Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8648
Peer-review started: January 19, 2022
First decision: April 8, 2022
Revised: April 22, 2022
Accepted: July 22, 2022
Article in press: July 22, 2022
Published online: August 26, 2022
Processing time: 208 Days and 10.1 Hours
Anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis is a treatable but frequently misdiagnosed autoimmune disease. Speech dysfunction, as one of the common manifestations of anti-NMDAR encephalitis, is usually reported as a symptom secondary to psychiatric symptoms or seizures rather than the initial symptom in a paroxysmal form. We report a case of anti-NMDAR encephalitis with paroxysmal speech disorder as a rare initial manifestation, and hope that it will contribute to the literature.
A 39-year-old man with anti-NMDAR encephalitis initially presented with paroxysmal nonfluent aphasia and was misdiagnosed with a transient ischemic attack and cerebral infarction successively. The patient subsequently presented with seizures, but no abnormalities were found on brain magnetic resonance imaging or electroencephalogram. Cerebrospinal fluid (CSF) analysis revealed mild pleocytosis and increased protein levels. Anti-NMDAR antibodies in serum and CSF were detected for a conclusive diagnosis. After immunotherapy, the patient made a full recovery.
This case suggests that paroxysmal speech disorder may be the presenting symptom of anti-NMDAR encephalitis in a young patient.
Core Tip: Anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis is a treatable but often misdiagnosed autoimmune disease. In this paper, we describe a 39-year-old man with anti-NMDAR encephalitis who initially presented with paroxysmal speech disorder and was subsequently misdiagnosed with a transient ischemic attack and cerebral infarction. The definitive diagnosis was made based on the detection of anti-NMDAR antibodies in serum and cerebrospinal fluid. The patient recovered completely after immunotherapy. This case suggests that paroxysmal speech disorder may be the first symptom of anti-NMDAR encephalitis in a young patient without risk factors for cerebrovascular disease.
- Citation: Hu CC, Pan XL, Zhang MX, Chen HF. Paroxysmal speech disorder as the initial symptom in a young adult with anti-N-methyl-D-aspartate receptor encephalitis: A case report. World J Clin Cases 2022; 10(24): 8648-8655
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8648.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8648
Anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis was initially reported as a paraneoplastic immune-mediated syndrome associated with ovarian teratoma[1]. Since Dalmau et al[2] discovered antibodies against N-methyl-D-aspartate receptor (NMDAR) in 2007, anti-NMDAR encephalitis has been gradually recognized worldwide. The exact incidence of the disease was unknown. A multicenter, population-based prospective study suggested that anti-NMDAR encephalitis accounts for 4% of all causes of encephalitis[3]. Data from the California Encephalitis Project regarding the cause of encephalitis revealed that the frequency of anti-NMDAR encephalitis surpassed that of individual viral etiologies in young individuals[4]. Anti-NMDAR encephalitis is a treatable but often misdiagnosed autoimmune disease[5]. It primarily affects children and young adults (a median age of 21 years), with a higher incidence among females (4:1) but a similar incidence between women and men after the age of 45 years[6]. We present a case of anti-NMDAR encephalitis with paroxysmal speech disorder as the presenting symptom. The patient was initially misdiagnosed with cerebrovascular disease due to a transient ischemic attack (TIA)-like onset, but anti-NMDAR antibodies in serum and cerebrospinal fluid (CSF) eventually validated the diagnosis.
A 39-year-old man presented to the emergency department of our hospital complaining of repeated episodes of speech impediment for one day. In addition, he experienced a generalized tonic-clonic seizure an hour before the visit.
The patient's symptoms began with paroxysmal speech disorder one day prior. Each attack lasted for dozens of seconds to several minutes and was not accompanied by other neurological deficits. The patient experienced convulsion with loss of consciousness during his first visit to another hospital. The epileptic attack lasted for two minutes and the patient regained consciousness after ten minutes. He was diagnosed with TIA and treated with 200 mg aspirin and 20 mg atorvastatin. Then, he presented to our hospital with persistent slurred speech lasted for more than one hour.
Except for a headache one month prior, the patient had no significant medical history and no drug in use.
The patient's personal and family history was unremarkable.
The patient's temperature was 36.5 °C, heart rate was 89 beats per minute, respiratory rate was 18 breaths per minute, blood pressure was 18.1/9.8 KPa and oxygen saturation in room air was 98%. No obvious abnormality was found on neurological examination when he was admitted to another hospital. However, we found nonfluent aphasia and deviation of the tongue to the right on neurological examination.
Routine laboratory studies, including a complete blood count, hepatic and renal function, blood glucose, glycosylated hemoglobin, coagulation testing, autoantibodies, autoantibody spectrum associated with anti-cardiolipin antibodies, thyroid function, homocysteine, serum tumor markers, human immunodeficiency virus antibody test and treponema pallidum hemagglutination assay, were all unremarkable.
An initial brain computed tomography (CT) scan showed no significant abnormalities. Brain computed tomography angiography (CTA) showed no intracranial hemorrhage, aneurysm, vascular malfor
Routine and ambulatory electroencephalogram (EEG) showed no epileptic discharges, and the diagnosis of epilepsy was untenable.
Lumbar puncture revealed a CSF pressure of 2.21 kPa. CSF analysis revealed a nucleated cell count of 28/μL (normal < 5/μL), which was dominated by lymphocytes (85% lymphocytes, 10% monocytes, 4% neutrophils, 1% eosinophils), lactate dehydrogenase of 56 U/L (normal < 40 U/L), and protein levels of 662 mg/L (normal 120-600 mg/L). The concentrations of glucose, chlorine and adenosine deaminase in the CSF were normal. Anti-NMDAR antibodies were detected in the CSF and serum by indirect immunofluorescence testing using a commercial kit (Euroimmune, Germany). The titer of anti-NMDAR antibody IgG was 1:10 (++) in CSF and 1:32 (++) in serum. Anti-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid 1, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid 2, gamma-aminobutyric acid-B, leucine-rich glioma-inactivated protein 1, contactin-associated protein-like 2 and glutamic acid decarboxylase-65 antibodies IgG in CSF and serum were negative. No tumor was found on chest CT or abdominal ultrasound.
The presence of anti-NMDAR antibodies in serum and CSF led to a final diagnosis of anti-NMDAR encephalitis in the presented case.
The patient was initially misdiagnosed with TIA and treated with 200 mg aspirin and 20 mg atorvastatin at another hospital. The patient was subsequently misdiagnosed with cerebral infarction and received intravenous thrombolytic therapy with 50 mg of recombinant tissue plasminogen activator at the emergency department of our hospital. Twenty-four hours after thrombolytic therapy, aspirin (200 mg/d) and atorvastatin (20 mg/d) were administered orally in the ward until the final diagnosis was reached.
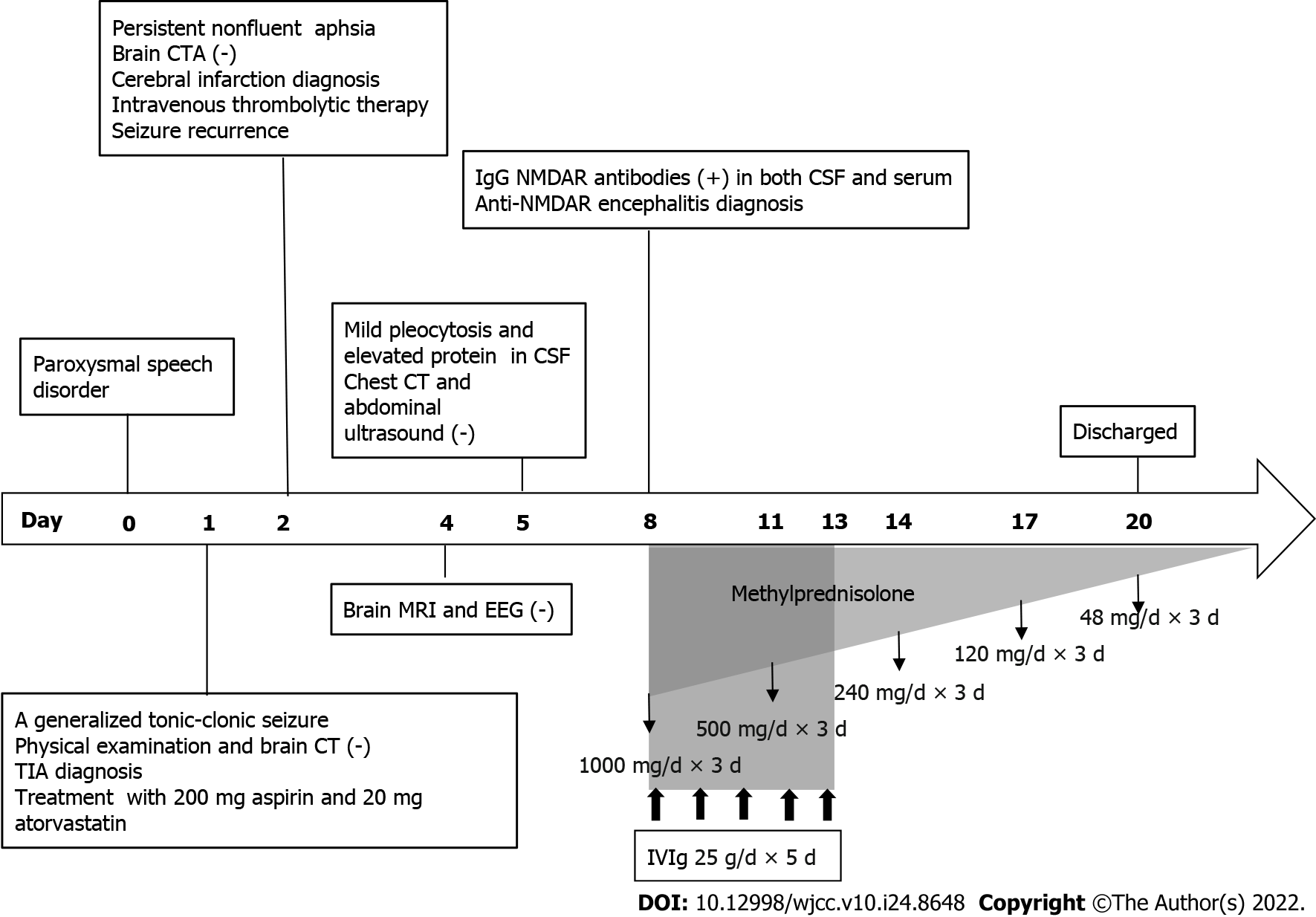
Ultimately, the patient was diagnosed with anti-NMDAR encephalitis and treated with intravenous immunoglobulin (25 g/d × 5 d) and intravenous methylprednisolone (1000 mg/d × 3 d to 500 mg/d × 3 d to 240 mg/d × 3 d to 120 mg/d × 3 d). Then the patient was discharged with slowly tapered oral methylprednisolone (48 mg qd × 2 wk to reduction of the dosage by 4 mg every 2 wk) (Figure 1).
The patient's speech disorder recovered after immunotherapy, and he no longer had seizures. No adverse effect was observed during the treatment. At the follow-up six months after discharge, he was asymptomatic.
The NMDAR is a member of the ionotropic glutamate receptor family, which plays a crucial role in neuronal communication[7]. NMDAR-mediated signals control diverse processes across the life course, including synaptogenesis and synaptic plasticity, and contribute to excitotoxic processes in neurological disorders[8]. NMDAR overactivity is the proposed underlying mechanism in epilepsy, dementia, and stroke, whereas decreased NMDAR activity results in symptoms of schizophrenia[9]. The antibodies in patients with anti-NMDAR encephalitis lead to selective and reversible loss of cell-surface NMDARs by capping and internalization, resulting in abrogation of NMDAR-mediated synaptic function, which can cause patients' symptoms, such as psychotic behavior, signs of involvement of dopaminergic pathways (rigidity, dystonia, orofacial movements, tremor) and autonomic dysfunction (cardiac dysrhythmia, hypertension, hypersalivation)[10,11].
Anti-NMDAR encephalitis is the most common cause of treatable autoimmune diseases and is characterized by prominent neuropsychiatric symptoms[12]. The clinical symptoms of the disease are mainly classified into eight groups: Psychiatric and behavioral symptoms, seizures, motor dysfunctions/involuntary movements, memory deficits, speech disorders, decreased levels of consciousness, autonomic dysfunctions and central hypoventilation[13]. Symptom presentations vary between children and adults; neurologic symptoms occur more often in children, while psychiatric symptoms are prevalent in adults[14], but in most cases, the progression of symptoms evolves toward a similar syndrome in days or weeks[6].
According to literature evaluations, over half of the patients with anti-NMDAR encephalitis had abnormal speech, including reduced verbal output or mutism, abnormal content, mumbling, echolalia, increased output or perseveration[15,16]. Speech dysfunction is one of the common symptoms of anti-NMDAR encephalitis but is frequently described as a symptom secondary to psychiatric symptoms or seizures rather than the main or initial symptom[17]. To our knowledge, paroxysmal speech disturbance as the first presentation has rarely been reported in anti-NMDAR encephalitis. Finke et al[18] described a patient with anti-NMDAR encephalitis who presented with recurrent aphasia. Episodes were accompanied by headache, hemianopia, and hemiparesis with pleocytosis, mimicking the syndrome of headache with neurological deficits and CSF lymphocytosis (Table 1). The patient in our report had recurrent speech dysfunction at onset but without typical psychiatric symptoms or movement disorders. Therefore, he was originally misdiagnosed with TIA and then with cerebral infarction due to his symptom lasting for more than one hour. Since brain MRI showed no structural abnormalities, we considered that paroxysmal speech disorder might be a form of seizure. However, subsequent EEG recorded no epileptic discharges. The hypothesis was disproved.
| Item | The present case | Finke et al[18] |
| Age (yr) | 39 | 67 |
| Gender | Male | Male |
| History of past illness | No | Migraine with aura |
| Vascular risk factors | No | No |
| Initial paroxysmal symptoms | Nonfluent aphasia | Right homonymous hemianopia, global aphasia and right hemiparesis |
| Accompanying symptoms | Generalized tonic-clonic seizures | Throbbing bilateral headaches, confusion and agitation |
| CSF analysis | Mild pleocytosis (28 cells/μL) dominated by lymphocytes (85%) and elevated protein (662 mg/L) | Lymphocytic pleocytosis (95 cells/mL) with few activated lymphocytes and plasma cells and elevated protein (96 mg/dL) |
| Brain MRI | No lesions | Mild frontoparietal microangiopathic leucoencephalopathy |
| EEG | No epileptic discharges | First: Moderate generalized slowing; r: Normal |
| Tumor screening | Negative | Negative |
| Testing for anti-NMDAR antibodies | IgG NMDAR antibodies in both CSF (titer, 1:10) and serum (titer, 1:32) | IgG NMDAR antibodies in CSF (titer, 1:32), but not serum |
| Treatment | Intravenous immunoglobulin and methylprednisolone, followed by oral methylprednisolone | Oral corticosteroids and plasma exchange, followed by azathioprine |
| Outcome | Asymptomatic | No further episodes occurred, but verbal long-term memory deficit persisted |
This case deserves our attention as we consider the diagnostic and treatment options. First, this patient was a young adult with no risk factors for cerebrovascular disease (e.g., hypertension, diabetes, cardiopathy, etc.) and no abnormalities on brain CTA. Furthermore, this patient had a headache a month before onset, which is exactly one of the common prodromal symptoms of anti-NMDAR encephalitis. Finally, the patient had a seizure during the visit, which is seldom observed in ischemic stroke but is a common symptom of anti-NMDAR encephalitis.
To date, the pathophysiological mechanism of speech impairments caused by anti-NMDAR encephalitis remains unclear. Hébert et al[17] reported a case of adult-onset anti-NMDAR encephalitis presenting primarily as progressive nonfluent aphasia. The patient's EEG showed the left frontotemporal slow wave activity, suggesting that the function of left frontal and opercular structures might be affected. Constantinides et al[19] described a case of an adult patient with anti-NMDAR encephalitis presenting with isolated, abrupt-onset aphasia. The patient's EEG revealed paroxysmal left temporal theta and delta waves. Deiva et al[20] reported a child with anti-NMDAR encephalitis who presented sudden and isolated Broca's aphasia following partial seizures and whose sleep EEG showed a repetitive pattern of focal theta rhythms spreading through the left hemisphere(Table 2). Similar electrical patterns were also described in previous anti-NMDAR encephalitis studies[21,22]. These studies suggested that these patterns did not necessarily correlate with seizures[17,21] but were probably the result of an increased frontotemporal-to-occipital gradient in cerebral glucose metabolism due to impaired NMDAR function[22]. Unfortunately, similar EEG abnormalities were not found in our case. However, the EEGs of the reported cases of anti-NMDAR encephalitis with aphasia provided neurophysiological evidence of left focal cortical dysfunction. Finke et al[18] speculated that cortical spreading depression (CSD) might be related to the patient's transient neurological symptoms. According to their hypothesis, CSD can be experimentally induced by glutamate, and it is assumed that an antibody-mediated decrease in NMDAR leads to increased glutamatergic activity by inactivating GABAergic neurons[10,18].
| Item | Constantinides et al[19] | Hébert et al[17] | Deiva et al[20] |
| Age (yr) | 29 | 29 | 4 |
| Gender | Female | Female | Female |
| Presenting symptoms | Isolated, abrupt-onset aphasia | A progressive nonfluent aphasia; simple partial seizures; confusion and emotional lability | Fever; repeated right partial motor seizures; sudden and isolated Broca's aphasia |
| Description of language difficulties | With a 6-mo history of aphasia; her prominent impairment, namely, non-fluent aphasic disturbances (effortful, halting speech with sound errors), had progressed rapidly and reached a peak in 72 h, at which point she was unable to speak and had difficulties in writing, but her ability to perceive verbal stimuli was relatively preserved | 6-d history of progressive word-finding difficulties | The patient suddenly presented isolated speech difficulties; speech evaluation showed that her receptive language was preserved but that expressive language was affected associated with anomia, and anarthria suggestive of Broca's aphasia |
| EEG | Paroxysmal left temporal theta and delta waves | Abundant intermittent polymorphic slow wave activity over the left lateral frontotemporal area | Waking EEG was characterized by unilateral left hemispheric slowing, and sleep EEG showed a repetitive pattern of focal theta rhythms over 10-15 s in the postero-temporal region which then spread to the whole left hemisphere for 45-60 s |
| Brain MRI | Normal | Normal | Normal |
| CSF analysis | Within normal limits (3 white blood cells × 106/L, protein 420 g/L), with negative cytology | Within normal limits (2 white blood cells × 106/L, 95% lymphocytes, protein 0.20 g/L, glucose 3.7 mmol/L) with normal cytology | 19 leukocytes, with 0.22 g/L of protein and no oligoclonal bands |
| Testing for anti-NMDAR antibodies | Positive in both serum and CSF | Positive in CSF | Positive (1:100) in both serum and CSF |
| Screening for ovarian teratoma | Negative | A 5.3 cm right adnexal cystic teratoma (confirmed by pathology) | Negative |
| Immunotherapy | A 5-d course of intravenous methylprednisolone 1 g/d, followed by slowly tapered oral methylprednisolone 1 mg/kg per day; six courses of plasmapheresis; azathioprine 50 mg bid | A 2d course of 2 mg/kg intravenous immunoglobulin | Intravenous rituximab (375 mg/m2) |
| Prognosis | Aphasia eventually resolved at the 1 yr follow-up | 10 mo after symptom onset, her language impairments completely resolved, but she had impaired recollection of the events surrounding her hospitalization | After 20 mo of follow-up, the child had completely recovered and was free of seizures |
From previous observations, approximately 90% of anti-NMDAR encephalitis patients had at least four symptoms by the fourth week of disease onset[6], and mono- or oligosymptomatic presentations of anti-NMDAR encephalitis were rare[6,10]. The atypical manifestations of our case might be due to early initiation of immunotherapy, which prevented the development of the complete clinical phenotype of anti-NMDAR encephalitis[18].
On physical examination, the patient's tongue deviated to the right when he was asked to extend it. As no involuntary movements of the patient's jaw, mouth, tongue, or lower face were observed, the phenomenon was thought to be functional or central hypoglossal palsy rather than oromandibular dystonia or orofacial dyskinesia.
No lesion was found on our patient's brain MRI scan. A systematic review indicated that MRI scans showed abnormal findings in less than 50% of patients with anti-NMDAR encephalitis[23]. The CSF test revealed a slight increase in cell count and protein. Dalmau et al[11] reported that 95% of patients had CSF abnormalities, 91% had a mild-to-moderate lymphocytic increase, and 32% had a mildly elevated level of protein. Anti-NMDAR encephalitis was diagnosed based on the clinical manifestations, evidence of CSF, brain MRI, EEG and the antibodies against the NR1 subunit of the NMDAR in CSF and/or serum[13]. Speech impairment and seizures, as well as positive anti-NMDAR IgG antibodies in CSF and serum, led to the patient's ultimate diagnosis. Despite the severity of anti-NMDAR encephalitis, patients tend to have a good prognosis after immunotherapy[6]. Our patient made a full recovery after intravenous immunoglobulin and steroid administration. Therefore, early diagnosis and immunotherapy are important to patients with anti-NMDAR encephalitis.
The present report describes a young patient with a peculiar initial manifestation, and it demonstrates that a patient with anti-NMDAR encephalitis can present solely paroxysmal speech dysfunction with no additional symptoms of limbic encephalitis at onset. This case may also help us to further understand the manifestation of speech dysfunction in patients with anti-NMDAR encephalitis. The likelihood of anti-NMDAR encephalitis should be considered in a young adult with paroxysmal speech disorder and clinical features of limbic encephalitis, such as psychiatric disorders, seizures, and cognitive impairment.
This case suggests that paroxysmal speech disorder may be the first symptom of anti-NMDAR encephalitis in a young patient without risk factors for cerebrovascular disease. Recognizing that is vital to early diagnosis and more timely treatment for future cases.
We sincerely thank the patient involved in this paper.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Huan C, United States; Kreisel W, Germany; Pitton Rissardo J, Brazil; Ullah K, Pakistan S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Vitaliani R, Mason W, Ances B, Zwerdling T, Jiang Z, Dalmau J. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 386] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 2. | Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2002] [Cited by in RCA: 1726] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 3. | Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, Cunningham R, Zuckerman M, Mutton KJ, Solomon T, Ward KN, Lunn MP, Irani SR, Vincent A, Brown DW, Crowcroft NS; UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1053] [Cited by in RCA: 873] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 4. | Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis. 2012;54:899-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 516] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 5. | Lu J, Zhang JH, Miao AL, Yin JX, Zhu DL, Lin XJ, Chen DW, Shi JP. Brain astrocytoma misdiagnosed as anti-NMDAR encephalitis: a case report. BMC Neurol. 2019;19:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I, Martinez-Hernandez E, Aguilar E, Gresa-Arribas N, Ryan-Florance N, Torrents A, Saiz A, Rosenfeld MR, Balice-Gordon R, Graus F, Dalmau J. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 2136] [Article Influence: 178.0] [Reference Citation Analysis (0)] |
| 7. | Goldsmith PJ. NMDAR PAMs: Multiple Chemotypes for Multiple Binding Sites. Curr Top Med Chem. 2019;19:2239-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Hardingham G. NMDA receptor C-terminal signaling in development, plasticity, and disease. F1000Res. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 335] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 9. | Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 639] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 10. | Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1879] [Cited by in RCA: 1652] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 11. | Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R, Lynch DR. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091-1098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2443] [Cited by in RCA: 2158] [Article Influence: 126.9] [Reference Citation Analysis (0)] |
| 12. | Huang Q, Xie Y, Hu Z, Tang X. Anti-N-methyl-D-aspartate receptor encephalitis: A review of pathogenic mechanisms, treatment, prognosis. Brain Res. 2020;1727:146549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Liu CY, Zhu J, Zheng XY, Ma C, Wang X. Anti-N-Methyl-D-aspartate Receptor Encephalitis: A Severe, Potentially Reversible Autoimmune Encephalitis. Mediators Inflamm. 2017;2017:6361479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Maggio MC, Mastrangelo G, Skabar A, Ventura A, Carrozzi M, Santangelo G, Vanadia F, Corsello G, Cimaz R. Atypical presentation of anti-N-methyl-D-aspartate receptor encephalitis: two case reports. J Med Case Rep. 2017;11:225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Warren N, Siskind D, O'Gorman C. Refining the psychiatric syndrome of anti-N-methyl-d-aspartate receptor encephalitis. Acta Psychiatr Scand. 2018;138:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 16. | Espinola-Nadurille M, Bustamante-Gomez P, Ramirez-Bermudez J, Bayliss L, Rivas-Alonso V, Flores-Rivera J. Frequency of neuropsychiatric disturbances in anti-NMDA receptor encephalitis. Acta Psychiatr Scand. 2018;138:483-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Hébert J, El-Sadi F, Maurice C, Wennberg RA, Tang-Wai DF. Adult-Onset Anti-N-methyl-D-aspartate-receptor Encephalitis Presenting as a Non-Fluent Aphasia. Can J Neurol Sci. 2018;45:248-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Finke C, Mengel A, Prüss H, Stöcker W, Meisel A, Ruprecht K. Anti-NMDAR encephalitis mimicking HaNDL syndrome. Cephalalgia. 2014;34:1012-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Constantinides VC, Kasselimis DS, Paraskevas GP, Zacharopoulou M, Andreadou E, Evangelopoulos ME, Kapaki E, Kilidireas C, Stamboulis E, Potagas C. Anti-NMDA receptor encephalitis presenting as isolated aphasia in an adult. Neurocase. 2018;24:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Deiva K, Pera MC, Maurey H, Chrétien P, Archambaud F, Bouilleret V, Tardieu M. Sudden and isolated Broca's aphasia: a new clinical phenotype of anti NMDA receptor antibodies encephalitis in children. Eur J Paediatr Neurol. 2014;18:790-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Gitiaux C, Simonnet H, Eisermann M, Leunen D, Dulac O, Nabbout R, Chevignard M, Honnorat J, Gataullina S, Musset L, Scalais E, Gauthier A, Hully M, Boddaert N, Kuchenbuch M, Desguerre I, Kaminska A. Early electro-clinical features may contribute to diagnosis of the anti-NMDA receptor encephalitis in children. Clin Neurophysiol. 2013;124:2354-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Leypoldt F, Buchert R, Kleiter I, Marienhagen J, Gelderblom M, Magnus T, Dalmau J, Gerloff C, Lewerenz J. Fluorodeoxyglucose positron emission tomography in anti-N-methyl-D-aspartate receptor encephalitis: distinct pattern of disease. J Neurol Neurosurg Psychiatry. 2012;83:681-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 23. | Bacchi S, Franke K, Wewegama D, Needham E, Patel S, Menon D. Magnetic resonance imaging and positron emission tomography in anti-NMDA receptor encephalitis: A systematic review. J Clin Neurosci. 2018;52:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |