Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8761
Peer-review started: April 5, 2022
First decision: June 16, 2022
Revised: July 5, 2022
Accepted: July 18, 2022
Article in press: July 18, 2022
Published online: August 26, 2022
Processing time: 132 Days and 1.8 Hours
Polyether ether ketone (PEEK) is a high-performance medical polymer, and there are some clinical cases of PEEK prosthesis implantation. However, application of 3D-printed injection-molded PEEK lunate prosthesis for treatment of stage III Kienböck’s disease has not been reported. This study’s purpose was to analyze the clinical efficacy of 3D-printed injection-molded PEEK lunate prosthesis in the treatment of stage III Kienböck’s disease and thus provide a good therapeutic choice for Kienböck’s disease.
We report a patient with stage III Kienböck’s disease. With the healthy lunate bone as reference, 3D lunate reconstruction was performed using a mirroring technique. A PEEK lunate prosthesis was prepared by 3D printing and injection molding, and then it was inserted into the original anatomical position after removing the necrotic lunate bone. Wrist pain and function, anatomical suitability of the lunate prosthesis, and complications were evaluated and analyzed postoperatively. At the last visit (one year after surgery), the range of motion, grasp force, visual analog scale score and Cooney score of the affected wrist were significantly improved, and postoperative X-ray examination indicated that the lunate prosthesis had good anatomical suitability for adjacent bony structures.
The 3D-printed injection-molded PEEK lunate prosthesis demonstrated definite efficacy in treating stage III Kienböck’s disease.
Core Tip: Polyether ether ketone (PEEK) has been widely used in the preparation of human bone tissue scaffolds and joint prostheses. This study evaluates the clinical efficacy of 3D-printed injection-molded PEEK lunate prosthesis designed by our team in the treatment of stage III Kienböck’s disease. Compared with other replacement methods, this technique can better reconstruct the anatomical structure of the wrist, alleviate pain, and restore the activity function and grip strength of the patient. Imaging examination confirmed that the lunar bone cement prosthesis has good anatomical adaptability. 3D-printed injection-molded PEEK lunate prosthesis is a safe and effective artificial replacement technique.
- Citation: Yuan CS, Tang Y, Xie HQ, Liang TT, Li HT, Tang KL. Application of 3 dimension-printed injection-molded polyether ether ketone lunate prosthesis in the treatment of stage III Kienböck’s disease: A case report. World J Clin Cases 2022; 10(24): 8761-8767
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8761.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8761
Kienböck’s disease is one of the main causes for wrist pain and movement limitation[1]. Stage III Kienböck’s disease is clinically characterized by lunate rupture, necrosis, atrophy and flattening[2], and its treatment aims to conserve normal structure, restore movement function, and relieve wrist symptoms. There are no uniform surgical procedures for Kienböck’s disease, and revascularization, biomechanical therapy, lunate resection and substitute implantation are all commonly used in clinical practice, but they have varying efficacy[3].
Polyether ether ketone (PEEK) is a special thermoplastic polymer, which has the advantages of high strength, high stiffness, corrosion resistance, and hydrolysis resistance. Compared with traditional metal materials, the elastic modulus of PEEK is closer to that of human cortical bone, and it has good plasticity. In the 1990s, as a substitute for metal implants, PEEK was increasingly used in the field of orthopedics. PEEK has good biocompatibility and biological inertia. It has no sensitization. Genotoxicity test results show that PEEK does not cause any chromosome aberration. It can maintain stable physical and chemical properties under the stimulation of various chemical and physical conditions. It is an ideal material for prosthesis fabrication[4-6], thus, it is more suitable for preparing human bone tissue scaffolds and joint prostheses. A hotspot issue in the recent years has been how to fabricate the implants with a precise anatomical structure by combining 3D printing technology, and some clinical cases of PEEK prosthesis implantation have been reported[7]. However, none of these has involved the application of 3D-printed injection-molded PEEK lunate prosthesis in the treatment of stage III Kienböck’s disease.
Based on the anatomical and biomechanical characteristics of the wrist and combining the physical and chemical properties of PEEK materials, we prepared a PEEK lunate prosthesis by 3D printing and injection molding to substitute the necrotic lunate bone. This is the first case report of a 3D-printed injection-molded PEEK lunate prosthesis in the treatment of stage III Kienböck’s disease, and aimed to present a new therapy and the clinical outcome of the disease.
A 42-yr-old female patient was admitted to hospital for left wrist pain with movement limitation for 5 mo.
Before surgery, her symptoms lasted 5 mo. The pain was persistent, became aggravated during loading, and was relieved after rest. The pain was associated with limited mobility of the wrist joint.
The patient had no previous medical history.
There is no history of genetic diseases and infectious diseases in the family.
The left wrist was slightly swollen, with obvious tenderness at the palmarar lunate, limited range of motion of wrist joint, decreased grip strength (Table 1), and normal peripheral blood flow and skin sensation.
| Affected wrist | Healthy wrist | ||
| Before operation | At last visit | ||
| Dorsal extension (°) | 0-25/32 | 0-50/55 | 0-50/59 |
| Palmar flexion (°) | 0-20/28 | 0-45/52 | 0-45/53 |
| Ulnar deviation (°) | 0-10/15 | 0-25/33 | 0-25/34 |
| Radical deviation (°) | 0-5/9 | 0-15/17 | 0-10/18 |
| GF (kg) | 15-20 | 30/38 | 35/40 |
The patient's preoperative laboratory examination was unremarkable.
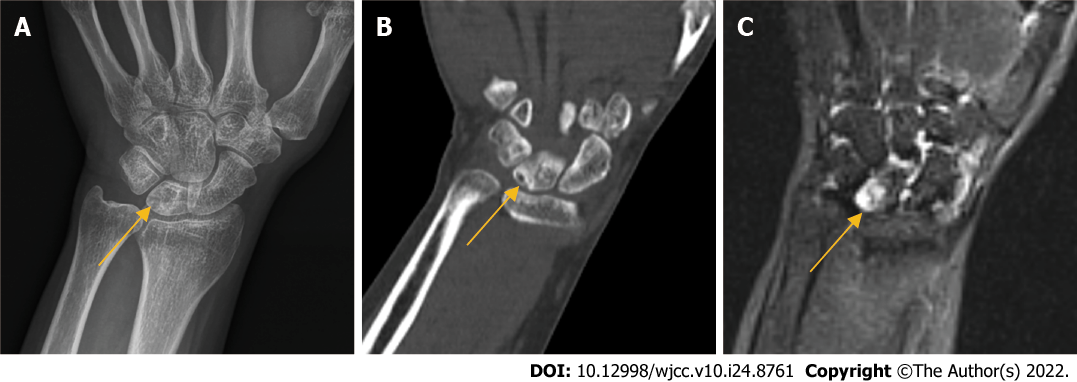
X-ray, computed tomography (CT), and magnetic resonance imaging were performed, and the imaging findings suggested lunate necrosis of the left wrist joint (Figure 1).
The patient was diagnosed with stage III Kienböck’s disease according to the Lichtman staging.
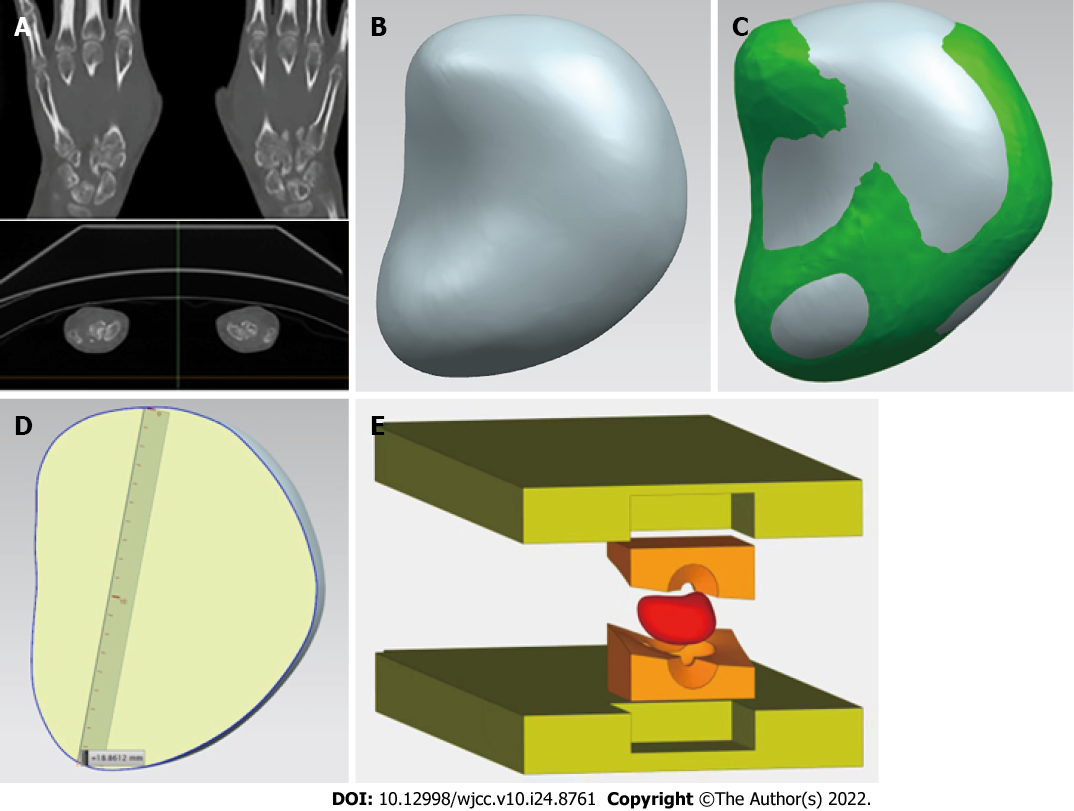
The precise 3D raw data of healthy lunate bone were acquired from the patient by CT examination, and then a 3D lunate model was constructed with reverse engineering methodology. Thereafter, a cutting plane of a 3D lunate model was created according to the individualized anatomical characteristics of lunate bone, and used to cut the 3D lunate model and obtain a 3D model of lunate prosthesis using a mirroring technique. Based on the 3D model of lunate prosthesis, a PEEK lunate prosthesis mold was prepared for injection molding by 3D printing. The mold, a high-strength prosthetic structure and a PEEK lunate prosthesis with a high surface smoothness (after good finishing with specific technology), a complete overall shape and dense material texture were fabricated by injection molding (Figure 2).
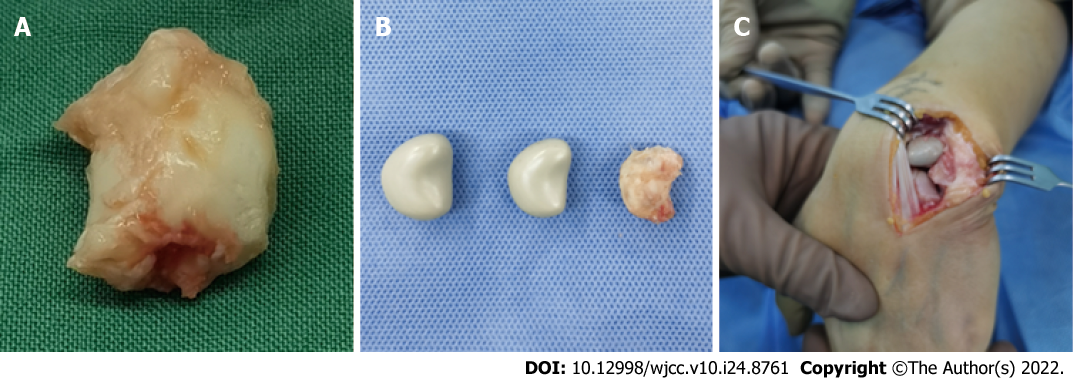
A longitudinal incision was made on the dorsal wrist, and the skin, subcutaneous tissue and dorsal extensor retinaculum were dissected layer by layer to expose the articular capsule of the lunate bone, and the perilunate ligaments were cut off to expose and completely remove the necrotic lunate bone (Figure 3A). Subsequently, the 3D-printed PEEK lunate prosthesis was inserted into the original anatomical position of the lunate bone (Figure 3B and C). After dorsal extension and palmar flexion reached a normal level through the repeated radioulnar deviation of the wrist and no prosthesis extrusion was observed, the lunate prosthesis was found with good matching by C-arm X-ray examination. The dorsal articular capsule and dorsal extensor retinaculum of the wrist were sutured layer by layer. After surgery, the wrist was fixed in the functional position using plaster slab for 4 wk.
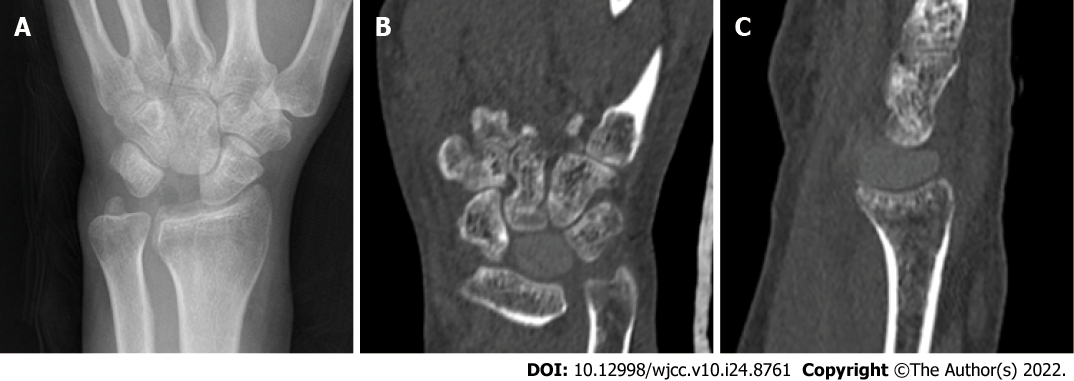
One year after surgery at the last visit, the range of motion and grasp force of the affected wrist were nearly normal, but VAS score and Cooney score were both improved in both resting and loading states (Tables 1 and 2). The carpal height ratio and radial scaphoid angle were nearly normal, the imaging examination confirmed that the PEEK lunate prosthesis had good anatomical suitability and no rupture, extrusion and detachment to other carpal bones (Figure 4).
| VAS score | Cooney score | ||
| Resting state | Loading state | ||
| Before operation | 6 | 7 | 50 |
| At last visit | 0 | 1 | 85 |
There are many therapies for Kienböck’s disease that have an uniform goal of conserving normal structure, restoring movement function, and relieving the clinical wrist symptoms[8]. Four surgical procedures are usually applied: Revascularization, biomechanical therapy, lunate resection, and substitute implantation[3]. Lunate resection often induces downward shift and disordered arrangement of the carpal bone, as well as causing wrist pain and dysfunction. Therefore, choosing a suitable substitute is important for reconstructing radiocarpal joint and restoring wrist function[9], thus lunate resection + substitute implantation is a good therapy for Kienböck’s disease. Many substitutes are available to be implanted after lunate resection, for example, tendon mass, capital bone shift, metal ball, pisiform bone shift, titanium alloy prosthesis, silicone prosthesis, and bone cement implant[10]. Tendon mass and arcuated transparent cartilage are soft in the texture, and have poor long-term efficacy as wrist instability will easily happen after their implantation[11]. Mariconda et al[12] reported that tendon flap could be used for sufficient interposition in the cavity. After surgery, however, the low-strength tendon is incapable to transmit the stress, and wrist synovitis and radial–scaphoid joint changes easily occur. The substitute implantation of capital and pisiform bone shift has complicated surgery and can damage the structure and partial function of the wrist. The hard metal ball causes abrasion against the adjacent carpal bones; the titanium alloy prosthesis is expensive and requires premature customization, and it is difficult to adjust the size of such prosthesis and achieve the simulation[13]; and the bone cement prosthesis presents an unavoidable friction against the adjacent bones, which may lead to the abrasion and injury of the articular facet. Viljakka et al[14] reported that the silicone prosthesis had high hardness but poor tissue compatibility; thus, prosthesis extrusion and silicone synovitis can happen after implantation, followed by prosthesis damage and abrasion, further aggravating injury of adjacent joints. Therefore, the silicone prosthesis cannot meet the long-term functional demand of patients. The above-mentioned surgical procedures all have some limitations and vary in their clinical efficacy.
Söhling et al[15] reported that implants made of biomaterials have become an integral part of modern medicine. Therefore, the ideal implant is reactive, but does not cause foreign body reaction. Elucidating the early immune response in the foreign body reaction is implant-material specific. Tanca et al[16] also reported application of 2D-fluorescence difference gel electrophoresis (2D-DIGE) to formalin-fixed diseased tissue samples from hospital repositories, and showed that 2D-DIGE can support biomarker discovery and validation studies on large sample cohorts. The associated patient information can considerably boost studies with limited sample availability or those involving long-distance exchange of samples. Kyriakides et al[17] reported the foreign body reaction to synthetic polymer biomaterials and the role of adaptive immunity, and suggested the existence of crosstalk between innate and adaptive immune cells that depends on the nature of the implants.
PEEK is a high-performance polymer that has been used for the manufacturing of dental implants. PEEK is a material with high biocompatibility, good mechanical properties, high temperature resistance, chemical stability, polishability, good wear resistance, low plaque affinity and high bond strength with veneering composites and luting cements. Compared to rigid framework materials such as zirconium oxide and metal alloys, PEEK has a low modulus of elasticity of 4 GPa and is as elastic as bone, providing a cushioning effect and reduction of stresses transferred[4,5]. So, it is more suitable for fabricating human bone tissue scaffolds and artificial joints. With such advantages as a short processing cycle, less material consumption and complex shaping, 3D printing technology better meets the clinical demand of individualized prosthesis customization. In addition, the prostheses may be fabricated with varying rigidity and elasticity by temperature control during 3D printing[4]; thus, a prosthesis can be designed which has a shape and size normally coincident to the original lunate bone and complies with the physiological characteristics of wrist. As a result, 3D-printed PEEK lunate prosthesis is a good choice. As the lunate bone has an irregular shape, the preoperative prosthesis design is important and focuses on the width, height and concave of lunate bone to keep the original size as much as possible. A prosthesis that is too big bears excessive pressure and thus become ruptured and extruded, while one that is too small size cannot stabilize the joint.
PEEK has outstanding tribological properties, excellent sliding and fretting wear resistance, especially high wear resistance and low friction coefficient at 250°C. It is easy for extrusion and injection molding, with excellent processing performance and high molding efficiency. As a medical implant, its elastic modulus is similar to that of bone, with good biocompatibility and transparent rays. The material is resistant to simulated in vivo degradation, including damage caused by lipid exposure. Although PEEK has these excellent characteristics[4], it still has some problems as a bone implant. One of them is that there is still a gap between PEEK and human bone in terms of modulus and mechanical strength. In terms of mechanical strength, PEEK still cannot meet the needs of human bones. Another problem with PEEK as a medical implant is that it is biologically inert[5]. Its low reactivity and integration with surrounding tissues will affect the successful transplantation of PEEK in vivo. In the next step of the treatment of lunar osteonecrosis, we plan to modify PEEK to better increase its mechanical strength and better match its elastic modulus.
In summary, the 3D-printed injection-molded PEEK lunate prosthesis demonstrates definite efficacy in treating stage III Kienböck’s disease, and it is an effective therapy worthy of being popularized. Although such prosthesis has good clinical efficacy, the subsequent large-sized long-term clinical case observation is needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Eccher A, Italy; Wang Y, China S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Chou J, Bacle G, Ek ETH, Tham SKY. Fixation of the Fractured Lunate in Kienböck Disease. J Hand Surg Am. 2019;44:67.e1-67.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Matsumoto T, Kakinoki R, Ikeguchi R, Ohta S, Akagi M, Matsuda S. Vascularized Bone Graft to the Lunate Combined With Temporary Scaphocapitate Fixation for Treatment of Stage III Kienböck Disease: A Report of the Results, a Minimum of 2 Years After Surgery. J Hand Surg Am. 2018;43:773.e1-773.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Li S, Sun C, Zhou X, Shi J, Han T, Yan H. Treatment of Intraosseous Ganglion Cyst of the Lunate: A Systematic Review. Ann Plast Surg. 2019;82:577-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Zhang J, Tian W, Chen J, Yu J, Zhang J. The application of polyetheretherketone (PEEK) implants in cranioplasty. Brain Res Bull. 2019;153:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Souza JCM, Pinho SS, Braz MP, Silva FS, Henriques B. Carbon fiber-reinforced PEEK in implant dentistry: A scoping review on the finite element method. Comput Methods Biomech Biomed Engin. 2021;24:1355-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Papathanasiou I, Kamposiora P, Papavasiliou G, Ferrari M. The use of PEEK in digital prosthodontics: A narrative review. BMC Oral Health. 2020;20:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 7. | Wang L, Huang L, Li X, Zhong D, Li D, Cao T, Yang S, Yan X, Zhao J, He J, Cao Y, Wang L. Three-Dimensional Printing PEEK Implant: A Novel Choice for the Reconstruction of Chest Wall Defect. Ann Thorac Surg. 2019;107:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Park JH, Jang WY, Kwak DH, Park JW. Lunate morphology as a risk factor of idiopathic ulnar impaction syndrome. Bone Joint J. 2017;99-B:1508-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Havulinna J, Jokihaara J, Paavilainen P, Leppänen OV. Keyhole Revascularization for Treatment of Coronal Plane Fracture of the Lunate in Kienböck Disease. J Hand Surg Am. 2016;41:e441-e445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Fontaine C. Kienböck's disease. Chir Main. 2015;34:4-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Kennedy C, Abrams R. In Brief: The Lichtman Classification for Kienböck Disease. Clin Orthop Relat Res. 2019;477:1516-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Mariconda M, Soscia E, Sirignano C, Smeraglia F, Soldati A, Balato G. Long-term clinical results and MRI changes after tendon ball arthroplasty for advanced Kienbock's disease. J Hand Surg Eur Vol. 2013;38:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Dewan AK, Chhabra AB, Khanna AJ, Anderson MW, Brunton LM. Magnetic resonance imaging of the hand and wrist: techniques and spectrum of disease: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95:e68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Viljakka T, Tallroth K, Vastamäki M. Long-term outcome (22-36 years) of silicone lunate arthroplasty for Kienbock's disease. J Hand Surg Eur Vol. 2014;39:405-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Söhling N, Ondreka M, Kontradowitz K, Reichel T, Marzi I, Henrich D. Early Immune Response in Foreign Body Reaction Is Implant/Material Specific. Materials (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Tanca A, Pisanu S, Biosa G, Pagnozzi D, Antuofermo E, Burrai GP, Canzonieri V, Cossu-Rocca P, De Re V, Eccher A, Fanciulli G, Rocca S, Uzzau S, Addis MF. Application of 2D-DIGE to formalin-fixed diseased tissue samples from hospital repositories: results from four case studies. Proteomics Clin Appl. 2013;7:252-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Kyriakides TR, Kim HJ, Zheng C, Harkins L, Tao W, Deschenes E. Foreign body response to synthetic polymer biomaterials and the role of adaptive immunity. Biomed Mater. 2022;17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |