Published online Sep 16, 2022. doi: 10.12998/wjcc.v10.i26.9241
Peer-review started: June 2, 2022
First decision: June 18, 2022
Revised: June 27, 2022
Accepted: August 6, 2022
Article in press: August 6, 2022
Published online: September 16, 2022
Processing time: 91 Days and 21.3 Hours
Hematopoietic stem cell transplantation (HSCT)-sinusoidal obstruction syndrome (SOS), also known as veno-occlusive disease, is a clinical syndrome characterized by symptoms, such as right upper quadrant pain, jaundice, fluid retention, and hepatomegaly, and is caused by pre-treatment-related hepatotoxicity during the early stages after HSCT. Clinical diagnosis of HSCT-SOS is based on the revised Seattle or Baltimore standards. The revised standard by the European Society for Bone Marrow Transplantation in 2016 has good practicability and can be used in combination with these two standards. Eight studies have shown the value of liver stiffness measurement (LSM) in the early diagnosis of HSCT-SOS. Four studies investigated LSM specificity and sensitivity for the early diagnosis of HSCT-SOS. LSM can distinguish SOS from other post-HSCT complications, enabling a clear differential diagnosis. It has been shown that median LSM of patients with SOS is significantly higher than that of patients with other treat
Core Tip: Clinical diagnosis of hematopoietic stem cell transplantation (HSCT)-sinusoidal obstruction syndrome (SOS) is based on the revised Seattle or Baltimore standards. Eight studies have shown the value of liver stiffness measurement (LSM) in the early diagnosis of HSCT-SOS. Four studies investigated the specificity and sensitivity of LSM for the early diagnosis of HSCT-SOS. Therefore, LSM can distinguish SOS from other post-HSCT complications, enabling a clear differential diagnosis. The early diagnosis of SOS is beneficial in preventing severe HSCT complications.
- Citation: Tan YW, Shi YC. Early diagnostic value of liver stiffness measurement in hepatic sinusoidal obstruction syndrome induced by hematopoietic stem cell transplantation. World J Clin Cases 2022; 10(26): 9241-9253
- URL: https://www.wjgnet.com/2307-8960/full/v10/i26/9241.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i26.9241
Hepatic sinusoidal obstruction syndrome (HSOS), also known as hepatic veno-occlusive disease (VOD), is a hepatic vascular disease characterized by hepatic sinusoidal, hepatic venule, and interlobular veinous endothelial cell injuries, leading to lumen stenosis or occlusion and eventually resulting in intrahepatic congestion, liver injury, and intrahepatic retrosinusoidal portal hypertension. Hepatic sinusoidal obstruction syndrome (SOS) manifests clinically as liver pain, jaundice, ascites, and hepatomegaly, and is a severe liver complication commonly observed in patients undergoing hematopoietic stem cell transplantation (HSCT)[1].
HSCT-SOS is a clinical syndrome characterized by symptoms, such as right upper quadrant pain, jaundice, fluid retention, and hepatomegaly, and is caused by pre-treatment-related hepatotoxicity in the early stages after HSCT. In the early stages, mortality rate of severe cases can be as high as 80%[2-4]. In recent years, defibrotide and defibrinopolysaccharide are the only approved drugs for SOS treatment[5,6]. The overall survival rates of patients with SOS at 100 d, 1 year, and 5 years after HSCT were 62%, 46%, and 27%, respectively, while those of patients without SOS were 92%, 77%, and 66% (P < 0.001), respectively with significant decreased in mortality rates[7-9].
The incidence of SOS varies greatly in studies, owing to factors, such as differences in patient characteristics, pre-treatment schemes, transplantation center experience, and diagnostic criteria. A meta-analysis showed that in 135 studies conducted between June 1979 and October 2007, the overall average incidence of SOS was 13.7% [95% confidence interval (CI): 13.3%-14.1%]. The average incidence of SOS between 1979 and 1994 was significantly lower than that between 1994 and 2007 [11.5% (95%CI: 10.9%-12.1%) vs 14.6% (95%CI: 14.0%-15.2%); P < 0.05][10]. Overall, the incidence of autologous HSCT (auto-HSCT) was 3.1%-8.7% and 8.9%-14.0% in allogeneic HSCT (allo-HSCT)[10-13]. The incidence of SOS is high in pediatric populations[14,15]. SOS is common in young patients (1-2-year-olds) with lower body weight, primary hemophagocytic lymphohistiocytosis, adrenal leukodystrophy osteolithiasis, severe thalassemia, or neuroblastoma undergoing autologous HSCT[16,17]. In recent years, the overall incidence and severity of this disease has decreased. However, the use of certain drugs, such as monoclonal antibodies (CD 33 and 22), raising the pre-treatment intensity, as well as secondary transplantation in these patients increases the risk to some extent. The severity of SOS ranges from remission within a few weeks to multiple organ failure and high mortality (> 80%). Therefore, a high index of suspicion along with early diagnosis and treatment is important.
Baixiaoan (1,4-bis[methanesulfonyl-y]butane) is a bifunctional alkylating compound. Over the past 20 years, baixiaoan has been widely used in combination with cyclophosphamide (CY) for the pre-treatment of HSCT. The therapeutic index of busulfan (BU) is very narrow, and acute toxicity may be related to the absorption and disposal of drugs and metabolites. Unstable gastrointestinal absorption can affect precise delivery of oral preparations, particularly in infants and young children. Nearly 40 years after oral preparation was approved, the formula for intravenous BU was approved. It has been reported that BU levels higher than 1500 µm/min increase the risk of SOS, whereas low levels may lead to implantation failure or disease recurrence. Measuring individual plasma BU levels during oral or intravenous administration may provide necessary protection for monitoring drug disposal, thereby ensuring efficacy and preventing toxicity in patients receiving HSCT[18].
Monitoring of therapeutic drug concentrations of BU began in 2000, and liver-supporting drugs, such as ursodeoxycholic acid and N-acetyl-L-cysteine (NAC) were used in 2002 and 2009, respectively. However, NAC is not routinely recommended for the treatment of venous occlusive disease[19]. This prospective randomized study included 160 patients. N-acetyl-L-cysteine showed no significant benefit in reducing post-HSCT hepatotoxicity[20].
Busulfan and CY are commonly used as chemotherapeutic drugs for allogeneic and autologous HSCT[21]. The BU-CY regimen is a commonly used HSCT pre-treatment regimen, but studies have found that administering CY immediately after BU treatment increases the active metabolites of CY[22]. The interval between CY and BU administration negatively correlated with the incidence of SOS[21]. A multicenter randomized controlled trial was designed by Seydoux et al[23] who showed that a 24-h interval between BU and CY administration reduced the incidence of SOS. According to recently published randomized controlled trials and other retrospective studies, the CY-BU protocol is recommended instead of BU-CY[24]. In a systematic evaluation of the efficacy and toxicity characteristics of busulfonamide-fludarabine (BU-flu) and BU-CY preparation program in patients undergoing allogeneic HSCT, patients in the BU-flu program had a lower risk of developing SOS and microbial record infections [risk ratio (RR), 0.34; 95%CI: 0.19-0.62: 8 trials: RR, 0.79; 95%CI: 0.64-0.97][24].
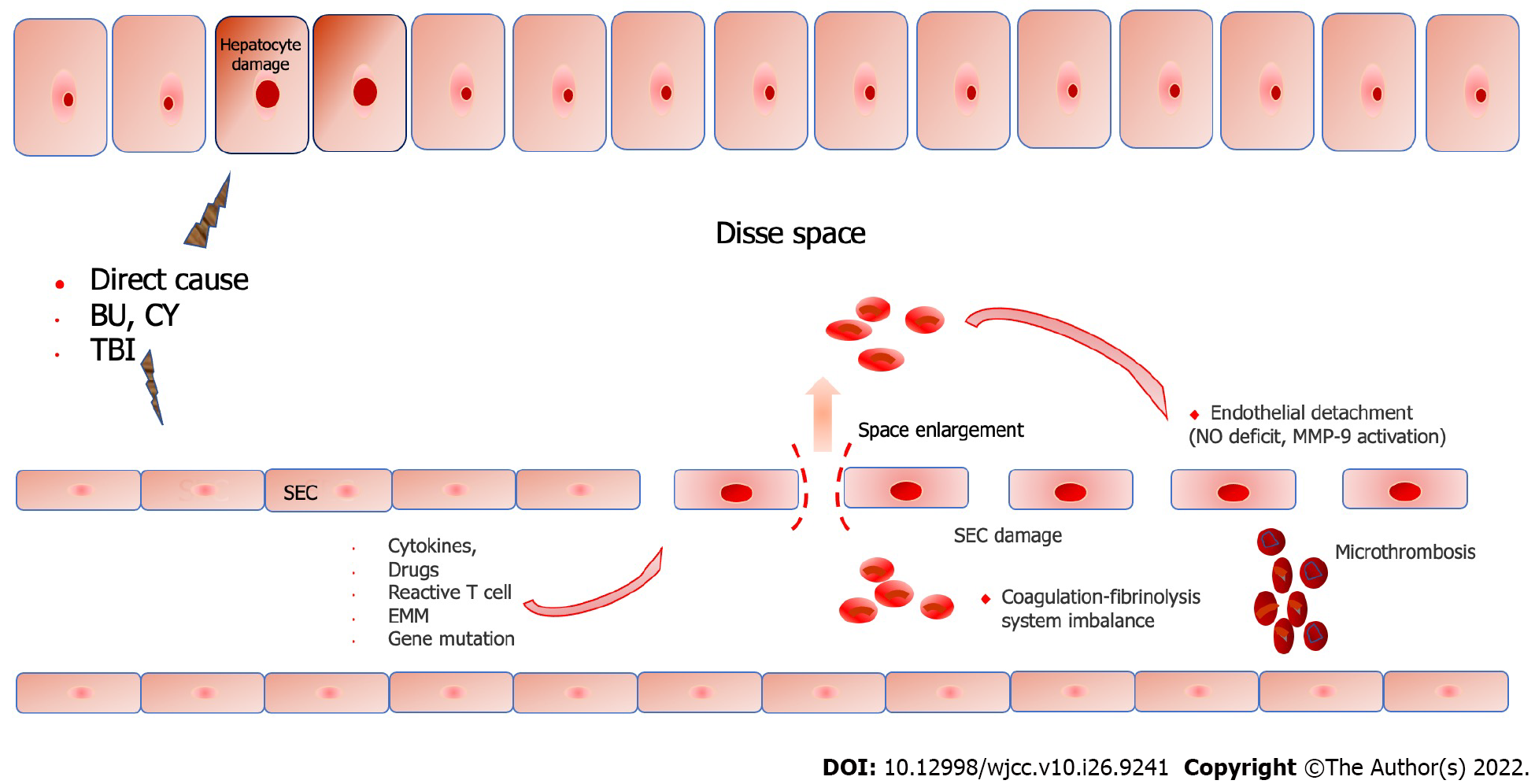
The pathogenesis of SOS remains unclear. However, the central role of SOS pathogenesis is the loss of endothelial cell cohesion caused by sinusoidal endothelial injury[25]. Gaps appear between endothelial cells, and red blood cells infiltrate the Disse space and aggregate followed by retrosinusoidal obstruction[26]. There are many reasons for sinusoidal endothelial cell (SEC) injury, including pre-treatment regimen[27], cytokines[28], endogenous microbial products[29], drugs [calcineurin inhibitor (CNI), monoclonal antibodies, and hematopoietic growth factor], heteroreactive T cells, mutations in glutathione (GSH) transferase-related genes, migration of endogenous microbial metabolites, hematopoietic implantation produced by tissue injury can also cause or aggravate secondary injury[28,30-33], and the implantation process itself[33]. Conditioning regimens play a crucial role in the pathogenesis, which highlights that the increased risk of SOS is associated with higher levels of cytotoxic drugs in the plasma, such as metabolites of BU[34] or CY[35]. Chemotherapeutic drugs are metabolized by the cytochrome P450 complex to produce toxic metabolites, which are then transformed into nontoxic metabolites and eliminated by the GSH enzyme system[36]. The central area of the hepatic lobule (hepatic acinus 3) is more vulnerable to injury, owing to the lack of GSH[21,27,37]. This also explains the pathological basis of serious damage to the central area of the hepatocytes[38,39]. Additionally, the GSH S-transferase M1 deletion genotype reduces the detoxification ability of the liver parenchyma and is prone to SOS[40]. The reduced detoxification ability caused by an immature enzyme system can at least partially explain the high incidence of SOS.
After HSCT, increased levels of endothelial activation markers, such as endothelial procoagulant factors and adhesion molecules[28], circulating endothelial cells[41], endothelial progenitor cells[42], and microparticles[43] were found in the circulatory system, and these pathophysiological changes suggested that endothelial cells showed signs of damage in pro-coagulant and pro-inflammatory states. Nitric oxide deficiency is also a promoter of endothelial cell isolation. Nitric oxide deficiency leads to an increase in matrix metalloproteinase-9, which is associated with isolation of SOS-formed endothelial cells. Metalloproteinase-9 inhibitors completely prevents its occurrence in vivo[44,45].
Damage to SEC leads to destruction of the endothelial cell barrier, loss of sinus wall integrity, infiltration of red blood cells into the Disse space, separation of endothelial cells, and obstruction of the hepatic sinus space[46]. Additionally, an imbalance in the coagulation fibrinolysis system can cause microthrombosis, aggravate obstruction of the central lobular vein, and ultimately result in retrosinus portal hypertension[47]. Central lobular necrosis, fibrosis, and liver failure may occur in severe cases. In view of the central role of endothelial cell injury in pathogenesis, some researchers have included SOS in the category of HSCT-related endothelial injury syndromes (Figure 1)[48].
Risk factors for SOS are divided into two categories: patient- and transplantation-related factors. The former mainly includes females (children)[34,49], physical condition, pre-transplant liver history, abnormal liver function, disease progression status, thalassemia, abdominal radiotherapy history, and use of targeted cancer drugs, such as gemtuzumab ozogamicin, or inotuzumab ozogamicin (IO)[50-52]. Transplantation-related risk factors include allo-HSCT vs auto-HSCT[53]. Some clinical observations have shown that allo-reactivity plays a role in SOS. Compared to autologous HSCT, the incidence of SOS was higher after allogeneic transplantation and in patients receiving mismatched unrelated donor transplantation. These observations are supported by experimental models in which endothelial cells are the targets of allo-reactive T cells[54]. Human leukocyte antigen incompatibility, haplotype transplantation, secondary transplantation, graft non-T cell removal, pre-treatment with BU or total-body radiotherapy (TBI), fludarabine, and CNI combined with sirolimus to prevent graft-versus-host disease (GVHD)[55-57].
In the past 7-10 years, studies assessing the risk factors of SOS in large HSCT recipients were inconsistent with previous data, which revealed potential risk factors that had not been noticed before[1,11,14,58-60]. In many of these studies, the total number of patients ranged between 75 and 5072, and the incidence of SOS ranged between 2.0% [the lowest incidence diagnosed according to the European Society for Bone Marrow Transplantation (EBMT) criteria] and 30.7%. After multivariate analysis, transplantation-related factors associated with an increased risk of SOS in adult HSCT recipients included the use of horse antithymocyte globulin[60], oral and intravenous serum level-adjusted administration of BU (adults and adolescents)[60], myeloablative pre-treatment (based on whole-body irradiation and BU), and two or more HSCTs[58]. Busulfan thiotepa conditioning[60] was used in adults with SOS who needed at least analgesic or diuretic drug treatment, and the serum tacrolimus level increased (above the target range of 5-10 ng/mL).
Liver disease is a major risk factor of SOS. For example, abnormal liver function indicators, or the existence of chronic liver disease (cirrhosis, chronic viral hepatitis, etc.)[13,61]. These liver diseases can be completely asymptomatic, but are risk factors for SOS during previous hepatotoxic treatment with gituzumab and ozone histamine, including iron overload. Identifying risk factors or building a forward-looking risk assessment model is helpful for early prediction and prevention of SOS[62].
Typical SOS usually occurs within 21 d after HSCT, whereas delayed SOS can occur after 21 d. The disease onset can be insidious or rapidly progressing. The main clinical manifestations include right upper abdominal tenderness, jaundice, painful hepatomegaly, ascites, weight gain, and edema. Laboratory examination revealed hyperbilirubinemia, elevated transaminase levels, and unexplained thrombocytopenia. Imaging (Doppler ultrasound is recommended) can detect hepatomegaly, ascites, gallbladder wall edema, slow or reverse flow of portal veinous blood, and portal vein widening[63,64]. The mild symptoms of the syndrome are self-limiting. Patients with severe disease may exhibit multiple organ failure in the kidneys, lungs, and heart with a poor prognosis. Ultrasound can easily detect ascites, hepatomegaly, slow or narrow hepatic veinous flow, and thickening of the gallbladder wall; although non-specifically, it may be helpful in the diagnosis of SOS[19]. Precise techniques to confirm the diagnosis of SOS, such as measurement of hepatic veinous gradient pressure through the jugular vein and liver biopsy, are invasive and difficult to perform in routine practice.
Currently, there are no biological markers of predictive or diagnostic significance. Bearman et al[65] established a model to predict the risk of SOS. However, this model is limited to myeloablative conditioning (TBI, CY, BU, or etoposide). Therefore, its utility is limited. Plasminogen activator inhibitor-1 and other hemagglutination markers have a predictive value. Markers of endothelial cell injury and inflammation are still being explored[34,66-68], and routine detection is not yet recom
The diagnosis of SOS is based on the presence of clinical manifestations within the first three weeks after HSCT and is not attributable to any other possible cause. However, neither set of criteria considers the late onset of SOS after 21 or 40-50 d. Additionally, the use of these criteria may be problematic when only edema and weight gain are present.
The clinical diagnosis of HSCT-SOS was based on the revised Seattle[61] or Baltimore standards[70]. The SOS standard revised by the EBMT in 2016 has good practicability[3], and can be used in conjunction with these two standards. The diagnostic criteria used are listed in Table 1.
| Standard | Definition |
| Revised Seattle standard | At least two of the following manifestations occurred within 20 d after HSCT: Bilirubin > 2 mg/dL, hepatomegaly with right upper abdominal pain, fluid retention, and weight gain ≥ 2% of baseline weight |
| Baltimore standard | At least two of the following manifestations occurred within 21 d after HSCT: Bilirubin > 2 mg/dL, hepatomegaly with right upper abdominal pain, fluid retention, and weight gain ≥ 5% of baseline weight |
| EBMT standard | Classic SOS: Bilirubin > 2 mg/dL within 21 d after HSCT, and at least two of the following manifestations are met: painful hepatomegaly, weight gain ≥ 5%, ascites |
| Delayed type of SOS: 21 d after HSCT, there was classical SOS or SOS confirmed by pathology, or ≥ 2 classical criteria with ultrasonic or hemodynamic evidence |
The EBMT diagnostic criteria for SOS in children mention that the onset does not have an age limit and is based on the occurrence of at least two of the following manifestations[15]: (1) Unexplained consumptive thrombocytopenia and ineffective infusion; (2) Unexplained body weight gain for three consecutive days, or weight gain > 5% of the baseline value, irrespective of the use of diuretics; (3) Liver enlargement above the baseline (confirmation using imaging is recommended); (4) Ascites above the baseline (confirmation using is recommended); and (5) Higher bilirubin levels than the baseline value for three consecutive days or values ≥ 2 mg/dL within 72 h. It is worth noting that 16%-20% of children with SOS have late onset, and nearly 30% have no jaundice[71,72].
Severity classification according to the EBMT classification standard is presented in Table 2.
| Mild | Moderate | Severe | Very severe - MOD/MOF | |
| Time since first clinical symptoms of SOS/VOD | > 7 d | 5-7 d | ≤ 4 d | Any time |
| Bilirubin (mg/dL) | ≥ 2 and < 3 | ≥ 3 and < 5 | ≥ 5 and < 8 | ≥ 8 |
| Bilirubin (μmol/L) | ≥ 34 and < 51 | ≥ 51 and < 85 | ≥ 85 and < 136 | ≥ 136 |
| Bilirubin kinetics | Doubling within 48 h | |||
| Transaminases | ≤ 2 ULN | > 2 and ≤ 5 ULN | > 5 and ≤ 8 ULN | > 8 ULN |
| Weight increase | < 5% | ≥ 5% and < 10% | ≥ 5% and < 10% | ≥ 10% |
| Renal function | < 1.2 × baseline at transplant | ≥ 1.2 and < 1.5 × baseline at transplant | ≥ 1.5 and < 2 × baseline at transplant | ≥ 2 × baseline at transplant |
The above diagnostic norms and standards depend on clinical symptoms, biochemical findings, and imaging data. However, acquiring all these data can take weeks, which could delay diagnosis and result in a poor prognosis. Liver biopsy is the gold standard; however, its invasive nature precludes its use in severe cases, which along with the risk of hemorrhage in severe thrombocytopenia, effectively limits its usability. Several non-invasive models have been developed for the diagnosis of SOS using clinical indicators[1,73]; however, it is still difficult to promote its application owing to its limitations[74]; less than half of those who met the SOS criteria were definitively diagnosed. The subjective difference in ultrasound examination is related to the operator's identification technology, especially in the early stage of SOS, and it is difficult to distinguish specific ultrasonic changes from other liver lesions. Elastography has been widely used to determine liver hardness in recent years, and it can effectively detect liver fibrosis and cirrhosis[75,76]. Liver stiffness measurement (LSM) includes transient elastic imaging, point shear waves with acoustic radiation force impulse (ARFI), and two-dimensional real-time shear wave elastography (2D-SWE)[77,78].
The elastography techniques used in clinical LSM mainly include TE, ARFI, and strain elastography (SE). Acoustic radiation force impulse technology includes point SWE (P-SWE) and 2D-SWE. Fontanilla et al[79] reported the use of quantitative ARFI elastography in two cases of HSCT-SOS, and described that when SOS was accurately diagnosed by ultrasound, acoustic radiation force pulse elastography showed medium and high shear wave velocities (case one, 2.75 m/s and case two, 2.58 m/s), which returned to normal after specific treatment. Therefore, ARFI elastography provides quantitative information to help diagnose this condition and monitor the treatment responses. In one of the above cases, contrast-enhanced ultrasound showed patch enhancement of the liver, which was related to high-speed patch distribution on ARFI elastography, whereas in the second case, nonhomogeneous images on contrast-enhanced computed tomography were noted. During the follow-up period after a specific treatment, the contrast-enhanced mode gradually returned to normal, demonstrating the role of ARFI elastography in early diagnosis and clinical follow-up of the disease.
Traditional ultrasonography has poor sensitivity and specificity. Two-dimensional-SWE is an ultrasound-based technique for staging liver fibrosis by measuring the liver stiffness. At present, it is widely used to evaluate fibrosis in various liver diseases[80-83]. The early diagnostic value of the SOS was unexpected. This single-center prospective cohort study evaluated 25 patients who underwent HCT. Five patients developed an SOS. Patients were evaluated using 2D-SWE at three predetermined time points. On day +5 of HSCT, SWE velocity in patients with SOS increased by 0.25 ± 0.21 m/s, while that in patients without SOS increased by 0.02 ± 0.18 (P = 0.020). On day +14, the SWE velocity increased significantly by 0.91 m/s ± 1.14 m/s in patients with SOS compared with 0.03 m/s ± 0.23 m/s in patients without SOS (P = 0.010). The SWE diagnosis was made 9 and 11 d before the clinical and routine ultrasound diagnoses, respectively. Patients with SOS had increased liver stiffness compared with those without SOS. Thus, SWE changes occur before other imaging and clinical findings of SOS[84].
Inotuzumab ozogamicin is an effective treatment for acute lymphoblastic leukemia (ALL) and is a risk factor for SOS. Ravaioli et al[85] observed 21 patients with ALL during their treatment period and 17.2 mo of follow-up. They observed a significant increase (P < 0.001) in median LSM from 6.7 kPa to 9.5 kPa after the IO cycle and > 8 kPa at the end of the second IO cycle in a dose-dependent kinetic model. Two patients developed SOS, and the LSM exceeded 21 kPa. Another prospective single-center study observed 78 patients receiving HSCT, four of whom developed SOS and found that LSM had good early diagnostic value[86]. It should be noted that increase in LSM after HSCT occurred 2-12 d earlier than that before HSCT. Compared to previous measurements, the increase in LSM value was significantly correlated with the diagnosis of SOS. In a prospective study of adult HSCT recipients, the increase in LSM value after HSCT was assessed using TE, and identified as an accurate predictor of SOS development for the first time. Colecchia et al[87] observed 22 children who underwent HSCT, for whom LSM was performed between 7–10 (T1), 17-20 (T2), and 27-30 (T3) d after the procedure. They reported that there were four individuals who developed SOS for whom LSM values were 4-6 kPa at baseline level, which sharply increased to < 12-20 kPa at T2. Alterations in LSM values were observed 3-6 d before the SOS diagnosis. Transient elastography-FibroScan quantitatively evaluates liver fibrosis by measuring LSM using a shear wave technique[88]. Its non-invasive diagnostic efficacy for liver fibrosis has been verified in a variety of causes of chronic liver disease[85,89].
The high LSM values reported in HSOS are not the result of increased liver hardness due to liver fibrosis and cirrhosis, but the result of liver congestion caused by congestion in the hepatic sinuses, occlusion of hepatic venules, and obstruction of hepatic venous reflux following damage to the hepatic SEC. Increase in LSM has been proven to be caused by inflammation, cholestasis, and congestion[90]. Ravaioli et al[85] found that LSM significantly increased in patients with ALL undergoing IO treatment. This observed trend was difficult to explain because liver fibrosis occurred in these patients. Thus, an inflammatory injury to the liver may be a plausible explanation.
LSMs have shown that the median LSM of patients with SOS is significantly higher than that of patients with other treatment-related liver complications (e.g., acute cholecystitis, cholangitis, antifungal drug-related liver injury, liver GVHD, isolated liver biochemical changes, and fulminant Epstein-Barr virus-related hepatitis reactivation)[85,91].
Four studies investigated the specificity and sensitivity of early diagnosis of HSCT-SOS using LSM (details are shown in Table 3)[84,86,92,93]. Two studies used SWE to detect LSM. In a prospective study, 5 of 25 HSCT patients developed SOS with an area under the curve (AUC) of 0.7762, sensitivity of 80%, and specificity of 67%[84]. Another retrospective study included 161 adult patients who underwent HCT for > 2 years, and 146 patients were analyzed. The ultrasound and 2D–SWE measurements were performed +7 and +14 d after the HCT. Within the first 100 days of the allo-HSCT, 81 patients (55%) developed liver injury. Baseline 2D-SWE could not predict the overall occurrence of liver abnormalities. Six patients were diagnosed with SOS, and we found a significant increase in 2D-SWE on day 14 (AUC = 0.84), with a 2D-SWE measurement greater than 8.1 kPa, increasing sensitivity (75%), specificity (99%), and positive predictive value (60%) in the Seattle criteria. Therefore, it is considered a promising and reproducible method for early diagnosis of SOS[93].
| Country | Year | Study design | Age | HSCT patients (n) | SOS patients (n) | LSM method | LSM (baseline) | LSM (peak) | AUC | Sensitivity | Specificity | Ref. |
| Italy | 2019 | Prospective | Adult | 78 | 4 | FibroScan | 3.7-8.8 | 15-34.3 | 0.997 | 75% | 98.70% | Colecchia et al[86] |
| Italy | 2016 | Retrospective | Children | 22 | 4 | FibroScan | 4-6 | 12-20 | Colecchia et al[87] | |||
| Italy | 2022 | Prospective | Adult | 21 | 2 | FibroScan | 6.7 | > 21 kPa | Ravaioli et al[85] | |||
| Turkey | 2022 | Prospective | 61 yr, 65 yr | 31 | 2 | FibroScan | 8.2 | 55, 17.7 | 0.569 | 100% | 55.17% | Özkan et al[92] |
| United States | 2017 | Prospective | Adult | 25 | 5 | SWE | 0.02 ± 0.18 m/s | 0.25 ± 0.21 m/s | 0.7762 | 80% | 67% | Reddivalla et al[84] |
| France | 2021 | Retrospective | Adult | 146 | 6 | 2D-SWE | > 8.1 kPa | 0.84 | 75% | 99% | Debureauxet al[93] | |
| Spain | 2011 | Retrospective | Adult | 2 | ARFI | 2.75 m/s, 2.58 m/s | Fontanilla et al[79] | |||||
| Italy | 2019 | Retrospective | Children | - | 3 | FibroScan | 2.8-4.6 | 34.3-36 | - | - | - | Zama et al[94] |
In two other prospective studies, a FibroScan was used to detect LSM. Seventy-eight adult patients with indications for allogeneic HSCT were prospectively enrolled. LSM was performed before HSCT and +9/10, +15/17, and +22/24 d after HSCT. Four patients (5.1%) developed SOS during the study period, and the median duration of SOS after the HSCT was +17 d. A sudden increase in LSM was observed in all patients with SOS compared with the values previously evaluated and those before HSCT. The LSM increased 2–12 days before the occurrence of clinical SOS. Compared to the pre-HSCT evaluation, the SOS diagnostic performance of LSM showed that the AUC of the patients was 0.997 (sensitivity, 75%; specificity, 98.7%). After a successful SOS-specific treatment, the LSM gradually decreased. Interestingly, except for SOS, there was no significant increase in LSM in the patients with hepatobiliary complications. The measurement of liver stiffness is considered a promising method for early diagnosis[86].
Another prospective study included 31 adult patients with two or more LSM values. SOS occurred in 2 of the 31 patients (6.4%) who received allogeneic HSCT. Very high LSM values were detected in patients with SOS with an AUC of 0.569, sensitivity of 100%, and specificity of 55.17%. Early and specific VOD/SOS processing can improve LSM values and other related characteristics[92].
The current HSCT-SOS diagnosis depends on the revised EBMT, Seattle, or Baltimore criteria, critically aiding which play an important role in the diagnosis and treatment of such potential serious complications of HSCT, but its though a serious disadvantage lies in its inaccuracy (inability) of an is that it is difficult to diagnose early diagnosis. As a non-invasive detection technology, LSM can detect abnormalities before clinical symptoms, which providing the possibility for an early diagnosis of SOS. Limitations of the current study is still a small sample study include its small size, thus further multi-center, prospective, large-sample studies are needed to verify our findings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: El-Serafi I, Sweden; Papadopoulos K, Thailand S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Roeker LE, Kim HT, Glotzbecker B, Nageshwar P, Nikiforow S, Koreth J, Armand P, Cutler C, Alyea EP, Antin JH, Richardson PG, Soiffer RJ, Ho VT. Early Clinical Predictors of Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome after Myeloablative Stem Cell Transplantation. Biol Blood Marrow Transplant. 2019;25:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 2. | Tewari P, Wallis W, Kebriaei P. Manifestations and management of veno-occlusive disease/sinusoidal obstruction syndrome in the era of contemporary therapies. Clin Adv Hematol Oncol. 2017;15:130-139. [PubMed] |
| 3. | Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, Arat M, Bader P, Baron F, Bazarbachi A, Blaise D, Ciceri F, Corbacioglu S, Dalle JH, Dignan F, Fukuda T, Huynh A, Masszi T, Michallet M, Nagler A, NiChonghaile M, Okamoto S, Pagliuca A, Peters C, Petersen FB, Richardson PG, Ruutu T, Savani BN, Wallhult E, Yakoub-Agha I, Duarte RF, Carreras E. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016;51:906-912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 329] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 4. | Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, Arat M, Bader P, Baron F, Bazarbachi A, Blaise D, Ciceri F, Corbacioglu S, Dalle JH, Duarte RF, Fukuda T, Huynh A, Masszi T, Michallet M, Nagler A, NiChonghaile M, Pagluica T, Peters C, Petersen FB, Richardson PG, Ruutu T, Savani BN, Wallhult E, Yakoub-Agha I, Carreras E. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2015;50:781-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 279] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 5. | Richardson PG, Riches ML, Kernan NA, Brochstein JA, Mineishi S, Termuhlen AM, Arai S, Grupp SA, Guinan EC, Martin PL, Steinbach G, Krishnan A, Nemecek ER, Giralt S, Rodriguez T, Duerst R, Doyle J, Antin JH, Smith A, Lehmann L, Champlin R, Gillio A, Bajwa R, D'Agostino RB Sr, Massaro J, Warren D, Miloslavsky M, Hume RL, Iacobelli M, Nejadnik B, Hannah AL, Soiffer RJ. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood. 2016;127:1656-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 228] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 6. | Richardson PG, Smith AR, Triplett BM, Kernan NA, Grupp SA, Antin JH, Lehmann L, Shore T, Iacobelli M, Miloslavsky M, Hume R, Hannah AL, Nejadnik B, Soiffer RJ; Defibrotide Study Group. Defibrotide for Patients with Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome: Interim Results from a Treatment IND Study. Biol Blood Marrow Transplant. 2017;23:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Richardson PG, Carreras E, Iacobelli M, Nejadnik B. The use of defibrotide in blood and marrow transplantation. Blood Adv. 2018;2:1495-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Kernan NA, Richardson PG, Smith AR, Triplett BM, Antin JH, Lehmann L, Messinger Y, Liang W, Hume R, Tappe W, Soiffer RJ, Grupp SA. Defibrotide for the treatment of hepatic veno-occlusive disease/sinusoidal obstruction syndrome following nontransplant-associated chemotherapy: Final results from a post hoc analysis of data from an expanded-access program. Pediatr Blood Cancer. 2018;65:e27269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Richardson PG, Triplett BM, Ho VT, Chao N, Dignan FL, Maglio M, Mohty M. Defibrotide sodium for the treatment of hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Expert Rev Clin Pharmacol. 2018;11:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, Guinan E, Vogelsang G, Krishnan A, Giralt S, Revta C, Carreau NA, Iacobelli M, Carreras E, Ruutu T, Barbui T, Antin JH, Niederwieser D. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 11. | Tsirigotis PD, Resnick IB, Avni B, Grisariu S, Stepensky P, Or R, Shapira MY. Incidence and risk factors for moderate-to-severe veno-occlusive disease of the liver after allogeneic stem cell transplantation using a reduced intensity conditioning regimen. Bone Marrow Transplant. 2014;49:1389-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Carreras E, Díaz-Beyá M, Rosiñol L, Martínez C, Fernández-Avilés F, Rovira M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transplant. 2011;17:1713-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Carreras E, Bertz H, Arcese W, Vernant JP, Tomás JF, Hagglund H, Bandini G, Esperou H, Russell J, de la Rubia J, Di Girolamo G, Demuynck H, Hartmann O, Clausen J, Ruutu T, Leblond V, Iriondo A, Bosi A, Ben-Bassat I, Koza V, Gratwohl A, Apperley JF. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukemia Working Party. Blood. 1998;92:3599-3604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Corbacioglu S, Cesaro S, Faraci M, Valteau-Couanet D, Gruhn B, Rovelli A, Boelens JJ, Hewitt A, Schrum J, Schulz AS, Müller I, Stein J, Wynn R, Greil J, Sykora KW, Matthes-Martin S, Führer M, O'Meara A, Toporski J, Sedlacek P, Schlegel PG, Ehlert K, Fasth A, Winiarski J, Arvidson J, Mauz-Körholz C, Ozsahin H, Schrauder A, Bader P, Massaro J, D'Agostino R, Hoyle M, Iacobelli M, Debatin KM, Peters C, Dini G. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomised controlled trial. Lancet. 2012;379:1301-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 265] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 15. | Corbacioglu S, Carreras E, Ansari M, Balduzzi A, Cesaro S, Dalle JH, Dignan F, Gibson B, Guengoer T, Gruhn B, Lankester A, Locatelli F, Pagliuca A, Peters C, Richardson PG, Schulz AS, Sedlacek P, Stein J, Sykora KW, Toporski J, Trigoso E, Vetteranta K, Wachowiak J, Wallhult E, Wynn R, Yaniv I, Yesilipek A, Mohty M, Bader P. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European society for blood and marrow transplantation. Bone Marrow Transplant. 2018;53:138-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 235] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 16. | Barker CC, Butzner JD, Anderson RA, Brant R, Sauve RS. Incidence, survival and risk factors for the development of veno-occlusive disease in pediatric hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2003;32:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Cesaro S, Pillon M, Talenti E, Toffolutti T, Calore E, Tridello G, Strugo L, Destro R, Gazzola MV, Varotto S, Errigo G, Carli M, Zanesco L, Messina C. A prospective survey on incidence, risk factors and therapy of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Haematologica. 2005;90:1396-1404. [PubMed] |
| 18. | Krivoy N, Hoffer E, Lurie Y, Bentur Y, Rowe JM. Busulfan use in hematopoietic stem cell transplantation: pharmacology, dose adjustment, safety and efficacy in adults and children. Curr Drug Saf. 2008;3:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Dignan FL, Wynn RF, Hadzic N, Karani J, Quaglia A, Pagliuca A, Veys P, Potter MN; Haemato-oncology Task Force of British Committee for Standards in Haematology; British Society for Blood and Marrow Transplantation. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol. 2013;163:444-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 20. | Barkholt L, Remberger M, Hassan Z, Fransson K, Omazic B, Svahn BM, Karlsson H, Brune M, Hassan M, Mattsson J, Ringdén O. A prospective randomized study using N-acetyl-L-cysteine for early liver toxicity after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:785-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Hassan M, Ljungman P, Ringdén O, Hassan Z, Oberg G, Nilsson C, Békassy A, Bielenstein M, Abdel-Rehim M, Georén S, Astner L. The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant. 2000;25:915-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 119] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Jethava YS, Sica S, Savani B, Socola F, Jagasia M, Mohty M, Nagler A, Bacigalupo A. Conditioning regimens for allogeneic hematopoietic stem cell transplants in acute myeloid leukemia. Bone Marrow Transplant. 2017;52:1504-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Seydoux C, Medinger M, Gerull S, Halter J, Heim D, Chalandon Y, Levrat SM, Schanz U, Nair G, Ansari M, Simon P, Passweg JR, Cantoni N. Busulfan-cyclophosphamide versus cyclophosphamide-busulfan as conditioning regimen before allogeneic hematopoietic cell transplantation: a prospective randomized trial. Ann Hematol. 2021;100:209-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Ben-Barouch S, Cohen O, Vidal L, Avivi I, Ram R. Busulfan fludarabine vs busulfan cyclophosphamide as a preparative regimen before allogeneic hematopoietic cell transplantation: systematic review and meta-analysis. Bone Marrow Transplant. 2016;51:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Bonifazi F, Barbato F, Ravaioli F, Sessa M, Defrancesco I, Arpinati M, Cavo M, Colecchia A. Diagnosis and Treatment of VOD/SOS After Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol. 2020;11:489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 26. | DeLeve LD, Wang X, Kuhlenkamp JF, Kaplowitz N. Toxicity of azathioprine and monocrotaline in murine sinusoidal endothelial cells and hepatocytes: the role of glutathione and relevance to hepatic venoocclusive disease. Hepatology. 1996;23:589-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 173] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Zeng L, Jia L, Xu S, Yan Z, Ding S, Xu K. Vascular endothelium changes after conditioning in hematopoietic stem cell transplantation: role of cyclophosphamide and busulfan. Transplant Proc. 2010;42:2720-2724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Palomo M, Diaz-Ricart M, Carbo C, Rovira M, Fernandez-Aviles F, Escolar G, Eissner G, Holler E, Carreras E. The release of soluble factors contributing to endothelial activation and damage after hematopoietic stem cell transplantation is not limited to the allogeneic setting and involves several pathogenic mechanisms. Biol Blood Marrow Transplant. 2009;15:537-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Eissner G, Multhoff G, Holler E. Influence of bacterial endotoxin on the allogenicity of human endothelial cells. Bone Marrow Transplant. 1998;21:1286-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Krause DS. Vaccination of health care workers. Arch Intern Med. 1992;152:208-209. [PubMed] |
| 31. | Mercanoglu F, Turkmen A, Kocaman O, Pinarbasi B, Dursun M, Selcukbiricik F, Sever MS. Endothelial dysfunction in renal transplant patients is closely related to serum cyclosporine levels. Transplant Proc. 2004;36:1357-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Zoja C, Furci L, Ghilardi F, Zilio P, Benigni A, Remuzzi G. Cyclosporin-induced endothelial cell injury. Lab Invest. 1986;55:455-462. [PubMed] [DOI] [Full Text] |
| 33. | Biedermann BC, Sahner S, Gregor M, Tsakiris DA, Jeanneret C, Pober JS, Gratwohl A. Endothelial injury mediated by cytotoxic T lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet. 2002;359:2078-2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 198] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 34. | Corbacioglu S, Jabbour EJ, Mohty M. Risk Factors for Development of and Progression of Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome. Biol Blood Marrow Transplant. 2019;25:1271-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 35. | Gómez-Centurión I, Bailén R, Oarbeascoa G, Muñoz C, Luque AÁ, Boyra ME, Calleja E, Rincón D, Dorado N, Barzallo P, Anguita J, Díez-Martín JL, Kwon M. Transjugular Intrahepatic Portosystemic Shunt for Very Severe Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome (VOD/SOS) after Unmanipulated Haploidentical Hematopoietic Stem Cell Transplantation with Post-transplantation Cyclophosphamide. Biol Blood Marrow Transplant. 2020;26:2089-2097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Wolf CR, Moll E, Friedberg T, Oesch F, Buchmann A, Kuhlmann WD, Kunz HW. Characterization, localization and regulation of a novel phenobarbital-inducible form of cytochrome P450, compared with three further P450-isoenzymes, NADPH P450-reductase, glutathione transferases and microsomal epoxide hydrolase. Carcinogenesis. 1984;5:993-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Carreras E, Diaz-Ricart M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant. 2011;46:1495-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 38. | DeLeve LD. Cellular target of cyclophosphamide toxicity in the murine liver: role of glutathione and site of metabolic activation. Hepatology. 1996;24:830-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 129] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis. 2002;22:27-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 427] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 40. | Srivastava A, Poonkuzhali B, Shaji RV, George B, Mathews V, Chandy M, Krishnamoorthy R. Glutathione S-transferase M1 polymorphism: a risk factor for hepatic venoocclusive disease in bone marrow transplantation. Blood. 2004;104:1574-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 41. | Sabatier F, Camoin-Jau L, Anfosso F, Sampol J, Dignat-George F. Circulating endothelial cells, microparticles and progenitors: key players towards the definition of vascular competence. J Cell Mol Med. 2009;13:454-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 42. | Pytlík R, Kideryová L, Benesová K, Cechová H, Veselá R, Rychtrmocová H, Trnený M. Circulating endothelial precursor cells (EPC) in patients undergoing allogeneic haematopoietic progenitor cell transplantation. Folia Biol (Praha). 2010;56:32-35. [PubMed] |
| 43. | Pihusch V, Rank A, Steber R, Pihusch M, Pihusch R, Toth B, Hiller E, Kolb HJ. Endothelial cell-derived microparticles in allogeneic hematopoietic stem cell recipients. Transplantation. 2006;81:1405-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | DeLeve LD, Wang X, Kanel GC, Ito Y, Bethea NW, McCuskey MK, Tokes ZA, Tsai J, McCuskey RS. Decreased hepatic nitric oxide production contributes to the development of rat sinusoidal obstruction syndrome. Hepatology. 2003;38:900-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Deleve LD, Wang X, Tsai J, Kanel G, Strasberg S, Tokes ZA. Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology. 2003;125:882-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 46. | Valla DC. Budd-Chiari syndrome and veno-occlusive disease/sinusoidal obstruction syndrome. Gut. 2008;57:1469-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol. 2016;64:179-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 529] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 48. | Otaka F, Ito Y, Goto T, Kojo K, Tanabe M, Hosono K, Majima M, Koizumi W, Amano H. Recovery of Liver Sinusoidal Endothelial Cells Following Monocrotaline-induced Liver Injury. In Vivo. 2021;35:2577-2587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Faraci M, Bertaina A, Luksch R, Calore E, Lanino E, Saglio F, Prete A, Menconi M, De Simone G, Tintori V, Cesaro S, Santarone S, Orofino MG, Locatelli F, Zecca M. Sinusoidal Obstruction Syndrome/Veno-Occlusive Disease after Autologous or Allogeneic Hematopoietic Stem Cell Transplantation in Children: a retrospective study of the Italian Hematology-Oncology Association-Hematopoietic Stem Cell Transplantation Group. Biol Blood Marrow Transplant. 2019;25:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 50. | Kantarjian HM, DeAngelo DJ, Advani AS, Stelljes M, Kebriaei P, Cassaday RD, Merchant AA, Fujishima N, Uchida T, Calbacho M, Ejduk AA, O'Brien SM, Jabbour EJ, Zhang H, Sleight BJ, Vandendries ER, Marks DI. Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: results from the open-label, randomised, phase 3 INO-VATE study. Lancet Haematol. 2017;4:e387-e398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 171] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 51. | Kantarjian HM, Vandendries E, Advani AS. Inotuzumab Ozogamicin for Acute Lymphoblastic Leukemia. N Engl J Med. 2016;375:2100-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Kebriaei P, Cutler C, de Lima M, Giralt S, Lee SJ, Marks D, Merchant A, Stock W, van Besien K, Stelljes M. Management of important adverse events associated with inotuzumab ozogamicin: expert panel review. Bone Marrow Transplant. 2018;53:449-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 53. | Giménez E, Torres I, Albert E, Piñana JL, Hernández-Boluda JC, Solano C, Navarro D. Cytomegalovirus (CMV) infection and risk of mortality in allogeneic hematopoietic stem cell transplantation (Allo-HSCT): A systematic review, meta-analysis, and meta-regression analysis. Am J Transplant. 2019;19:2479-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 54. | Biedermann BC. Vascular endothelium and graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Takami A. Hematopoietic stem cell transplantation for acute myeloid leukemia. Int J Hematol. 2018;107:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 56. | Bazinet A, Popradi G. A general practitioner's guide to hematopoietic stem-cell transplantation. Curr Oncol. 2019;26:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 57. | Radujkovic A, Hegenbart U, Müller-Tidow C, Herfarth K, Dreger P, Luft T. High leukemia-free survival after TBI-based conditioning and mycophenolate mofetil-containing immunosuppression in patients allografted for chronic myelomonocytic leukemia: a single-center experience. Ann Hematol. 2020;99:855-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Yakushijin K, Atsuta Y, Doki N, Yokota A, Kanamori H, Miyamoto T, Ohwada C, Miyamura K, Nawa Y, Kurokawa M, Mizuno I, Mori T, Onizuka M, Taguchi J, Ichinohe T, Yabe H, Morishima Y, Kato K, Suzuki R, Fukuda T. Sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation: Incidence, risk factors and outcomes. Bone Marrow Transplant. 2016;51:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 59. | Hwang DY, Kim SJ, Cheong JW, Kim Y, Jang JE, Lee JY, Min YH, Yang WI, Kim JS. High pre-transplant serum ferritin and busulfan-thiotepa conditioning regimen as risk factors for hepatic sinusoidal obstructive syndrome after autologous stem cell transplantation in patients with malignant lymphoma. Leuk Lymphoma. 2016;57:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Kim H, Lee KH, Sohn SK, Jung CW, Joo YD, Kim SH, Kim BS, Choi JH, Kwak JY, Kim MK, Bae SH, Shin HJ, Won JH, Oh S, Lee WS, Park JH, Yoon SS. Hepatic sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation in adult patients with idiopathic aplastic anemia. Leuk Res. 2013;37:1241-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, Hardin BJ, Shulman HM, Clift RA. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 827] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 62. | Strouse C, Zhang Y, Zhang MJ, DiGilio A, Pasquini M, Horowitz MM, Lee S, Ho V, Ramanathan M, Chinratanalab W, Loren A, Burns LJ, Artz A, Villa KF, Saber W. Risk Score for the Development of Veno-Occlusive Disease after Allogeneic Hematopoietic Cell Transplant. Biol Blood Marrow Transplant. 2018;24:2072-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 63. | Chan SS, Colecchia A, Duarte RF, Bonifazi F, Ravaioli F, Bourhis JH. Imaging in Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome. Biol Blood Marrow Transplant. 2020;26:1770-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 64. | Nishida M, Kahata K, Hayase E, Shigematsu A, Sato M, Kudo Y, Omotehara S, Iwai T, Sugita J, Shibuya H, Shimizu C, Teshima T. Novel Ultrasonographic Scoring System of Sinusoidal Obstruction Syndrome after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2018;24:1896-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 65. | Bearman SI, Anderson GL, Mori M, Hinds MS, Shulman HM, McDonald GB. Venoocclusive disease of the liver: development of a model for predicting fatal outcome after marrow transplantation. J Clin Oncol. 1993;11:1729-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 173] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 66. | Lee JH, Lee KH, Lee JH, Kim S, Seol M, Park CJ, Chi HS, Kang W, Kim ST, Kim WK, Lee JS. Plasminogen activator inhibitor-1 is an independent diagnostic marker as well as severity predictor of hepatic veno-occlusive disease after allogeneic bone marrow transplantation in adults conditioned with busulphan and cyclophosphamide. Br J Haematol. 2002;118:1087-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 67. | Pihusch M, Wegner H, Goehring P, Salat C, Pihusch V, Hiller E, Andreesen R, Kolb HJ, Holler E, Pihusch R. Diagnosis of hepatic veno-occlusive disease by plasminogen activator inhibitor-1 plasma antigen levels: a prospective analysis in 350 allogeneic hematopoietic stem cell recipients. Transplantation. 2005;80:1376-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Sartori MT, Cesaro S, Peruzzo M, Messina C, Saggiorato G, Calore E, Pillon M, Varotto S, Spiezia L, Cella G. Contribution of fibrinolytic tests to the differential diagnosis of veno-occlusive disease complicating pediatric hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2012;58:791-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Piccin A, Sartori MT, Bisogno G, Van Schilfgaarde M, Saggiorato G, Pierro AMD, Corvetta D, Marcheselli L, Mega A, Gastl G, Cesaro S. New insights into sinusoidal obstruction syndrome. Intern Med J. 2017;47:1173-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 70. | Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, Vogelsang GB, Sensenbrenner LL, Santos GW, Saral R. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 667] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 71. | Kernan NA, Grupp S, Smith AR, Arai S, Triplett B, Antin JH, Lehmann L, Shore T, Ho VT, Bunin N, Iacobelli M, Liang W, Hume R, Tappe W, Soiffer R, Richardson P. Final results from a defibrotide treatment-IND study for patients with hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Br J Haematol. 2018;181:816-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 72. | Myers KC, Dandoy C, El-Bietar J, Davies SM, Jodele S. Veno-occlusive disease of the liver in the absence of elevation in bilirubin in pediatric patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:379-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 73. | Kaya N, Erbey F, Atay D, Akçay A, Bozkurt C, Ozturk G. The Diagnostic Value of Hepatic Arterial Velocity in Venoocclusive Disease After Pediatric Hematopoietic Stem Cell Transplantation. J Pediatr Hematol Oncol. 2017;39:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | Kaya N. Grayscale and Spectral Doppler Ultrasound in the Diagnosis of Hepatic Veno-occlusive Disease/Sinusoidal Obstruction Syndrome After Hematopoietic Stem Cell Transplantation in Children. J Pediatr Hematol Oncol. 2021;43:e1105-e1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Wang H, Zheng P, Wang X, Sang L. Effect of Q-Box size on liver stiffness measurement by two-dimensional shear wave elastography. J Clin Ultrasound. 2021;49:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 76. | Lorée H, Bastard C, Miette V, Sandrin L. Vibration-Guided Transient Elastography: A Novel Fibroscan® Examination with Improved Guidance for Liver Stiffness Measurement. Ultrasound Med Biol. 2020;46:2193-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 77. | Zayed N, Darweesh SK, Mousa S, Atef M, Ramzy E, Yosry A. Liver stiffness measurement by acoustic radiation forced impulse and transient elastography in patients with intrahepatic cholestasis. Eur J Gastroenterol Hepatol. 2019;31:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Li JH, Zhu N, Min YB, Shi XZ, Duan YY, Yang YL. Ultrasonic assessment of liver stiffness and carotid artery elasticity in patients with chronic viral hepatitis. BMC Gastroenterol. 2018;18:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 79. | Fontanilla T, Hernando CG, Claros JC, Bautista G, Minaya J, Del Carmen Vega M, Piazza A, Méndez S, Rodriguez C, Arangüena RP. Acoustic radiation force impulse elastography and contrast-enhanced sonography of sinusoidal obstructive syndrome (Veno-occlusive Disease): preliminary results. J Ultrasound Med. 2011;30:1593-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | Zaleska-Dorobisz U, Pawluś A, Kucharska M, Inglot M. [SWE elastography in assessment of liver fibrosis]. Postepy Hig Med Dosw (Online). 2015;69:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 81. | Yan Y, Xing X, Lu Q, Wang X, Luo X, Yang L. Assessment of biopsy proven liver fibrosis by two-dimensional shear wave elastography in patients with primary biliary cholangitis. Dig Liver Dis. 2020;52:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 82. | Boeken T, Lucidarme O, Mbarki E, Scatton O, Savier E, Wagner M. Association of shear-wave elastography with clinical outcomes post-liver transplantation. Clin Res Hepatol Gastroenterol. 2021;45:101554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | Alawaji G, Alhothali W, Albakr A, Amer A, Al-Habib A, Ajlan A. Shear wave elastography for intracranial epidermoid tumors. Clin Neurol Neurosurg. 2021;207:106531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 84. | Reddivalla N, Robinson AL, Reid KJ, Radhi MA, Dalal J, Opfer EK, Chan SS. Using liver elastography to diagnose sinusoidal obstruction syndrome in pediatric patients undergoing hematopoetic stem cell transplant. Bone Marrow Transplant. 2020;55:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 85. | Ravaioli F, Marconi G, Martinelli G, Dajti E, Sartor C, Abbenante MC, Alemanni LV, Nanni J, Rossini B, Parisi S, Colecchia L, Cristiano G, Marasco G, Vestito A, Paolini S, Bonifazi F, Curti A, Festi D, Cavo M, Colecchia A, Papayannidis C. Assessment of liver stiffness measurement and ultrasound findings change during inotuzumab ozogamicin cycles for relapsed or refractory acute lymphoblastic leukemia. Cancer Med. 2022;11:618-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 86. | Colecchia A, Ravaioli F, Sessa M, Alemanni VL, Dajti E, Marasco G, Vestito A, Zagari RM, Barbato F, Arpinati M, Cavo M, Festi D, Bonifazi F. Liver Stiffness Measurement Allows Early Diagnosis of Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome in Adult Patients Who Undergo Hematopoietic Stem Cell Transplantation: Results from a Monocentric Prospective Study. Biol Blood Marrow Transplant. 2019;25:995-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 87. | Colecchia A, Marasco G, Ravaioli F, Kleinschmidt K, Masetti R, Prete A, Pession A, Festi D. Usefulness of liver stiffness measurement in predicting hepatic veno-occlusive disease development in patients who undergo HSCT. Bone Marrow Transplant. 2017;52:494-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 88. | Muñoz-Codoceo C, Amo M, Martín A, Martín-Arriscado Arroba C, Cuevas Del Campo L, Manzano ML, Muñoz R, Castellano G, Fernández I. Diagnostic accuracy of liver and spleen stiffness measured by fibroscan® in the prediction of esophageal varices in HCV-related cirrhosis patients treated with oral antivirals. Gastroenterol Hepatol. 2021;44:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Silva CF, Nardelli MJ, Barbosa FA, Galizzi HO, Cal TCMF, Ferrari TCA, Faria LC, Couto CA. Liver stiffness is able to differentiate hepatosplenic Schistosomiasis mansoni from liver cirrhosis and spleen stiffness may be a predictor of variceal bleeding in hepatosplenic schistosomiasis. Trans R Soc Trop Med Hyg. 2022;116:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 90. | Qi X, Berzigotti A, Cardenas A, Sarin SK. Emerging non-invasive approaches for diagnosis and monitoring of portal hypertension. Lancet Gastroenterol Hepatol. 2018;3:708-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 91. | Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017;38:e16-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 575] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 92. | Özkan SG, Pata C, Şekuri A, Çınar Y, Özkan HA. Transient elastography of liver: Could it be a guide for diagnosis and management strategy in hepatic veno-occlusive disease (sinusoidal obstruction syndrome)? Transfus Apher Sci. 2022;61:103370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Debureaux PE, Bourrier P, Rautou PE, Zagdanski AM, De Boutiny M, Pagliuca S, Sutra Del Galy A, Robin M, Peffault de Latour R, Plessier A, Sicre de Fontbrune F, Xhaard A, de Lima Prata PH, Valla D, Socié G, Michonneau D. Elastography improves accuracy of early hepato-biliary complications diagnosis after allogeneic stem cell transplantation. Haematologica. 2021;106:2374-2383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 94. | Zama D, Bossù G, Ravaioli F, Masetti R, Prete A, Festi D, Pession A. Longitudinal evaluation of liver stiffness in three pediatric patients with veno-occlusive disease. Pediatr Transplant. 2019;23:e13456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |