Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8755
Peer-review started: March 30, 2022
First decision: June 16, 2022
Revised: June 29, 2022
Accepted: July 20, 2022
Article in press: July 20, 2022
Published online: August 26, 2022
Processing time: 138 Days and 6.9 Hours
Cardiac arrhythmias, including bradyarrhythmias, have been described as manifestations of coronavirus disease 2019 (COVID-19). Herein, we present a case of junctional bradycardia secondary to possible sinus node dysfunction in a patient with COVID-19.
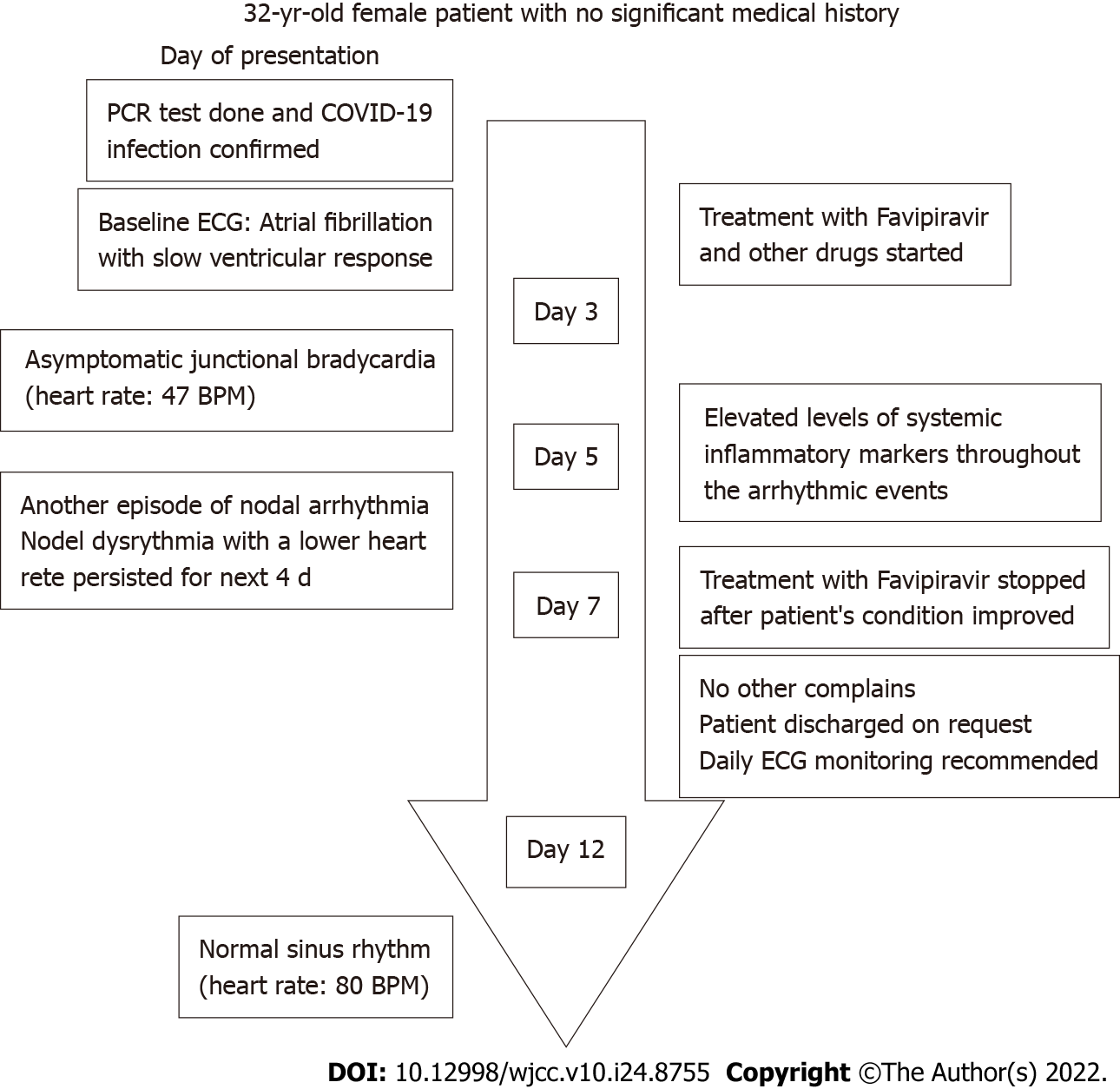
The patient was a 32-year-old woman with no significant medical history. On the third day of hospitalization, she developed junctional bradycardia while being hemodynamically stable. The episodes of nodal dysrhythmia with a low heart rate persisted for the next few days and were associated with elevated levels of systemic inflammatory markers. The patient received antiviral and anti-inflammatory treatments for the viral infection but no antiarrhythmic medications. She had a normal sinus rhythm on day 12.
Cardiac rhythm monitoring, focusing on the association between cardiac arrhythmias and the systemic inflammatory response, is important in COVID-19 patients.
Core Tip: A variety of cardiovascular manifestations of COVID-19 have been described. Among these, bradyarrhythmias should be considered one of the main complications and clinical features of COVID-19. Herein, we present a case of junctional bradycardia in a patient with COVID-19. Bradyarrhythmias in COVID-19 patients has been linked to increased systemic inflammatory response and the subsequent dysfunctioning of the sino-atrial node. The inflammatory biomarker and cardiac rhythm monitoring should be considered in patients with COVID-19 during different stages of care.
- Citation: Aedh AI. Junctional bradycardia in a patient with COVID-19: A case report. World J Clin Cases 2022; 10(24): 8755-8760
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8755.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8755
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and was declared a pandemic in March 2020 by the World Health Organization (WHO)[1]. Patients with COVID-19 have had various cardiovascular consequences throughout the pandemic, including acute cardiac damage, myocardial infarction, cardiogenic shock, acute congestive heart failure, and cardiac arrhythmias[2].
Bradyarrhythmia has been identified as a cardiac manifestation of COVID-19 in several studies. In a retrospective case series, Amaratunga et al[3] reported sinus bradycardia in four patients. These episodes lasted for 14 d. Transient sinus bradycardia may be a possible manifestation of COVID-19, and its development may be indicative of the onset of a severe cytokine storm. In another case series, severe bradyarrhythmias were reported in seven patients with COVID-19. The patients had or developed severe bradyarrhythmias during hospitalization and required pacing assistance. None of the patients had pre-existing cardiac diseases. Inflammatory marker levels were significantly elevated in all patients[4]. In another report, new-onset sinus bradycardia resulting from sinus node dysfunction in two elderly patients with COVID-19 was described by Peigh et al[5]Both patients had persistent sinus bradycardia 2 wk after the onset of sinus node dysfunction. In a recent report from the United Arab Emirates, electrocardiogram (ECG) of a 36-year-old patient with COVID-19 revealed a junctional rhythm with atrial escape capture beats. A Holter study confirmed sinus node dysfunction with frequent pauses of the sinus node block alternating with sinus node arrest[6]. In a case series from India, bradyarrhythmias were reported in seven patients with COVID-19. Complete heart block was noted in five patients. Additionally, sick sinus syndrome was reported in two patients. Four of these patients had ventricular escape beats, and the remaining three had a junctional escape rhythm[7]. Herein, we describe a case of a patient with COVID-19 who developed junctional bradycardia.
A patient presented with chief complaints of headache, sore throat, dry cough, and fever for 3 d.
A 32-year old female presented with complaints of headache, sore throat, dry cough, and fever for 3 d. On presentation, the patient also complained of bone ache, fatigue, and malaise.
The patient had no significant past medical history.
There was no family history of heart rhythm disorders.
The patient's physical examination revealed typical vital signs except for a body temperature of 38.9°C. The patient was hemodynamically stable and was not receiving any atrioventricular (AV) node blocking medications.
Reverse transcription polymerase chain reaction for SARS-CoV-2 using a nasopharyngeal swab confirmed the presence of COVID-19. Laboratory findings revealed lymphopenia with a lymphocyte count of 0.88 × 103/μL (reference range 1.0–4.0 × 103/μL), increased C-reactive protein (CRP) levels (95.79 mg/L; reference range < 10 mg/L), high serum ferritin levels (171 μg/L; reference range 12–150 μg/L), and increased D-dimer levels (0.54 mg/L; reference range 0–0.5 mg/L). The patient’s alanine aminotransferase (115.1 U/L; reference range 0–33 U/L) and aspartate aminotransferase (54.8 U/L; reference range 0–32 U/L) levels were also elevated. There were no significant electrolyte abnormalities.
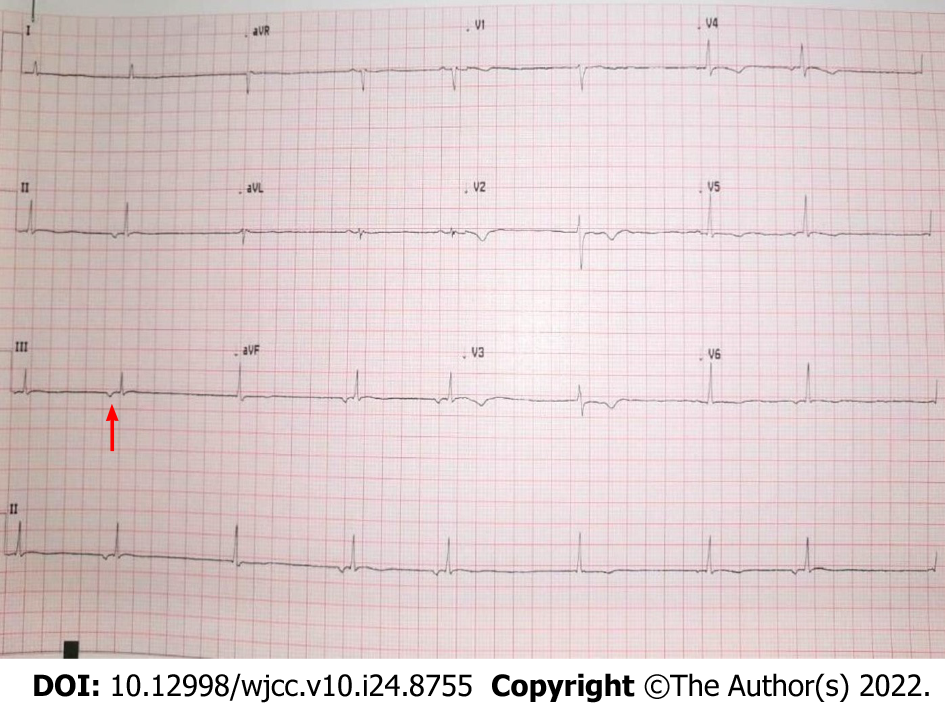
At baseline, the patient's ECG revealed atrial fibrillation with a slow ventricular response accompanied by moderately abnormal T waves. Three days later, the patient developed asymptomatic junctional bradycardia (Figure 1) with a heart rate of 47 beats per minute (BPM) while being awake. Her heart rate further dropped to 38 BPM during sleep. Moderate T wave abnormalities were observed. On day 5 after hospitalization, the ECG again revealed nodal arrhythmia. However, the patient was still asymptomatic and had good mobility and agility. Cardiac enzyme levels were normal, with a creatine kinase level of 48 U/L (reference range 39–308 U/L) and a creatine kinase-MB level of 12.3 U/L (reference range 0–25 U/L). Nodal dysrhythmia with bradycardia persisted for the next 4 days. The patient’s CRP (216.15 mg/L; reference range < 10 mg/L) and D-dimer (0.84 mg/L; reference range 0–0.5 mg/L) levels remained elevated during these episodesand became normal on day 12. Her CRP (4.38 mg/L; reference range < 10 mg/L) and D-dimer (0.36 mg/L; reference range 0–0.5 mg/L) levels coincided with normal sinus rhythm.
The patient’s chest computed tomography scan was normal.
The presence of clinical symptoms, such as new-onset fever, cough, and fatigue, along with these laboratory findings, are now known manifestations of COVID-19. The patient’s ECG revealed junctional bradycardia.
After confirmation of the diagnosis, the patient was started on the antiviral drug favipiravir at a dose of 1600 mg twice a day for 1 d, followed by 600 mg twice a day. She was also administered 8 mg of dexamethasone intravenously daily for its anti-inflammatory effects and 40 mg of subcutaneous enoxaparin daily as an anticoagulant. Additionally, the patient received intravenous paracetamol for fever, omeprazole as prophylaxis for gastrointestinal bleeding, and ondansetron for nausea (two doses).
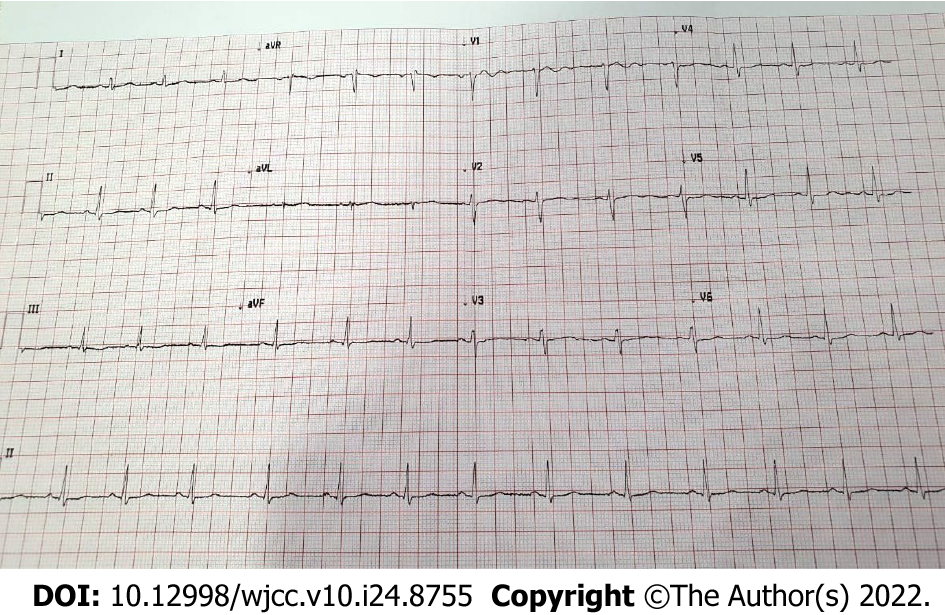
The patient’s symptoms significantly improved over the course of treatment. Besides the episodes of bradycardia, she was doing fine. On day 7, favipiravir was stopped following improvement in patient’s condition. On day 9, she was discharged upon her request with the recommendation of daily ECG monitoring. The patient’s heart rate gradually normalized and ECG on day 12 revealed a normal sinus rhythm (Figure 2). Figure 3 depicted the chronological flowchart of the patient’s treatment course.
An array of cardiovascular complications of COVID-19, including cardiac arrhythmias, has been previously reported. However, the mechanisms underlying cardiac involvement in COVID-19 remain poorly understood. Multiple mechanisms may contribute to the development of arrhythmias in these patients. COVID-19 may cause acute bradycardia events by the following mechanisms: direct infiltration of myocardial cells and the conduction system via angiotensin-converting enzyme-2 (ACE2) receptors, aggravation of pre-existing conduction diseases during acute illness, cardiac injury, hemodynamic instability, alteration in the intrinsic cardiac nervous system leading to autonomic dysfunction, a secondary effect of hypoxia caused by pulmonary injury, electrolyte abnormalities, and an enhanced systemic inflammatory response[7-10]. SARS-CoV-2 may also activate the ACE2 receptor by utilizing the spike protein to enter the host cell. This event leads to down-regulation of ACE2 receptors and has been associated with conduction disturbances subsequent to adverse myocardial remodeling[9,10].
The patient developed junctional bradycardia, a slow heart rhythm that originates from the AV node instead of the sinoatrial (SA) node. It can develop from pathology in the SA node causing it to fail as a pacemaker, or from pathology in the AV node, causing it to misfire. While the patient's cardiac biomarker levels were normal, she had elevated levels of systemic inflammatory markers throughout the arrhythmic events. High levels of inflammatory mediators, such as cytokines, may directly affect the SA node and the conduction system, leading to the development of bradycardia. This event might have been the cause of bradycardia in our patient. Notably, the patient developed junctional bradycardia on day 6 of the illness, which is within the cytokine storm’s onset timetable, similar to the case reported by Amaratunga et al[3].
The incidence of arrhythmias is often associated with disease severity[9,10]. However, in our case, the patient's condition was not severe and did not require admission to the intensive care unit. The patient did not receive any corrective treatment for bradycardia, as the episodes were asymptomatic and short-lived.
The patient had no pre-existing cardiac conduction abnormalities or structural heart disease, and she was not taking any AV node blocking medications that could have provoked bradycardia. The episodes of bradycardia in our patient may partly result from possible dysfunction of the sinus node by the direct or indirect effect of SARS-CoV-2 and systemic illness on the cardiovascular system.
Drugs used to treat COVID-19 may also contribute to conduction system disturbances[7-10]. Since dexamethasone was first assumed to be the cause of bradycardia, it was stopped on day 3. Dexamethasone was restarted after nodal dysrhythmia with bradycardia persisted from day 5 onwards, along with increased systemic inflammatory markers. In a recent case report from Qatar, favipiravir was attributed to asymptomatic severe sinus bradycardia with a heart rate of 30 BPM in a middle-aged woman. After the drug was stopped, the event resolved[11]. In addition, in the WHO database, bradycardia has been reported as a suspected adverse drug event to favipiravir[12]. However, in our case, the event of junctional bradycardia persisted even after the favipiravir was stopped on day 7 and the patient's heart rate gradually normalized as her condition improved on day 12 of presentation.
Patients with COVID-19 are increasingly presenting with bradyarrhythmias. The specific mechanisms fundamental to the development of bradyarrhythmias in these patients remain indistinct, and they may be multifactorial. The development of bradyarrhythmias can be considered a clinical feature of COVID-19, which could imply cardiac involvement. Therefore, cardiac rhythm monitoring should be performed during therapy, recovery, and discharge and cardiac and inflammatory biomarker monitoring should also be considered.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hassan M, United States; Moreno-Gómez-Toledano R, Spain; Peng XC, China S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | World Health Organization. Director-General's opening remarks at the media briefing on COVID-19. 2020 Mar 11 [cited 4 October 2021]. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-openingremarks-at-the-media-briefing-on-covid-19---11-march-2020. |
| 2. | Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38:1504-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 668] [Cited by in RCA: 648] [Article Influence: 129.6] [Reference Citation Analysis (0)] |
| 3. | Amaratunga EA, Corwin DS, Moran L, Snyder R. Bradycardia in Patients With COVID-19: A Calm Before the Storm? Cureus. 2020;12:e8599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Chinitz JS, Goyal R, Harding M, Veseli G, Gruberg L, Jadonath R, Maccaro P, Gandotra P, Ong L, Epstein LM. Bradyarrhythmias in patients with COVID-19: Marker of poor prognosis? Pacing Clin Electrophysiol. 2020;43:1199-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Peigh G, Leya MV, Baman JR, Cantey EP, Knight BP, Flaherty JD. Novel coronavirus 19 (COVID-19) associated sinus node dysfunction: a case series. Eur Heart J Case Rep. 2020;4:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Eid MM. COVID-19 patient with symptomatic bradycardia. Vis J Emerg Med. 2021;22:100920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Gupta MD, Qamar A, Mp G, Safal S, Batra V, Basia D, Mandal SK, Yusuf J, Mukhopadhyay S, Bansal A. Bradyarrhythmias in patients with COVID-19: A case series. Indian Pacing Electrophysiol J. 2020;20:211-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 9. | Dherange P, Lang J, Qian P, Oberfeld B, Sauer WH, Koplan B, Tedrow U. Arrhythmias and COVID-19: A Review. JACC Clin Electrophysiol. 2020;6:1193-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 10. | Babapoor-Farrokhran S, Rasekhi RT, Gill D, Babapoor S, Amanullah A. Arrhythmia in COVID-19. SN Compr Clin Med. 2020;2:1430-1435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 11. | Habib MB, Elshafei M, Rahhal A, Mohamed MFH. Severe sinus bradycardia associated with favipiravir in a COVID-19 patient. Clin Case Rep. 2021;9:e04566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Kaur RJ, Charan J, Dutta S, Sharma P, Bhardwaj P, Lugova H, Krishnapillai A, Islam S, Haque M, Misra S. Favipiravir Use in COVID-19: Analysis of Suspected Adverse Drug Events Reported in the WHO Database. Infect Drug Resist. 2020;13:4427-4438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |