomyocytes in Drug Discovery and Research: New Opportunities in Translational Sciences. Curr Pharm Biotechnol. 2020;21:787-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Mazzola M, Di Pasquale E. Toward Cardiac Regeneration: Combination of Pluripotent Stem Cell-Based Therapies and Bioengineering Strategies. Front Bioeng Biotechnol. 2020;8:455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Andrysiak K, Stępniewski J, Dulak J. Human-induced pluripotent stem cell-derived cardiomyocytes, 3D cardiac structures, and heart-on-a-chip as tools for drug research. Pflugers Arch. 2021;473:1061-1085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Breckwoldt K, Letuffe-Brenière D, Mannhardt I, Schulze T, Ulmer B, Werner T, Benzin A, Klampe B, Reinsch MC, Laufer S, Shibamiya A, Prondzynski M, Mearini G, Schade D, Fuchs S, Neuber C, Krämer E, Saleem U, Schulze ML, Rodriguez ML, Eschenhagen T, Hansen A. Differentiation of cardiomyocytes and generation of human engineered heart tissue. Nat Protoc. 2017;12:1177-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 20. | Huch M, Knoblich JA, Lutolf MP, Martinez-Arias A. The hope and the hype of organoid research. Development. 2017;144:938-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 261] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 21. | Chen Z, Xian W, Bellin M, Dorn T, Tian Q, Goedel A, Dreizehnter L, Schneider CM, Ward-van Oostwaard D, Ng JK, Hinkel R, Pane LS, Mummery CL, Lipp P, Moretti A, Laugwitz KL, Sinnecker D. Subtype-specific promoter-driven action potential imaging for precise disease modelling and drug testing in hiPSC-derived cardiomyocytes. Eur Heart J. 2017;38:292-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Cyganek L, Tiburcy M, Sekeres K, Gerstenberg K, Bohnenberger H, Lenz C, Henze S, Stauske M, Salinas G, Zimmermann WH, Hasenfuss G, Guan K. Deep phenotyping of human induced pluripotent stem cell-derived atrial and ventricular cardiomyocytes. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 23. | Devalla HD, Schwach V, Ford JW, Milnes JT, El-Haou S, Jackson C, Gkatzis K, Elliott DA, Chuva de Sousa Lopes SM, Mummery CL, Verkerk AO, Passier R. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol Med. 2015;7:394-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 269] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 24. | Lee JH, Protze SI, Laksman Z, Backx PH, Keller GM. Human Pluripotent Stem Cell-Derived Atrial and Ventricular Cardiomyocytes Develop from Distinct Mesoderm Populations. Cell Stem Cell. 2017;21:179-194.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 285] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 25. | Zhang Q, Jiang J, Han P, Yuan Q, Zhang J, Zhang X, Xu Y, Cao H, Meng Q, Chen L, Tian T, Wang X, Li P, Hescheler J, Ji G, Ma Y. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011;21:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 268] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 26. | Protze SI, Lee JH, Keller GM. Human Pluripotent Stem Cell-Derived Cardiovascular Cells: From Developmental Biology to Therapeutic Applications. Cell Stem Cell. 2019;25:311-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 27. | Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 378] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 28. | El Robrini N, Etchevers HC, Ryckebüsch L, Faure E, Eudes N, Niederreither K, Zaffran S, Bertrand N. Cardiac outflow morphogenesis depends on effects of retinoic acid signaling on multiple cell lineages. Dev Dyn. 2016;245:388-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Drowley L, McPheat J, Nordqvist A, Peel S, Karlsson U, Martinsson S, Müllers E, Dellsén A, Knight S, Barrett I, Sánchez J, Magnusson B, Greber B, Wang QD, Plowright AT. Discovery of retinoic acid receptor agonists as proliferators of cardiac progenitor cells through a phenotypic screening approach. Stem Cells Transl Med. 2020;9:47-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Blankesteijn WM. Interventions in WNT Signaling to Induce Cardiomyocyte Proliferation: Crosstalk with Other Pathways. Mol Pharmacol. 2020;97:90-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | van Wijk B, Moorman AF, van den Hoff MJ. Role of bone morphogenetic proteins in cardiac differentiation. Cardiovasc Res. 2007;74:244-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 868] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 33. | Sirbu IO, Chiş AR, Moise AR. Role of carotenoids and retinoids during heart development. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Gassanov N, Er F, Zagidullin N, Jankowski M, Gutkowska J, Hoppe UC. Retinoid acid-induced effects on atrial and pacemaker cell differentiation and expression of cardiac ion channels. Differentiation. 2008;76:971-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Chirikian O, Goodyer WR, Dzilic E, Serpooshan V, Buikema JW, McKeithan W, Wu H, Li G, Lee S, Merk M, Galdos F, Beck A, Ribeiro AJS, Paige S, Mercola M, Wu JC, Pruitt BL, Wu SM. CRISPR/Cas9-based targeting of fluorescent reporters to human iPSCs to isolate atrial and ventricular-specific cardiomyocytes. Sci Rep. 2021;11:3026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Josowitz R, Lu J, Falce C, D'Souza SL, Wu M, Cohen N, Dubois NC, Zhao Y, Sobie EA, Fishman GI, Gelb BD. Identification and purification of human induced pluripotent stem cell-derived atrial-like cardiomyocytes based on sarcolipin expression. PLoS One. 2014;9:e101316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Snir M, Kehat I, Gepstein A, Coleman R, Itskovitz-Eldor J, Livne E, Gepstein L. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;285:H2355-H2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 240] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 38. | Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22:1991-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 567] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 39. | McDevitt TC, Angello JC, Whitney ML, Reinecke H, Hauschka SD, Murry CE, Stayton PS. In vitro generation of differentiated cardiac myofibers on micropatterned laminin surfaces. J Biomed Mater Res. 2002;60:472-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Ribeiro AJ, Ang YS, Fu JD, Rivas RN, Mohamed TM, Higgs GC, Srivastava D, Pruitt BL. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci U S A. 2015;112:12705-12710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 351] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 41. | Carson D, Hnilova M, Yang X, Nemeth CL, Tsui JH, Smith AS, Jiao A, Regnier M, Murry CE, Tamerler C, Kim DH. Nanotopography-Induced Structural Anisotropy and Sarcomere Development in Human Cardiomyocytes Derived from Induced Pluripotent Stem Cells. ACS Appl Mater Interfaces. 2016;8:21923-21932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 42. | Ma R, Liang J, Huang W, Guo L, Cai W, Wang L, Paul C, Yang HT, Kim HW, Wang Y. Electrical Stimulation Enhances Cardiac Differentiation of Human Induced Pluripotent Stem Cells for Myocardial Infarction Therapy. Antioxid Redox Signal. 2018;28:371-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 43. | LaBarge W, Mattappally S, Kannappan R, Fast VG, Pretorius D, Berry JL, Zhang J. Maturation of three-dimensional, hiPSC-derived cardiomyocyte spheroids utilizing cyclic, uniaxial stretch and electrical stimulation. PLoS One. 2019;14:e0219442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 44. | Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, Aggarwal P, Zhang B, Conant G, Ronaldson-Bouchard K, Pahnke A, Protze S, Lee JH, Davenport Huyer L, Jekic D, Wickeler A, Naguib HE, Keller GM, Vunjak-Novakovic G, Broeckel U, Backx PH, Radisic M. A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell. 2019;176:913-927.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 414] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 45. | Ruan JL, Tulloch NL, Razumova MV, Saiget M, Muskheli V, Pabon L, Reinecke H, Regnier M, Murry CE. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation. 2016;134:1557-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 331] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 46. | Richards DJ, Tan Y, Coyle R, Li Y, Xu R, Yeung N, Parker A, Menick DR, Tian B, Mei Y. Nanowires and Electrical Stimulation Synergistically Improve Functions of hiPSC Cardiac Spheroids. Nano Lett. 2016;16:4670-4678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 47. | Li M, Iismaa SE, Naqvi N, Nicks A, Husain A, Graham RM. Thyroid hormone action in postnatal heart development. Stem Cell Res. 2014;13:582-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 48. | Parikh SS, Blackwell DJ, Gomez-Hurtado N, Frisk M, Wang L, Kim K, Dahl CP, Fiane A, Tønnessen T, Kryshtal DO, Louch WE, Knollmann BC. Thyroid and Glucocorticoid Hormones Promote Functional T-Tubule Development in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ Res. 2017;121:1323-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 292] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 49. | Kim C, Majdi M, Xia P, Wei KA, Talantova M, Spiering S, Nelson B, Mercola M, Chen HS. Non-cardiomyocytes influence the electrophysiological maturation of human embryonic stem cell-derived cardiomyocytes during differentiation. Stem Cells Dev. 2010;19:783-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 50. | Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H, Murry CE. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109:47-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 581] [Cited by in RCA: 507] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 51. | Giacomelli E, Meraviglia V, Campostrini G, Cochrane A, Cao X, van Helden RWJ, Krotenberg Garcia A, Mircea M, Kostidis S, Davis RP, van Meer BJ, Jost CR, Koster AJ, Mei H, Míguez DG, Mulder AA, Ledesma-Terrón M, Pompilio G, Sala L, Salvatori DCF, Slieker RC, Sommariva E, de Vries AAF, Giera M, Semrau S, Tertoolen LGJ, Orlova VV, Bellin M, Mummery CL. Human-iPSC-Derived Cardiac Stromal Cells Enhance Maturation in 3D Cardiac Microtissues and Reveal Non-cardiomyocyte Contributions to Heart Disease. Cell Stem Cell. 2020;26:862-879.e11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 391] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 52. | Yoshida S, Miyagawa S, Fukushima S, Kawamura T, Kashiyama N, Ohashi F, Toyofuku T, Toda K, Sawa Y. Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes by Soluble Factors from Human Mesenchymal Stem Cells. Mol Ther. 2018;26:2681-2695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 53. | Bian W, Badie N, Himel HD 4th, Bursac N. Robust T-tubulation and maturation of cardiomyocytes using tissue-engineered epicardial mimetics. Biomaterials. 2014;35:3819-3828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 54. | Lemme M, Ulmer BM, Lemoine MD, Zech ATL, Flenner F, Ravens U, Reichenspurner H, Rol-Garcia M, Smith G, Hansen A, Christ T, Eschenhagen T. Atrial-like Engineered Heart Tissue: An In Vitro Model of the Human Atrium. Stem Cell Reports. 2018;11:1378-1390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 55. | Llach A, Molina CE, Fernandes J, Padró J, Cinca J, Hove-Madsen L. Sarcoplasmic reticulum and L-type Ca²⁺ channel activity regulate the beat-to-beat stability of calcium handling in human atrial myocytes. J Physiol. 2011;589:3247-3262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Asp J, Synnergren J, Jonsson M, Dellgren G, Jeppsson A. Comparison of human cardiac gene expression profiles in paired samples of right atrium and left ventricle collected in vivo. Physiol Genomics. 2012;44:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Brandenburg S, Kohl T, Williams GS, Gusev K, Wagner E, Rog-Zielinska EA, Hebisch E, Dura M, Didié M, Gotthardt M, Nikolaev VO, Hasenfuss G, Kohl P, Ward CW, Lederer WJ, Lehnart SE. Axial tubule junctions control rapid calcium signaling in atria. J Clin Invest. 2016;126:3999-4015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 58. | Dobrev D, Wehrens XH. Calmodulin kinase II, sarcoplasmic reticulum Ca2+ leak, and atrial fibrillation. Trends Cardiovasc Med. 2010;20:30-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Zhang H, Garratt CJ, Zhu J, Holden AV. Role of up-regulation of IK1 in action potential shortening associated with atrial fibrillation in humans. Cardiovasc Res. 2005;66:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 60. | Ehrlich JR, Biliczki P, Hohnloser SH, Nattel S. Atrial-selective approaches for the treatment of atrial fibrillation. J Am Coll Cardiol. 2008;51:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Zhao Y, Rafatian N, Wang EY, Wu Q, Lai BFL, Lu RX, Savoji H, Radisic M. Towards chamber specific heart-on-a-chip for drug testing applications. Adv Drug Deliv Rev. 2020;165-166:60-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 62. | Wu SP, Cheng CM, Lanz RB, Wang T, Respress JL, Ather S, Chen W, Tsai SJ, Wehrens XH, Tsai MJ, Tsai SY. Atrial identity is determined by a COUP-TFII regulatory network. Dev Cell. 2013;25:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 63. | Benzoni P, Campostrini G, Landi S, Bertini V, Marchina E, Iascone M, Ahlberg G, Olesen MS, Crescini E, Mora C, Bisleri G, Muneretto C, Ronca R, Presta M, Poliani PL, Piovani G, Verardi R, Di Pasquale E, Consiglio A, Raya A, Torre E, Lodrini AM, Milanesi R, Rocchetti M, Baruscotti M, DiFrancesco D, Memo M, Barbuti A, Dell'Era P. Human iPSC modelling of a familial form of atrial fibrillation reveals a gain of function of If and ICaL in patient-derived cardiomyocytes. Cardiovasc Res. 2020;116:1147-1160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 64. | Argenziano M, Lambers E, Hong L, Sridhar A, Zhang M, Chalazan B, Menon A, Savio-Galimberti E, Wu JC, Rehman J, Darbar D. Electrophysiologic Characterization of Calcium Handling in Human Induced Pluripotent Stem Cell-Derived Atrial Cardiomyocytes. Stem Cell Reports. 2018;10:1867-1878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 65. | Nakanishi H, Lee JK, Miwa K, Masuyama K, Yasutake H, Li J, Tomoyama S, Honda Y, Deguchi J, Tsujimoto S, Hidaka K, Miyagawa S, Sawa Y, Komuro I, Sakata Y. Geometrical Patterning and Constituent Cell Heterogeneity Facilitate Electrical Conduction Disturbances in a Human Induced Pluripotent Stem Cell-Based Platform: An In vitro Disease Model of Atrial Arrhythmias. Front Physiol. 2019;10:818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Lemoine MD, Lemme M, Ulmer BM, Braren I, Krasemann S, Hansen A, Kirchhof P, Meyer C, Eschenhagen T, Christ T. Intermittent Optogenetic Tachypacing of Atrial Engineered Heart Tissue Induces Only Limited Electrical Remodelling. J Cardiovasc Pharmacol. 2020;77:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Hong L, Zhang M, Ly OT, Chen H, Sridhar A, Lambers E, Chalazan B, Youn SW, Maienschein-Cline M, Feferman L, Ong SG, Wu JC, Rehman J, Darbar D. Human induced pluripotent stem cell-derived atrial cardiomyocytes carrying an SCN5A mutation identify nitric oxide signaling as a mediator of atrial fibrillation. Stem Cell Reports. 2021;16:1542-1554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 68. | Soepriatna AH, Kim TY, Daley MC, Song E, Choi BR, Coulombe KLK. Human Atrial Cardiac Microtissues for Chamber-Specific Arrhythmic Risk Assessment. Cell Mol Bioeng. 2021;14:441-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 69. | Honda Y, Li J, Hino A, Tsujimoto S, Lee JK. High-Throughput Drug Screening System Based on Human Induced Pluripotent Stem Cell-Derived Atrial Myocytes ~ A Novel Platform to Detect Cardiac Toxicity for Atrial Arrhythmias. Front Pharmacol. 2021;12:680618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 70. | Schmid C, Abi-Gerges N, Leitner MG, Zellner D, Rast G. Ion Channel Expression and Electrophysiology of Singular Human (Primary and Induced Pluripotent Stem Cell-Derived) Cardiomyocytes. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Wang J, Ye Q, Bai S, Chen P, Zhao Y, Ma X, Bai C, Liu Y, Xin M, Zeng C, Liu Q, Zhao C, Yao Y, Ma Y. Inhibiting microRNA-155 attenuates atrial fibrillation by targeting CACNA1C. J Mol Cell Cardiol. 2021;155:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 72. | Xie D, Xiong K, Su X, Wang G, Ji Q, Zou Q, Wang L, Liu Y, Liang D, Xue J, Gao X, Gu X, Liu H, He X, Li L, Yang J, Lu Y, Peng L, Chen YH. Identification of an endogenous glutamatergic transmitter system controlling excitability and conductivity of atrial cardiomyocytes. Cell Res. 2021;31:951-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |