Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8709
Peer-review started: March 10, 2022
First decision: April 10, 2022
Revised: April 23, 2022
Accepted: July 18, 2022
Article in press: July 18, 2022
Published online: August 26, 2022
Processing time: 158 Days and 18.7 Hours
Bronchogenic cysts are congenital cysts caused by abnormal sprouting from the ventral foregut during fetal life. They usually occur in the mediastinum or lung, but there are very rare cases of ectopic bronchogenic cysts that develop in the abdominal cavity. A unique intra-abdominal ectopic bronchogenic cyst with a mucinous neoplasm that was producing carcinoembryonic antigen (CEA), harboring a GNAS mutation, is reported. The present case may contribute to clarifying the mechanism of tumorigenesis and malignant transformation of ectopic bronchogenic cysts.
In 2007, a man in his 50s was incidentally found to have an intra-abdominal cystic mass, 8 cm in diameter. Surgical resection was recommended, but he preferred to remain under observation. In 2020, his serum CEA level increased to 26.7 ng/mL, and abdominal computed tomography showed a 15 cm × 12 cm, multifocal, cystic mass located predominantly on the lesser curvature of the stomach. Since malignancy could not be ruled out, he finally underwent surgical resection. Histologically, the cystic wall was lined by ciliated columnar epithelium, accompanied by bronchial gland-like tissue, bronchial cartilage, and smooth muscle. Part of the cyst consisted of atypical columnar epithelium with an MIB-1 index of 5% and positive for CEA. Moreover, a GNAS mutation (p.R201C) was detected in the atypical epithelium, leading to a diagnosis of an ectopic bronchogenic cyst with a low-grade mucinous neoplasm. The patient is currently undergoing outpatient follow-up without recurrence.
An extremely rare case of an abdominal bronchogenic cyst with a low-grade mucinous neoplasm harboring a GNAS mutation was reported.
Core Tip: A man in his 60s had an intra-abdominal, 15-cm, multifocal, cystic mass located on the lesser curvature of the stomach, and he underwent surgical resection. Histologically, the resected cystic wall was lined by ciliated columnar epithelium, accompanied by bronchial gland-like tissue, bronchial cartilage, and smooth muscle. Part of the cyst consisted of atypical columnar epithelium with an MIB-1 index of 5% that was positive for carcinoembryonic antigen. Moreover, a GNAS mutation (p.R201C) was detected in the atypical epithelium, leading to a diagnosis of an ectopic bronchogenic cyst with a low-grade mucinous neoplasm.
- Citation: Murakami T, Shimizu H, Yamazaki K, Nojima H, Usui A, Kosugi C, Shuto K, Obi S, Sato T, Yamazaki M, Koda K. Intra-abdominal ectopic bronchogenic cyst with a mucinous neoplasm harboring a GNAS mutation: A case report. World J Clin Cases 2022; 10(24): 8709-8717
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8709.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8709
Bronchogenic cysts are congenital cysts caused by abnormal sprouting from the ventral foregut during fetal life from the 3rd to 7th weeks[1]. Bronchogenic cysts usually occur in the mediastinum or lung, but there are rare cases of ectopic bronchogenic cysts that develop in the abdominal cavity or retroperitoneum[2,3]. There are some reports of malignant transformation in bronchogenic cysts, but the mechanism of tumorigenesis and malignant transformation is still unknown[1]. An extremely rare case of an intra-abdominal ectopic bronchogenic cyst with carcinoembryonic antigen (CEA) production, in which a mucinous neoplasm harboring a GNAS gene mutation was observed, is presented. The present case may contribute to clarifying the mechanism of tumorigenesis and malignant transformation of ectopic bronchogenic cysts.
The patient had no symptoms.
A man in his 50s underwent magnetic resonance imaging (MRI) for the follow-up of chronic pancreatitis in 2007 and was incidentally found to have an 8-cm-diameter intra-abdominal mass. His physician referred him to the Department of Surgery, but he preferred to remain under observation because he had no symptoms. In 2020, his serum CEA level increased to 26.7 ng/mL, suggesting possible malignancy, and he was finally referred to our department.
The patient had a history of gastroesophageal reflux disease and thoracic compression fracture.
The patient had no relevant family history.
A large, soft mass was palpable in the left upper quadrant.
The CEA level was elevated to 26.7 ng/mL, whereas the carbohydrate antigen (CA)19-9 level was within the normal limit. Blood counts, blood biochemistry, and coagulation function were all normal.
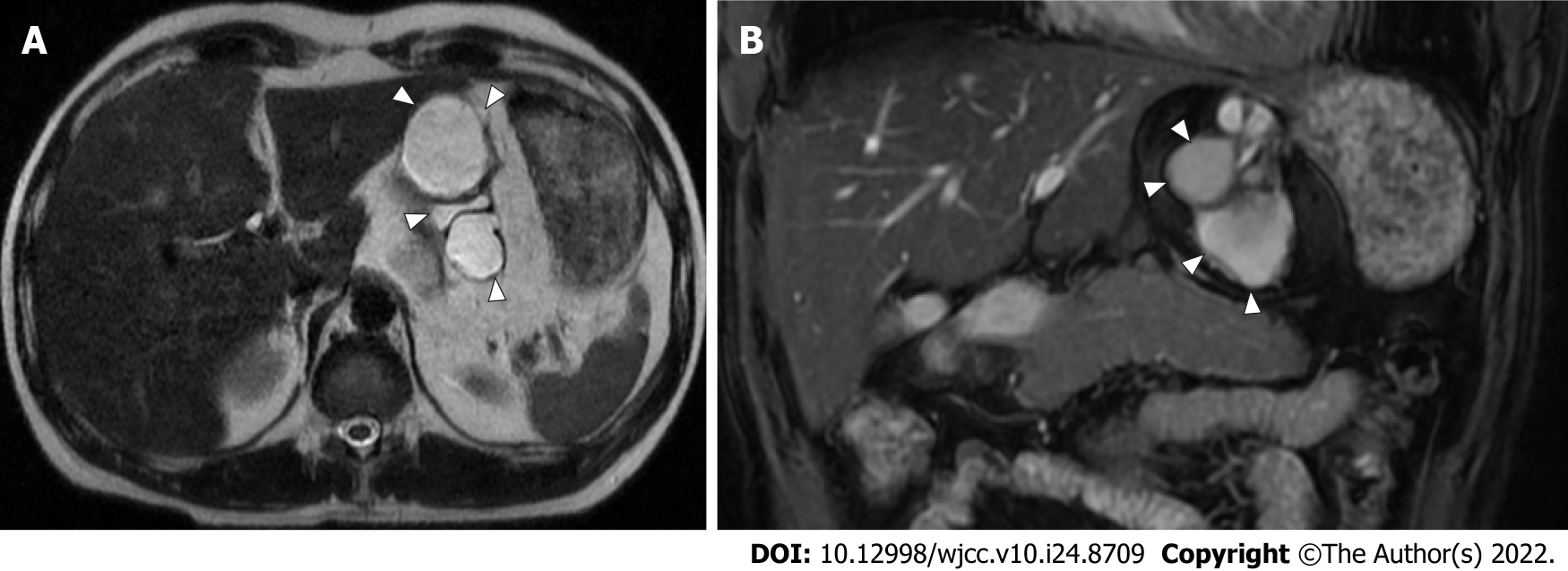
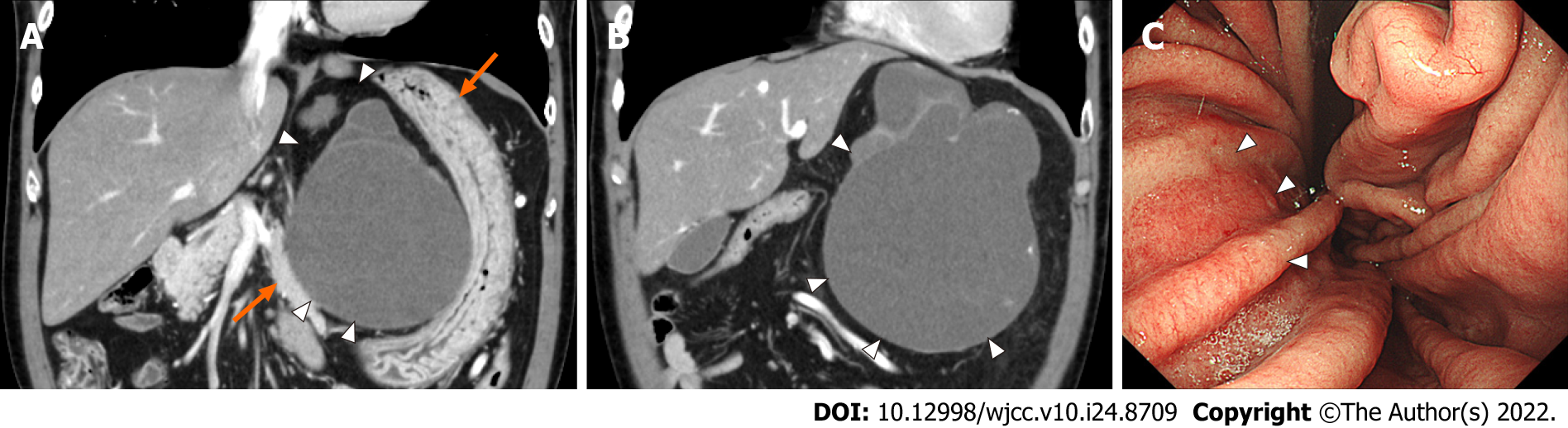
MRI T2-weighted imaging showed a high-intensity, multifocal, cystic mass between the stomach and lateral lobe of the liver (Figure 1). A mass the size of a child’s head was palpable in the left upper quadrant, but he had no symptoms. Contrast-enhanced computed tomography (CT) showed that the multifocal cystic mass had increased to 15 cm in diameter, and part of the cystic wall showed calcification and partial thickness, but there were no obvious intra-cystic nodules (Figure 2A and B). The surrounding organs such as the stomach, pancreas, and liver were markedly compressed by the mass, but the borders of the mass were clear.
Esophagogastroduodenoscopy showed compression of the lesser curvature, but no neoplastic lesions were found in the gastric mucosa (Figure 2C). Colonoscopy did not show any neoplastic lesions.
The final diagnosis was an intra-abdominal ectopic bronchogenic cyst with a low-grade mucinous neoplasm harboring a GNAS mutation.
These findings suggested that the primary site of the tumor was within the lesser omentum. Although he was not diagnosed definitely, malignancy was suspected due to the increasing size of the mass and elevated CEA level. Therefore, he finally underwent surgical resection after his informed consent was obtained.
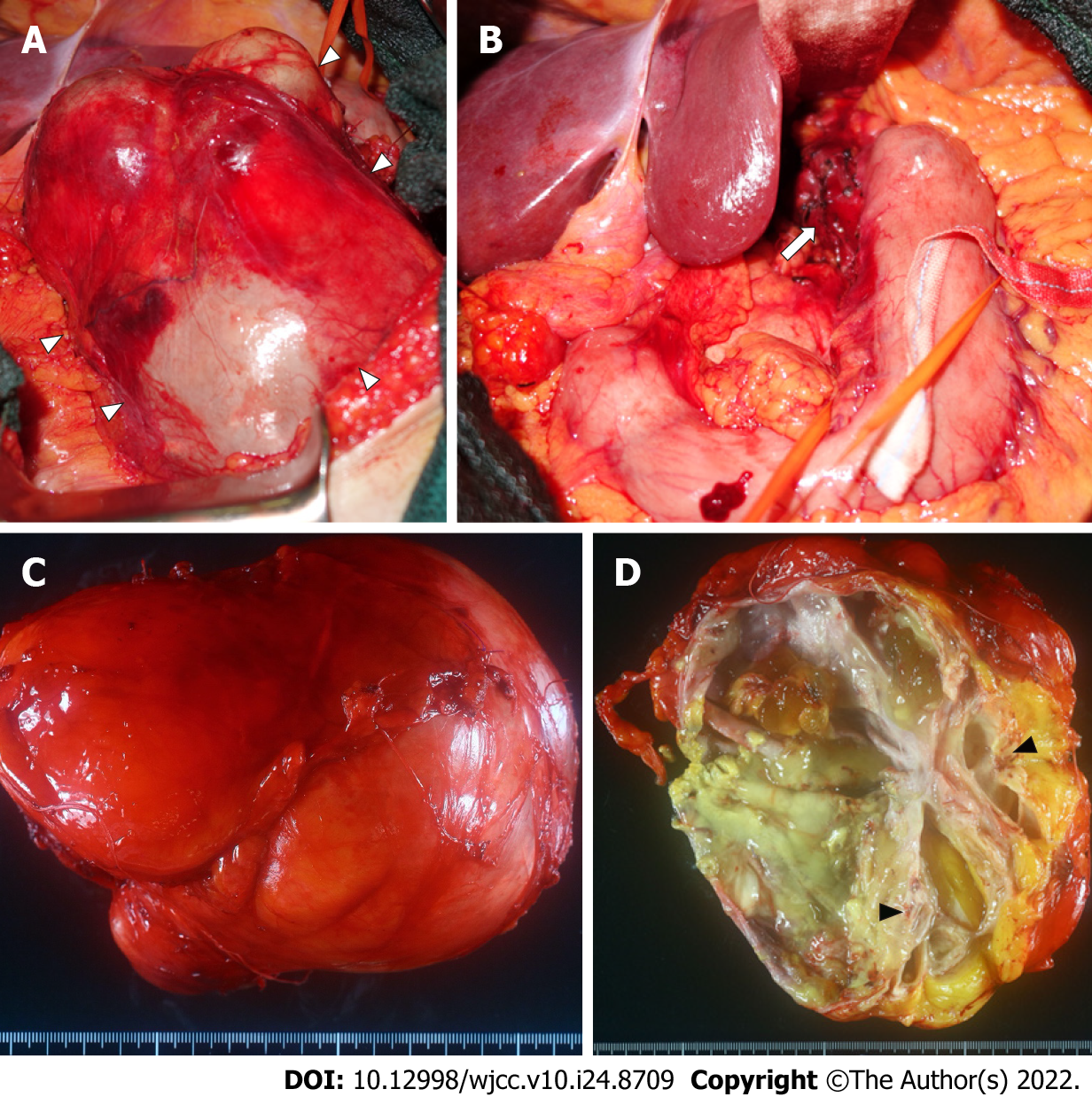
After laparotomy and resection of the lesser omentum, a smooth-surfaced mass, 15 cm in diameter, was exposed. Most of the mass was loosely attached to surrounding organs and easily dissected, though the mass adhered to the lesser curvature of the stomach (Figure 3A). Therefore, partial resection of the seromuscular layer of the stomach was required to remove the mass (Figure 3B). The defected seromuscular layer was sutured. The operation time was 2 h and 46 min, and intraoperative bleeding was 450 g.
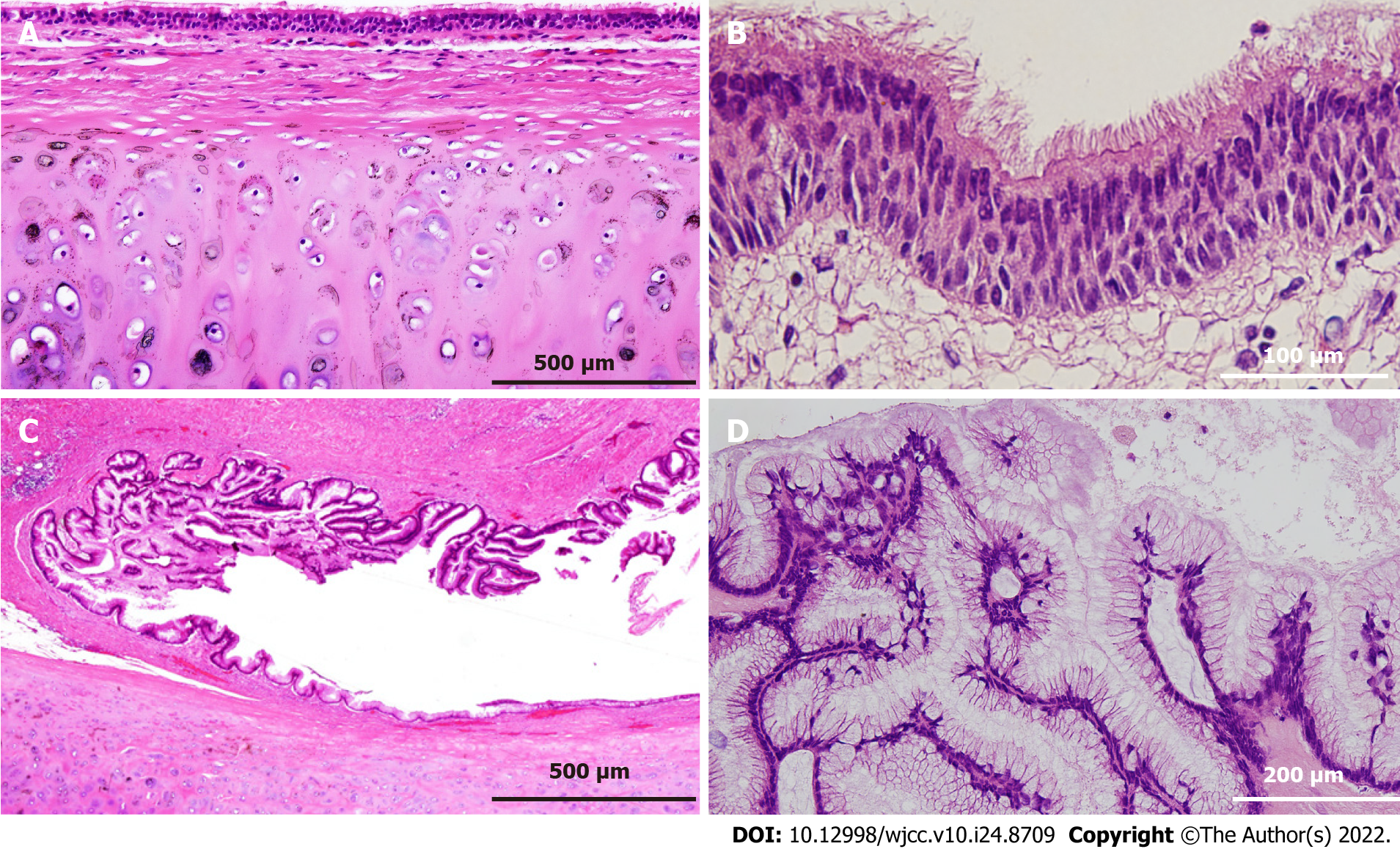
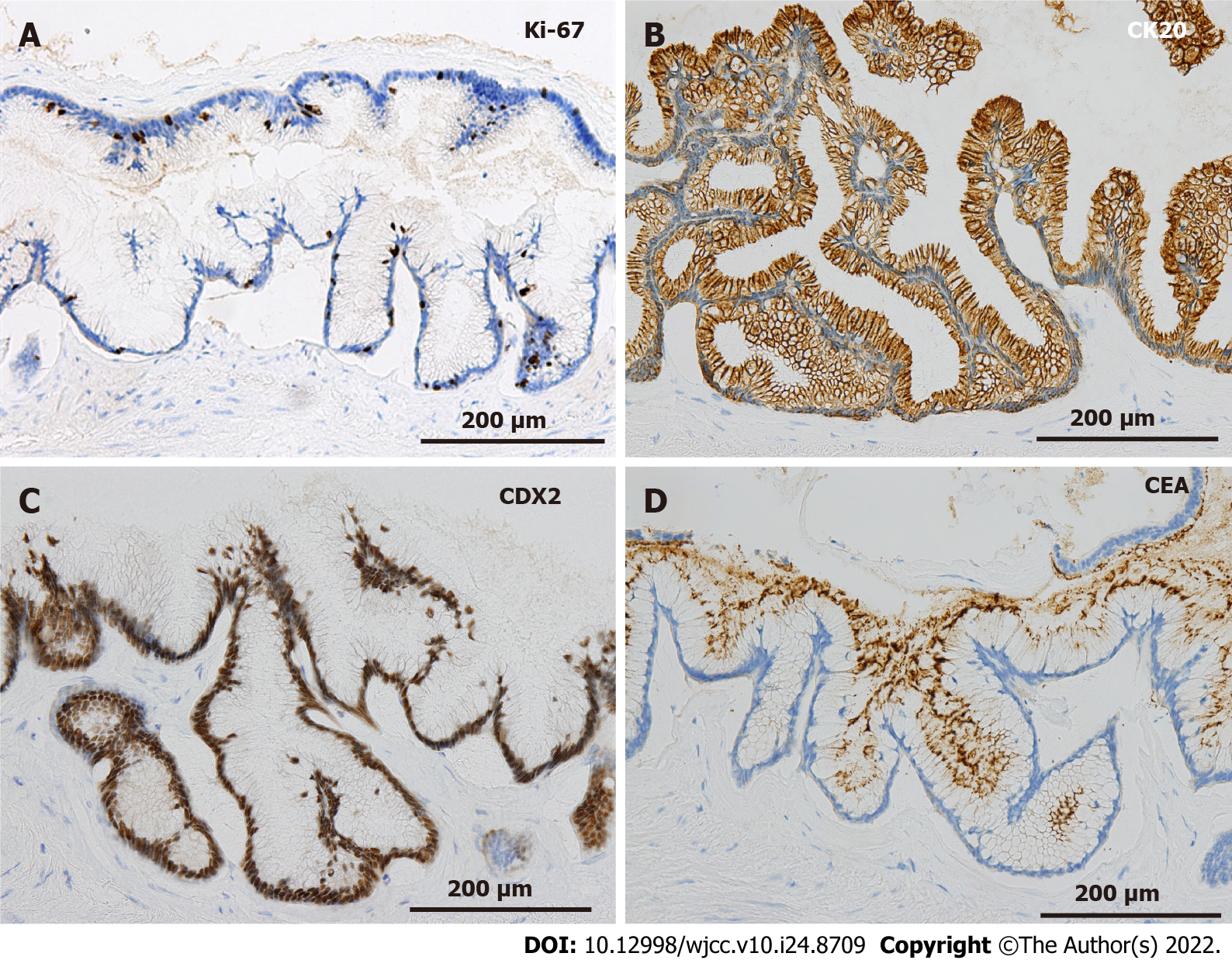
On gross examination of the resected specimen, the mass was multifocal and filled with viscous mucus, with size of 15 cm × 12 cm × 12 cm and weight of 1240 g (Figure 3C). Part of the cystic wall consisted of cartilage (Figure 3D). Histologically, the majority of the cystic wall was lined by ciliated columnar epithelium, and bronchial gland-like tissue, cartilage, and smooth muscle were observed in the deeper layer, leading to the diagnosis of an ectopic bronchogenic cyst (Figure 4A and B). Meanwhile, part of the cystic wall, about 5 cm in size, was lined by high columnar epithelium containing mucus in the cytoplasm (Figure 4C and D). This columnar epithelium was folded, making papillary structures, which was considered to be a low-grade mucinous neoplasm. The MIB-1 index was 5% at the site of the low-grade mucinous neoplasm (Figure 5A). Immunohistochemical staining showed that the area with the low-grade mucinous neoplasm was positive for CK20 and CDX2, and negative for CK7, indicating intestinal metaplasia (Figure 5B and C). In addition, the neoplastic lesion showed positive staining for CEA, suggesting CEA production (Figure 5D).
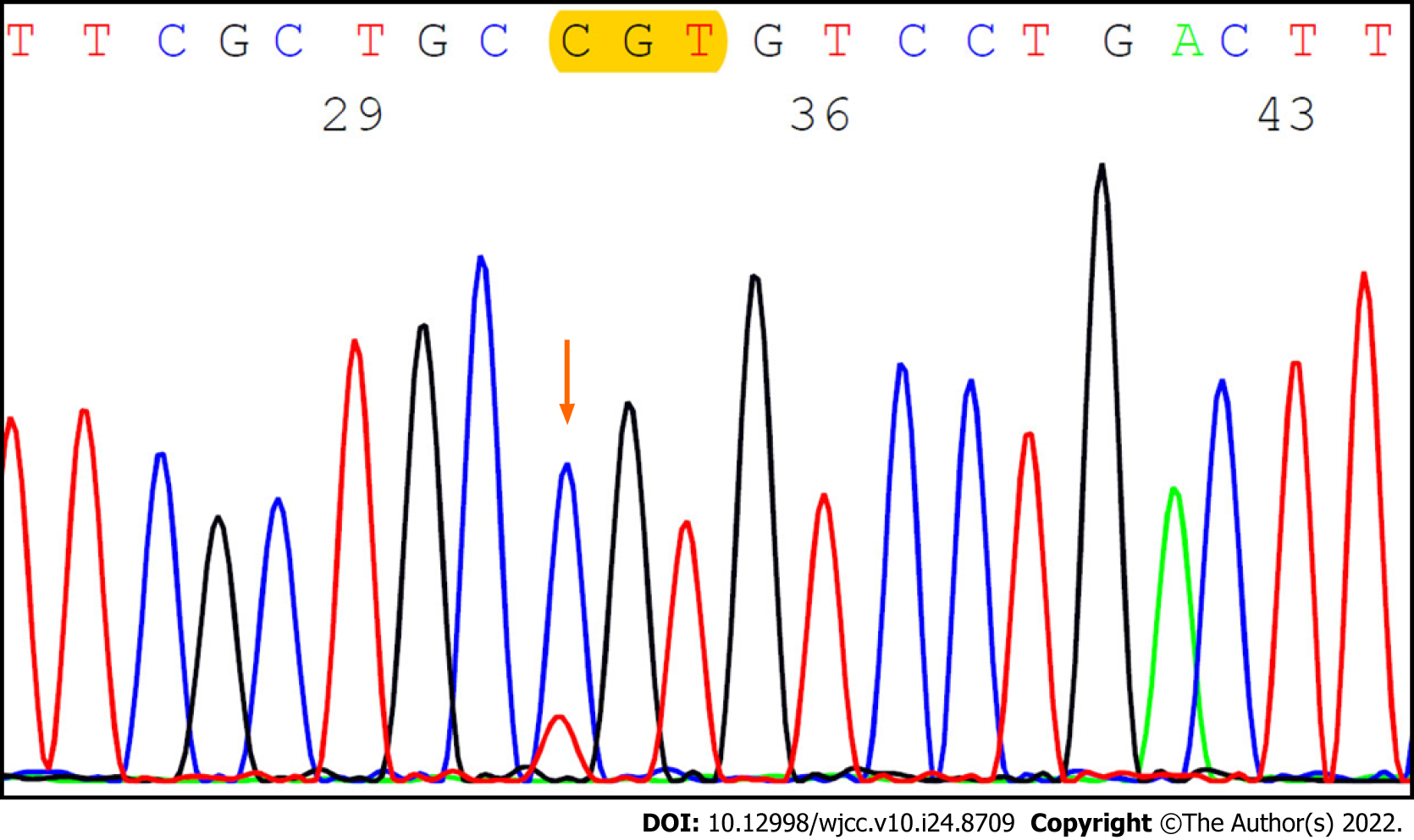
GNAS mutation analysis by the Sanger method of the lesion with the low-grade mucinous neoplasm showed a missense mutation at codon 201 (p.R201C, c.601C>T; c.601C>T; Figure 6).
The patient had grade II delayed gastric emptying, but he recovered and was discharged on the 25th postoperative day. His serum CEA level normalized. He continues to be under observation without evidence of recurrence at 1-year follow-up.
This is the first report of a case of an intra-abdominal ectopic bronchogenic cyst with a mucinous neoplasm that was producing CEA, harboring a GNAS mutation.
Bronchogenic cysts are congenital cysts that are established by persistent secretion from abnormal sprouting lung buds that lack continuity with the tracheobronchial system during fetal life[4,5]. Most bronchogenic cysts develop in the lungs or mediastinum; if abnormal lung buds sprout before 4 wk of fetal life, bronchogenic cysts are located in the mediastinum, and if they sprout after 4 wk of fetal life, bronchogenic cysts are located in the lungs[6,7]. The thoracic cavity and abdominal cavity are connected by the pericardioperitoneal canal, but they become separated by the diaphragm, which develops from the septum transversum, dorsal esophageal mesentery, and pleuroperitoneal fold by 7 wk of fetal life[6,8,9]. Therefore, intra-abdominal bronchogenic cysts are considered to be caused by abnormally sprouted lung buds that wandered into the abdominal cavity prior to diaphragmatic formation through the pericardioperitoneal canal.
Ectopic bronchogenic cysts are very rare and have been reported to occur in the abdominal cavity, retroperitoneum, neck, tongue, and scapula[10]. Subphrenic ectopic bronchogenic cysts develop more often on the left side of the patient, with fewer intra-abdominal cases than retroperitoneal cases[5]. In 2011, Ubukata et al[5] summarized 12 cases of intra-abdominal bronchogenic cysts, 9 of which were located close to the stomach, suggesting that intra-abdominal bronchogenic cysts tend to develop around the stomach. In a report of a rare case, a bronchogenic cyst attached to the gastric wall was invaded by adjacent gastric cancer[11].
Bronchogenic cysts are visualized as spherical masses with clear borders, but CT values of the cystic contents vary from as low as normal cysts to as high as solid tumors[12]. With regard to MRI, Murakami et al[13] and Martín et al[14] stated that high intensity of cystic contents on T1-weighted images is more useful than CT for differential diagnosis, but there are also some cases with low intensity of cystic contents on T1-weighted imaging. Due to the rarity of intra-abdominal bronchogenic cysts and the diversity of imaging findings, preoperative diagnosis is considered to be extremely difficult[12]. Due to the risk of tumor cell dissemination, histological or cytological diagnosis by puncture of cysts is not indicated. The definite diagnosis of bronchogenic cysts is based on histological examination of resected specimens, and they are characterized by a bronchial-like histology such as ciliated columnar epithelium, cartilage, bronchial gland, and smooth muscle in the cystic wall[1].
Since ectopic bronchogenic cysts are extremely rare, details of their prognosis are still unclear. Malignant transformation has been reported in a few cases of retroperitoneal bronchogenic cysts[1]. The tumor diameter of malignant cases (11.7 ± 4.7 cm) was reported to be larger than that of benign cases (5.5 ± 2.9 cm)[12]. Moreover, levels of serum tumor markers such as CEA, CA19-9, or CA125 were high in malignant cases[1,12]. The increasing serum CEA level in the present case suggested potential malignant transformation.
Regarding the indications for surgery, cases with possible malignant transformation, such as those with increasing tumor diameter or elevated serum tumor marker levels, should be treated with surgical resection[1,12]. Moreover, there are reports recommending complete surgical resection for all bronchogenic cysts, because malignancy can be found after surgical resection, and recurrence or infection can occur even in benign cases[15,16]. Similar to the present case, there was a report of a patient with an intra-abdominal bronchogenic cyst that developed in the gastric cardia who underwent partial resection of the seromuscular layer of the stomach[17]. Some cases of intra-abdominal bronchogenic cysts underwent laparoscopic tumor resection[18,19]. Since the tumor was large and suspected to have malignant transformation in the present case, open laparotomy was preferred to maintain the intraoperative field of view and to avoid incidental tumor cell dissemination due to accidental perforation of the mass.
As for pathological findings, CK20-positive and CK7-negative are specific for gastric, small intestinal, and colonic epithelium, and CDX2 encodes an intestine-specific transcription factor that is expressed in the nuclei of epithelial cells in the small intestine and colorectum[20]. In the present case, the area with low-grade adenoma was positive for CK20 and CDX2 and negative for CK7, suggesting a background intestinal metaplasia. GNAS is a proto-oncogene encoding a stimulatory G protein α subunit (GSα), which is a signal mediator of the G protein-coupled receptor signaling pathway located at 20q13.3[21]. GNAS mutations have been found in intraductal papillary mucinous neoplasms (IPMNs) of the pancreas, colorectal villous adenomas, gastric pyloric gland adenomas, appendiceal mucinous neoplasms, and a bronchial mucous gland adenoma[22-25]. Most GNAS mutations observed in IPMN are caused by R201H or R201C mutations, missense mutations at codon 201, which result in reducing intrinsic hydrolytic activity of GSα, prolonging activation of cell proliferation signals[23]. The GNAS mutation is considered to play a central role in mucus production in appendiceal mucinous neoplasms[25]. In the present case, a low-grade mucinous neoplasm was thought to be caused by the GNAS mutation at the site of intestinal metaplasia. Since the GNAS mutation has also been observed in some appendiceal mucinous carcinomas, a low-grade mucinous neoplasm in bronchogenic cysts may undergo malignant transform to mucinous carcinoma[26]. Since this is the first report describing a GNAS mutation in a bronchogenic cyst, further investigation of the association between GNAS mutations and tumorigenesis both in ectopic and normotopic bronchogenic cysts through accumulation of additional cases is expected.
An extremely rare case of a huge abdominal bronchogenic cyst with a low-grade mucinous neoplasm harboring a GNAS mutation was reported. The present case may contribute to elucidating the pathogenesis and natural history of ectopic bronchogenic cysts.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Japanese Society of Hepato-Biliary-Pancreatic Surgery.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feng J, China; Navarrete Arellano M, Mexico S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Sullivan SM, Okada S, Kudo M, Ebihara Y. A retroperitoneal bronchogenic cyst with malignant change. Pathol Int. 1999;49:338-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Subramanian JB, K S S, Selvarangam S. A case report- retroperitoneal bronchogenic cyst in relation to the hindgut. Int J Surg Case Rep. 2020;75:140-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Choi KK, Sung JY, Kim JS, Kim MJ, Park H, Choi DW, Choi SH, Heo JS. Intra-abdominal bronchogenic cyst: report of five cases. Korean J Hepatobiliary Pancreat Surg. 2012;16:75-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Coselli MP, de Ipolyi P, Bloss RS, Diaz RF, Fitzgerald JB. Bronchogenic cysts above and below the diaphragm: report of eight cases. Ann Thorac Surg. 1987;44:491-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Ubukata H, Satani T, Motohashi G, Konishi S, Goto Y, Watanabe Y, Nakada I, Tabuchi T. Intra-abdominal bronchogenic cyst with gastric attachment: report of a case. Surg Today. 2011;41:1095-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Tanaka G, Yamashita H, Yasui A, Suka Y, Nagai M, Ohira Y, Suzuki Y. Laparoscopic resection of a retroperitoneal bronchogenic cyst: A case report. J Jpn Surg Assoc. 2020;81:367-373. [DOI] [Full Text] |
| 7. | Gomes MN, Hufnagel CA. Intrapericardial bronchogenic cysts. Am J Cardiol. 1975;36:817-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Sumiyoshi K, Shimizu S, Enjoji M, Iwashita A, Kawakami K. Bronchogenic cyst in the abdomen. Virchows Arch A Pathol Anat Histopathol. 1985;408:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Merrell AJ, Kardon G. Development of the diaphragm -- a skeletal muscle essential for mammalian respiration. FEBS J. 2013;280:4026-4035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Tang J, Zeng Z, Deng S, Lin F. Ectopic bronchogenic cyst arising from the diaphragm: a rare case report and literature review. BMC Surg. 2021;21:321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Shibahara H, Arai T, Yokoi S, Hayakawa S. Bronchogenic cyst of the stomach involved with gastric adenocarcinoma. Clin J Gastroenterol. 2009;2:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Eguchi H, Ohigashi H, Ishikawa O, Kasugai T, Yokoyama S, Sasaki Y, Miyashiro I, Doki Y, Murata K, Imaoka S. A case of retroperitoneal bronchogenic cysts with malignant regeneration. Jpn J Gastroenterol Surg. 2004;37:584-589. [DOI] [Full Text] |
| 13. | Murakami R, Machida M, Kobayashi Y, Ogura J, Ichikawa T, Kumazaki T. Retroperitoneal bronchogenic cyst: CT and MR imaging. Abdom Imaging. 2000;25:444-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Martín R, Sanz E, de Vicente E, Ortega P, Labrador E, Paumard A, Gómez-Durán J, Quijano Y, Santamaría L. Differential diagnosis of asymptomatic retroperitoneal cystic lesion: a new case of retroperitoneal bronchogenic cyst. Eur Radiol. 2002;12:949-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Cuypers P, De Leyn P, Cappelle L, Verougstraete L, Demedts M, Deneffe G. Bronchogenic cysts: a review of 20 cases. Eur J Cardiothorac Surg. 1996;10:393-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Hasegawa T, Murayama F, Endo S, Sohara Y. Recurrent bronchogenic cyst 15 years after incomplete excision. Interact Cardiovasc Thorac Surg. 2003;2:685-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Xiao J, Zhang R, Chen W, Wu B. Ectopic bronchogenic cyst of the gastric cardia considered to be a gastrointestinal stromal tumor before surgery: a case report. BMC Surg. 2020;20:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Trehan M, Singla S, Singh J, Garg N, Mahajan A. A Rare Case of Intra-Abdominal Bronchogenic Cyst- A Case Report. J Clin Diagn Res. 2015;9:PD03-PD04. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Díaz Nieto R, Naranjo Torres A, Gómez Alvarez M, Ruiz Rabelo JF, Pérez Manrique MC, Ciria Bru R, Valverde Martínez A, Roldán de la Rúa J, Alonso Gómez J, Rufián Peña S. Intraabdominal bronchogenic cyst. J Gastrointest Surg. 2010;14:756-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Lee BH, Kim N, Lee HS, Kang JM, Park HK, Jo HJ, Shin CM, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, Jung HC, Song IS. The Role of CDX2 in Intestinal Metaplasia Evaluated Using Immunohistochemistry. Gut Liver. 2012;6:71-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Ritterhouse LL, Vivero M, Mino-Kenudson M, Sholl LM, Iafrate AJ, Nardi V, Dong F. GNAS mutations in primary mucinous and non-mucinous lung adenocarcinomas. Mod Pathol. 2017;30:1720-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Kojima R, Morimoto J, Ishida Y, Sugano I, Yamazaki K. A bronchial mucous gland adenoma harboring GNAS R201C mutation. Diagn Cytopathol. 2021;49:E203-E206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Furukawa T, Kuboki Y, Tanji E, Yoshida S, Hatori T, Yamamoto M, Shibata N, Shimizu K, Kamatani N, Shiratori K. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep. 2011;1:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 338] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 24. | Matsubara A, Sekine S, Kushima R, Ogawa R, Taniguchi H, Tsuda H, Kanai Y. Frequent GNAS and KRAS mutations in pyloric gland adenoma of the stomach and duodenum. J Pathol. 2013;229:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Nishikawa G, Sekine S, Ogawa R, Matsubara A, Mori T, Taniguchi H, Kushima R, Hiraoka N, Tsuta K, Tsuda H, Kanai Y. Frequent GNAS mutations in low-grade appendiceal mucinous neoplasms. Br J Cancer. 2013;108:951-958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 26. | Garland-Kledzik M, Scholer A, Ensenyat-Mendez M, Orozco JIJ, Khader A, Santamaria-Barria J, Fischer T, Pigazzi A, Marzese DM. Establishing Novel Molecular Subtypes of Appendiceal Cancer. Ann Surg Oncol. 2022;29:2118-2125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |