Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8749
Peer-review started: March 25, 2022
First decision: June 27, 2022
Revised: July 2, 2022
Accepted: July 20, 2022
Article in press: July 20, 2022
Published online: August 26, 2022
Processing time: 143 Days and 18.7 Hours
The mitochondrial respiratory chain defects have become the most common cause of neurometabolic disorders in children and adults, which can occur at any time in life, often associated with neurological dysfunction, and lead to chronic disability and premature death. Approximately one-third of patients with mitochondrial disease have biochemical defects involving multiple respiratory chain complexes, suggesting defects in protein synthesis within the mitochondria. We here report a child with VARS2 gene mutations causing mitochondrial disease.
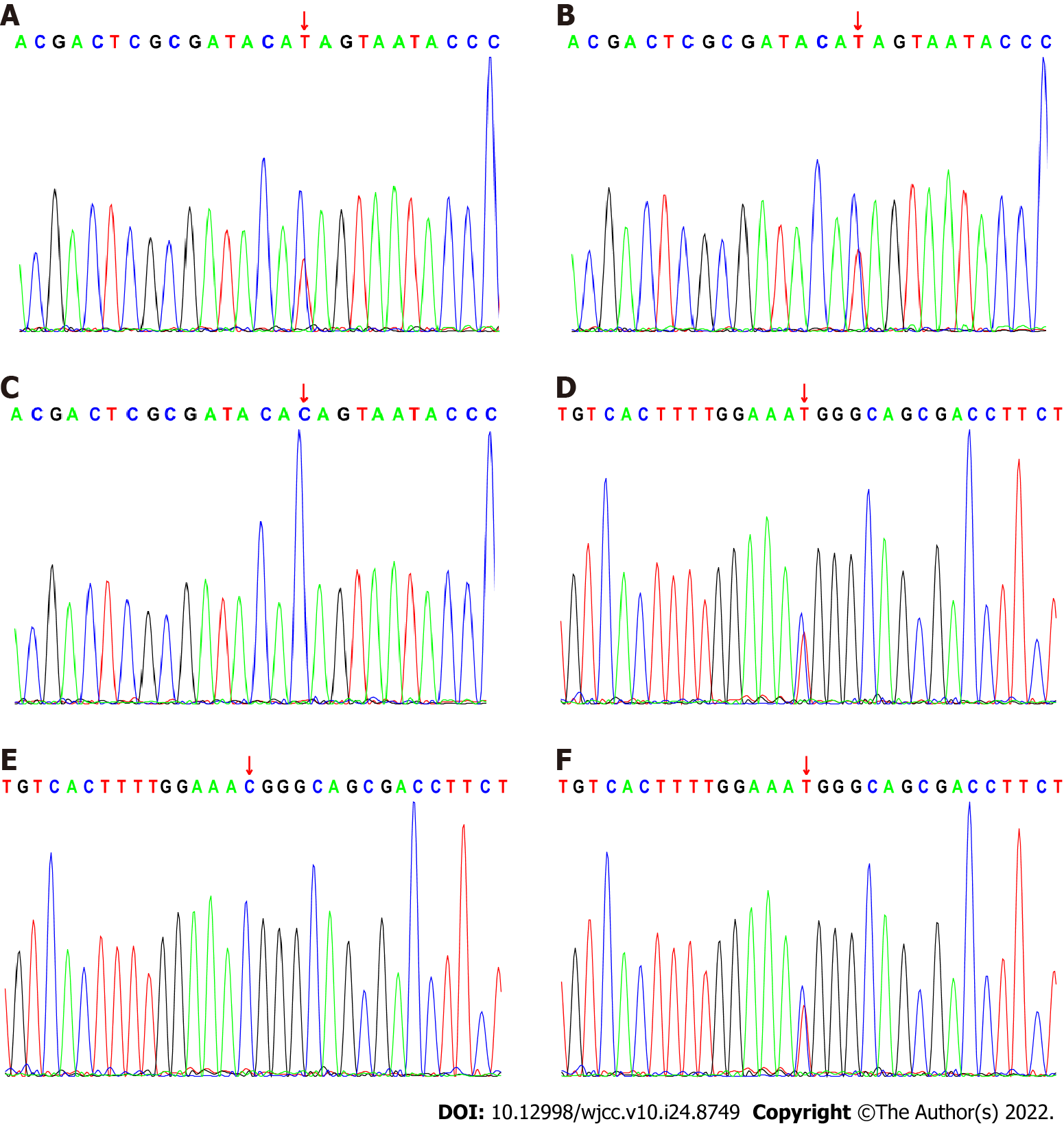
A girl, aged 3 years and 4 mo, had been unable to sit and crawl alone since birth, with obvious seizures and microcephaly. Brain magnetic resonance imaging showed symmetrical, flaky, long T1-weighted and low T2-weighted signals in the posterior part of the bilateral putamen with a high signal shadow. T2 fluid-attenuated inversion recovery imaging showed a slightly high signal and diffusion-weighted imaging showed an obvious high signal. Whole-exome gene sequencing revealed a compound heterozygous mutation in the VARS2 gene, c.1163(exon11)C>T and c.1940(exon20)C>T, which was derived from the parents. The child was diagnosed with combined oxidative phosphorylation deficiency type 20.
In this patient, mitochondrial disorders including Leigh syndrome and MELAS syndrome (mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes) were ruled out, and combined oxidative phosphorylation deficiency type 20 was diagnosed, expanding the phenotypic spectrum of the disease.
Core Tip: The clinical manifestation of the child was remarkable. Through the comprehensive analysis of symptoms, physical examination, biochemical examination and gene sequencing, the child was confirmed to have combined oxidative phosphorylation deficiency type 20, and the phenotypic spectrum of the disease was thus expanded.
- Citation: Wu XH, Lin SZ, Zhou YQ, Wang WQ, Li JY, Chen QD. VARS2 gene mutation leading to overall developmental delay in a child with epilepsy: A case report. World J Clin Cases 2022; 10(24): 8749-8754
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8749.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8749
Mitochondrial disorders include widely heterogeneous clinical syndromes, frequently presenting as encephalo- and/or cardiomyopathies, associated with a broad range of causative genes[1,2]. Their biochemical signature is the presence of defective activity in the mitochondrial respiratory chain complexes, resulting in faulty oxidative phosphorylation (OXPHOS), which can impair ATP production. Mutations in several genes associated with defects of mitochondrial protein synthesis, affecting either mitochondrial DNA (mtDNA) or nucleus-encoded genes, have been reported in a range of mitochondrial syndromes[3].
In recent years, an increasing number of mitochondrial diseases have been associated with mutations in the mitochondrial aminoacyl-tRNA synthetase, a key enzyme for mitochondrial protein synthesis[4]. Mutations in this enzyme have also been associated with diverse clinical presentations, usually inherited as early-onset autosomal recessive traits. The synthesis of mitochondrial proteins involves complex interactions between mtDNA-encoded RNA and nuclear DNA-encoded proteins, such as elongation factors, ribosomal proteins, and aminoacyl-tRNA synthetase. Among the 17 mitochondria-specific aminoacyl-tRNA synthases, VARS2 encodes the mitochondrial valine-tRNA synthase, a class I enzyme that catalyzes the attachment of a valine to its cognate tRNA molecule in a highly specific reaction[5].
Bruni et al[4] studied 13 patients with VARS2 gene mutations from different families, the clinical manifestations are severe cerebral myopathy and cardiomyopathy at birth. Features include hypotonia, psychomotor delay, seizures, feeding difficulties, cranial magnetic resonance imaging (MRI) abnormalities, and elevated lactate levels.
A girl aged 3 years and 4 mo, was admitted to Quanzhou Children’s Hospital on July 14, 2021 due to three convulsions in a day.
Her convulsions occurred on the day of admission and manifested as eyes staring rightward, mouth tilted to the right, clonic twitching of the right limbs, and loss of consciousness. Each convulsion lasted ~20 min and relieved after Anticonvulsant therapy with diazepam without fever or vomiting.
The child’s weight at birth was 3.3 kg and height was 50 cm. Intellectual and motor development was retarded after birth. She could track using her eyes and responded to voices at 3 mo. She could hold her head up steadily at 5 mo, turn over to the prone position, and could say “eee, eee, eee, eee”. At 7 mo, she could take the initiative and reach out to grab objects. At almost 1 year old she could turn over freely, but at 13 mo could still not sit alone, crawl or stand alone; she could move objects from one hand to the other, but could only make “babbling” vocalizations, and she underwent short-term rehabilitation training. By the age of 3 years and 4 mo, she still could not sit alone, crawl or stand alone; she could move objects from one hand to the other, but could only say “papa, mama, nana”.
The child is the third child and the second birth (the first led to abortion), and was delivered vaginally at 39 + 4 wk of gestation. The parents denied a history of intrauterine hypoxia and birth asphyxia, and the mother was 43 years old, healthy during pregnancy and denied taking medicine, with no history of poisoning. The parents and older brother were healthy, with no family history of epilepsy, and psychomotor retardation.
The child’s head circumference was 47 cm, height 95 cm, and weight 12 kg, with no special facial features, clear consciousness, good spirit, no café-au-lait spots or depigmentation on the entire skin, no neck resistance, clear breath sounds in both lungs, and no dry or wet rales. Her heart rate was 116 bpm, the heart rhythm was consistent, heart sounds were strong, no murmur was heard, the abdomen was soft, and no masses were palpable. The liver and spleen were unpalpable, and muscle strength of the limbs could not be determined. The muscle tone in the limbs was increased, bilateral ankle joint contractures, knee tendon reflexes were active and symmetrical, and the Brudzinski sign was negative bilaterally.
Auditory evoked potential (April 10, 2019) showed that the bilateral auditory brainstem-evoked potential results were normal and the critical bilateral V/I (ratio of the highest and lowest potential) peak ratios were 0.67 for the left ear and 0.68 for the right ear, and the normal bilateral threshold was 30 dBnHL.
Urine filter paper organic acid detection and analysis (April 10, 2019) showed no abnormalities.
TORCH examination (including toxoplasma, cytomegalovirus and herpes simplex virus) IgM was negative (April 12, 2019).
Blood tandem mass spectrometry analysis (April 28, 2019) showed that C5OH (0.84) and the ratio of ion mass to charge were still elevated, and that blood ammonia, liver function, blood gas, lactic acid, and urine organic acid had improved. Gene analysis was planned to exclude 3-methylcrotonyl-CoA hydroxylation enzyme deficiency and perhydroxylase/biotinidase deficiency.
Genetic testing with trio whole exome sequencing (April 29, 2019) showed the following: (1) Chromosomal aneuploidy; the chromosome composition was 46, XX, and the number of chromosomes was normal; and (2) Chromosome microdeletion or microduplication was negative. No clinically identifiable pathogenic copy number variations were found within the scope of this assay.
Thyroid function tests (August 19, 2019) showed that triiodothyronine (T3), thyroxine (T4), free T3, free T4 and thyroid-stimulating hormone were normal.
Color Doppler echocardiography (August 19, 2019) showed that the intracardiac structure and cardiac function were normal.
Blood ammonia (July 15, 2021) was normal at 21 μmol/L.
Eelectroencephalography (July 15, 2021) was abnormal. Each lead in the wake–sleep phase showed diffuse 2-4 Hz mixed spread activity, basically symmetrical in left and right, with no obvious dominant rhythm in the occipital region in the waking period and no typical peaks in the sleep period. The sleep spindle was affected, and the sleep cycle was not easy to identify.
Electromyography (July 31, 2019) showed peripheral nerve damage (motor fiber involvement) in both upper extremities, which was most severe in the left upper extremity.
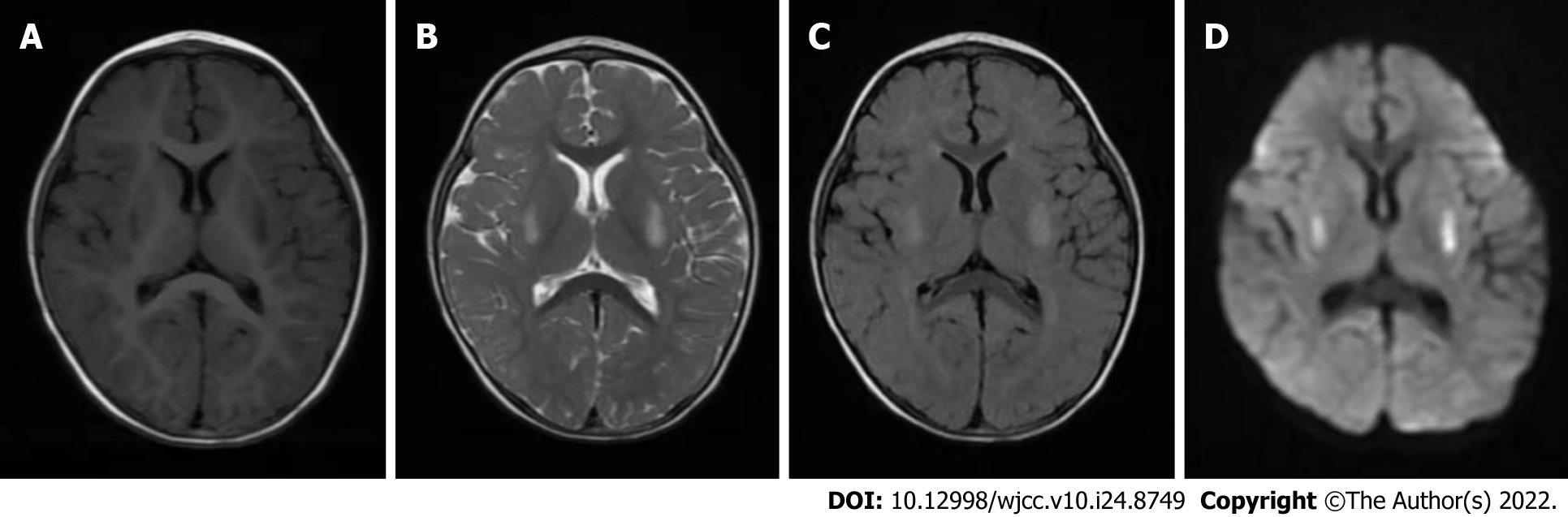
Brain MRI (April 3, 2019) showed symmetrical, patchy, long T1-weighted (Figure 1A) and low T2-weighted (Figure 1B) high signal shadow in the posterior part of the bilateral putamen, slightly high signal on T2 fluid-attenuated inversion recovery (Figure 1C) and an obvious high signal on diffusion-weighted imaging (Figure 1D).
Brain MRI (July 19, 2021) showed bilateral putamen symmetric lesions, and the length and diameter of the lesions were larger than before.
The child had clinical characteristics of comprehensive psychomotor developmental delay, microcephaly, seizures, cerebral magnetic brain with obvious symmetrical putamen lesions, and whole exon testing showed compound heterozygous mutations in VARS2 gene (Figure 2), so the child was diagnosed with combined oxidative phosphorylation deficiency 20 (COXPD20).
There is no specific treatment for this disease, and we provided the patient with nutritional supportive care and topiramate to control seizures.
During the initial 6-mo follow-up period, the number of seizures decreased, and there was no progression or regression in the psychomotor development of the patient.
The VARS2 gene contains 30 exons and encodes mitochondrial valine tRNA synthetase, which participates in mitochondrial protein synthesis. Rare biallelic variants in VARS2 are associated with mitochondrial encephalopathy or cardiomyopathy[6]. Mitochondrial disorders are caused by inherited defects of the pyruvate oxidation route. The core of this pathway consists of the OXPHOS system. Components of this system are encoded by the nuclear and the mitochondrial genome. Aside from mitochondrial DNA-encoded tRNAs and rRNAs, the mitochondrial translation apparatus consists of over 100 nuclear-encoded proteins, which are synthesized in the cytoplasm and then imported into the mitochondrial matrix[7].
COXPD20 is an autosomal recessive mitochondrial encephalocardiomyopathy caused by variants in the VARS2 gene located on chromosome 6p21. Presently, there are 17 reported pathogenic variants in the VARS2 gene (https://www.ncbi.nlm.nih.gov/clinvar/?term=VARS2[all])[8].
Our patient aged 3 years and 4 mo, was admitted to hospital due to three convulsions in a day. The clinical presentation of comprehensive development delay and epileptic seizures attracted our attention. We strongly suspected that the child had mitochondrial disease. Nuclear and mitochondrial genes were tested 2 years ago, but the child did not have seizures at that time. Therefore, whole-exon testing was advised again, and in this test, the sequencing depth was increased and a compound heterozygous variant of the VARS2 gene was found. The pathogenicity grade of this variant was uncertain according to the American College of Medical Genetics and Genomics guidelines. But this also led us to confirm a diagnosis of COXPD20, because the biallelic variant of VARS2 is a nuclear gene encoding valine tRNA synthase. Mutations of the VARS2 gene suggest COXPD20 disease. The typical manifestations of that disease include general growth retardation, hypotonia, seizures, and microcephaly.
The patient’s main clinical manifestations were consistent with COXPD20, but unlike the typical disease, the child had increased muscle tone but did not have hypotonia. We did not conduct functional studies of VARS2 gene-associated proteins; thus, we could not determine that the clinical manifestations were caused by mutations in the VARS2 gene. We considered the possibility of other mitochondrial diseases, including Leigh syndrome and MELAS syndrome (mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes) during the course of clinical diagnosis and treatment of this patient, but the clinical presentation of the patient and the results of laboratory tests ruled out the possibility of both diseases.
The VARS2 gene is one of the genes encoding mitochondrial aminoacyl-tRNA synthetase. Few patients with VARS2 deficiencies have been described with specific phenotypes. The phenotypes are present at any time during life and demonstrate clinical heterogeneity[9]. We will continue to monitor the patient’s disease changes and conduct related protein function studies if conditions permit.
We would like to thank the child and her family members for their contributions to this report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Genetics and heredity
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ozden F, Turkey; Sood M, India S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Taylor RW, Pyle A, Griffin H, Blakely EL, Duff J, He L, Smertenko T, Alston CL, Neeve VC, Best A, Yarham JW, Kirschner J, Schara U, Talim B, Topaloglu H, Baric I, Holinski-Feder E, Abicht A, Czermin B, Kleinle S, Morris AA, Vassallo G, Gorman GS, Ramesh V, Turnbull DM, Santibanez-Koref M, McFarland R, Horvath R, Chinnery PF. Use of whole-exome sequencing to determine the genetic basis of multiple mitochondrial respiratory chain complex deficiencies. JAMA. 2014;312:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 284] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 2. | Ghezzi D, Zeviani M. Assembly factors of human mitochondrial respiratory chain complexes: physiology and pathophysiology. Adv Exp Med Biol. 2012;748:65-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Diodato D, Melchionda L, Haack TB, Dallabona C, Baruffini E, Donnini C, Granata T, Ragona F, Balestri P, Margollicci M, Lamantea E, Nasca A, Powell CA, Minczuk M, Strom TM, Meitinger T, Prokisch H, Lamperti C, Zeviani M, Ghezzi D. VARS2 and TARS2 mutations in patients with mitochondrial encephalomyopathies. Hum Mutat. 2014;35:983-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Bruni F, Di Meo I, Bellacchio E, Webb BD, McFarland R, Chrzanowska-Lightowlers ZMA, He L, Skorupa E, Moroni I, Ardissone A, Walczak A, Tyynismaa H, Isohanni P, Mandel H, Prokisch H, Haack T, Bonnen PE, Enrico B, Pronicka E, Ghezzi D, Taylor RW, Diodato D. Clinical, biochemical, and genetic features associated with VARS2-related mitochondrial disease. Hum Mutat. 2018;39:563-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 5. | Begliuomini C, Magli G, Di Rocco M, Santorelli FM, Cassandrini D, Nesti C, Deodato F, Diodato D, Casellato S, Simula DM, Dessì V, Eusebi A, Carta A, Sotgiu S. VARS2-linked mitochondrial encephalopathy: two case reports enlarging the clinical phenotype. BMC Med Genet. 2019;20:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Bonnefond L, Fender A, Rudinger-Thirion J, Giegé R, Florentz C, Sissler M. Toward the full set of human mitochondrial aminoacyl-tRNA synthetases: characterization of AspRS and TyrRS. Biochemistry. 2005;44:4805-4816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Baertling F, Alhaddad B, Seibt A, Budaeus S, Meitinger T, Strom TM, Mayatepek E, Schaper J, Prokisch H, Haack TB, Distelmaier F. Neonatal encephalocardiomyopathy caused by mutations in VARS2. Metab Brain Dis. 2017;32:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Ruzman L, Kolic I, Radic Nisevic J, Ruzic Barsic A, Skarpa Prpic I, Prpic I. A novel VARS2 gene variant in a patient with epileptic encephalopathy. Ups J Med Sci. 2019;124:273-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Ma K, Xie M, He X, Liu G, Lu X, Peng Q, Zhong B, Li N. A novel compound heterozygous mutation in VARS2 in a newborn with mitochondrial cardiomyopathy: a case report of a Chinese family. BMC Med Genet. 2018;19:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |