Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8568
Peer-review started: February 26, 2022
First decision: May 11, 2022
Revised: May 13, 2022
Accepted: July 22, 2022
Article in press: July 22, 2022
Published online: August 26, 2022
Processing time: 170 Days and 12.3 Hours
Pancreatic segmental portal hypertension (PSPH) is the only type of portal hypertension that can be completely cured. However, it can easily cause varicose veins in the esophagus and stomach and hemorrhage in the digestive tract.
To explore the application of computed tomography (CT) to examine the characteristics of PSPH and assess the risk level.
This was a retrospective analysis of CT images of 22 patients diagnosed with PSPH at our center. Spearman correlation analysis was performed using the range of esophageal and gastric varices (measured by the vertical gastric wall), the ratio of the width of the splenic portal vein to that of the compression site (S/C ratio), the degree of splenomegaly, and the stage determined by gastroscopy. This study examined whether patients experienced gastrointestinal bleeding within 2 wk and combined CT and gastroscopy to explore the connection between bleeding and CT findings.
The range of esophageal and gastric varices showed the best correlation in the diagnosis of PSPH (P < 0.001), and the S/C ratio (P = 0.007) was correlated with the degree of splenomegaly (P = 0.021) and PSPH (P < 0.05). This study revealed that male patients were more likely than females to progress to grade 2 or grade 3 as determined by gastroscopy. CT demonstrated excellent performance, with an area under the curve of 0.879.
CT can be used to effectively analyze the imaging signs of PSPH, and CT combined with gastro
Core Tip: This is a detailed clinical imaging study (computed tomography, CT) of pancreatic segmental portal hypertension (PSPH), the only curable form of portal hypertension. CT is of great significance in the diagnosis and treatment of PSPH.
- Citation: Wang YL, Zhang HW, Lin F. Computed tomography combined with gastroscopy for assessment of pancreatic segmental portal hypertension. World J Clin Cases 2022; 10(24): 8568-8577
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8568.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8568
Pancreatic segmental portal hypertension (PSPH) is a rare regional condition, accounting for only 8% of extrahepatic portal hypertension cases[1]. PSPH is usually caused by pancreatic tumors, pancreatitis, and IgG4-related diseases that cause pancreatic vein compression. Clinically, it manifests as regional portal hypertension on the left side; usually, the right portal system is not significantly affected[2]. At the same time, it is now the only curable form of portal hypertension. However, if PSPH is not detected early, continuous compression of the pancreatic veins will cause esophageal and gastric varices, which may lead to hemorrhage in the gastrointestinal tract. In severe cases, hemorrhagic shock or death may occur. Therefore, it is essential to diagnose PSPH as soon as possible and assess whether there is a tendency for gastrointestinal bleeding[3].
Because other underlying diseases of the pancreas usually cause this type of disease, it can generally be cured by removing the underlying disease[4,5]. This approach ignores the risk of gastrointestinal bleeding in this type of patient. Clinicians usually only focus on curing the underlying disease, and iatrogenic secondary injuries after tumor resection or pancreatitis treatment may also cause a continuous increase in the pressure of the already narrowed pancreatic veins, leading to an increased risk of gastrointestinal bleeding[6-8]. At the same time, splenomegaly or hypersplenism caused by PSPH can also cause increased blood cell destruction and decreased resistance. Therefore, this disease is receiving increasing attention in clinical practice[9].
The diagnostic criteria for this type of disease are as follows: (1) A history of primary pancreatic disease; (2) No history of liver cirrhosis, blood system disease, or schistosomiasis disease and normal liver function; (3) Splenomegaly or hypersplenism; (4) Doppler ultrasound results showing that the portal vein is not wide [only the splenic vein is obstructed with or without widening of the splenic portal vein, and there are regional varicose veins on the left side (usually esophageal and gastric varices)]; and (5) varicose vascular masses observable under gastroscopy. PSPH can be diagnosed when three of the above five criteria are satisfied[10]. Among the current diagnostic criteria, criteria (1) and (2) are met by almost all diagnosed patients. However, splenomegaly does not appear in all patients. Gastroscopy can only elucidate varicose veins that have compressed the fundus or esophagus. Pancreatic effusion or gas will also obscure the site analyzed by Doppler ultrasound. Therefore, the diagnosis of varicose veins under gastroscopy combined with the clinical history can usually be used to diagnose the disease.
Due to the relatively rare nature of this type of disease, clear computed tomography (CT) findings of this type of disease have not yet been determined. However, CT has apparent advantages over ultrasound and gastroscopy. It is not affected by gas and can reflect and measure deeper varicose veins. In addition, contrast enhanced CT during the portal phase can also facilitate an excellent preliminary judgment regarding stenosis of the splenic portal vein. It can also be used to evaluate enlargement of the spleen. Moreover, CT is more convenient than digital subtraction angiography and can reflect the initial vascular condition[11]. In this study, CT signs were combined with the manifestations of gastric fundal and esophageal varices to explore the appearance of PSPH on CT and the risk of gastrointestinal bleeding.
CT images of 22 patients who had clinically confirmed PSPH from 2007 to 2021 at our center were collected. Among them, 14 were men and 8 were women, aged 26-69 years old. These patients had a history of pancreatic or pancreatic area disease and had complete gastroscopic image data. The current study was approved by the Institutional Review Board, and the requirement for informed consent was waived (Clinical Research Ethics Committee of Shenzhen Second People's Hospital, approval ID: 20211108009).
All patients were examined using a 32-section CT system (Siemens Healthcare Sector, Germany). Before the CT examination, the patient fasted for 4–6 h without intramuscular antispasmodic drug injection or oral contrast agent administration. The scanning range was from the top of the diaphragm to the mid-abdominal level. The scanning parameters used were as follows: Collimator width, 64 mm × 0.625 mm; tube voltage, 100 kVp; tube current, automatically controlled at 85-150 mA; and X-ray tube rotation speed, 0.5 s/cycle. The reconstruction layer thickness was 2.0 mm, and the layer spacing was 0 mm. The enhanced scan was performed using contrast agent intelligent tracking threshold trigger technology. The trigger point was set in the abdominal aortic lumen at the celiac trunk. The trigger threshold was 120 HU. The venous phase started to be collected 30 s after the end of the arterial-phase scan. Iopromide (iodine concentration, 350 mg/mL) was used as the contrast agent at a dose of 1.2 mL/kg, and the injection rate was 3.0 mL/s.
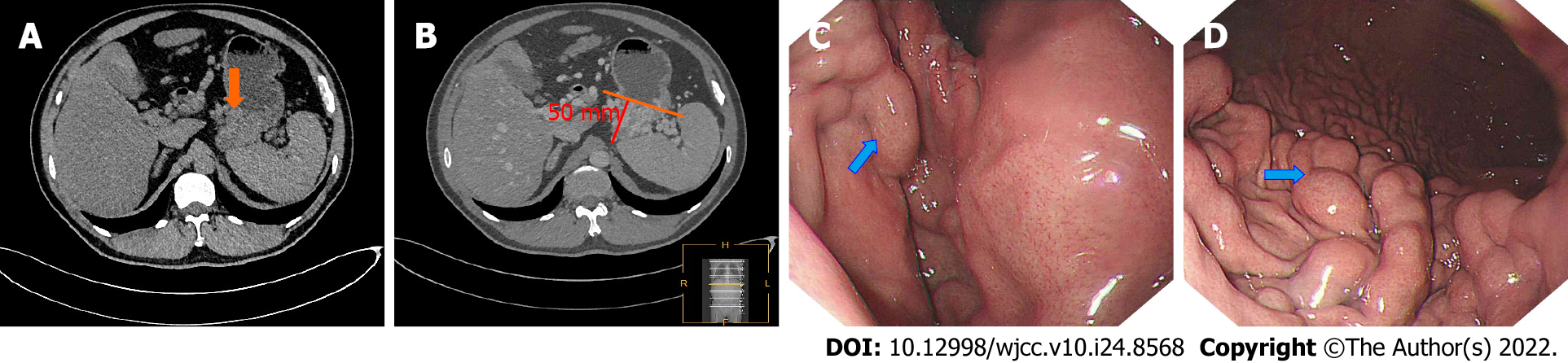
Because the portal phase can better reflect the pancreatic veins, the enhanced portal phase of the CT images of the above PSPH patients was analyzed. Three senior doctors interpreted the images and measured the diameter of the splenic vein in the compressed area and the diameter of the splenic vein at the splenic hilum. The rib unit was measured at the largest level of the spleen, and the largest side of the varicose vein was measured. The vertical distance from the outermost edge to the stomach wall was taken as the range of varicose veins (Figure 1, patient 1). To reduce differences between men and women and individual patients, the splenic meridian was not directly measured; instead, the number of rib units was used. The degree of vascular stenosis was determined by the ratio of the diameter of the splenic portal vein to that at the site of splenic vein compression (S/C).
At the same time, we combined the findings from the abovementioned analyses with those of the gastroscopy report from the Department of Gastroscopy to classify these PSPH patients with esophageal varices by gastroscopy, as follows: Grade 1: The varicose veins are straight or slightly tortuous, and there is no "red sign"; grade 2: The veins are straight or somewhat tortuous with the red sign or exhibit a snake-like tortuousness without the red sign; and grade 3 (G3): The varicose veins are serpentine with the red sign or the vascular abnormalities are beaded and nodular and exhibit a tumor-like bulge accompanied (or not) by the red sign. The red sign represents a high risk of bleeding in the digestive tract[12]. The patients were followed, and whether bleeding occurred within 2 wk after the scan was recorded.
The above patient clinical information and imaging measurement information are presented in Table 1. SPSS 19.0 (SPSS v. 19, Chicago, Illinois) was used for statistical analyses. Because the data did not conform to a normal distribution, the Spearman test combined with the stage diagnosed by gastroscopy were employed to compare and test various measurement parameters. P < 0.05 indicated statistical significance. All statistical graphs were created using GraphPad Prism 7 (Prism 7, La Jolla, California).
| Patient ID (n = 22) | Sex | Age | Clinical history | Splenic portal vein width (mm) | Compression area vein width (mm) | S/C ratio | Varicose vein range (mm) | Rib unit | Gastroscopy grade | Gastrointestinal bleeding within 2 wk |
| Patient 1 | Male | 26 | CP | 6 | 2 | 3 | 50 | 7 | G3 | Yes |
| Patient 2 | Male | 58 | AP | 6 | 2.5 | 2.4 | 36 | 9 | G2 | No |
| Patient 3 | Male | 57 | CP | 4.5 | 2 | 2.25 | 36 | 7.5 | G3 | Yes |
| Patient 4 | Male | 53 | PTC | 6 | 3 | 2 | 37 | 7.5 | G3 | Yes |
| Patient 5 | Male | 63 | PTC | 4 | 3 | 1.333 | 21 | 5 | G1 | No |
| Patient 6 | Male | 34 | CP | 6.5 | 4.5 | 1.444 | 24 | 5.5 | G2 | Yes |
| Patient 7 | Female | 67 | PBC | 4 | 4 | 1 | 10 | 7 | G1 | No |
| Patient 8 | Male | 69 | PTC | 5 | 6 | 0.833 | 16 | 6 | G2 | No |
| Patient 9 | Female | 56 | PBC | 5 | 2 | 2.5 | 5 | 5 | G1 | No |
| Patient 10 | Male | 65 | PHC | 6 | 4 | 1.5 | 11 | 8 | G2 | No |
| Patient 11 | Female | 67 | PHC | 4 | 3 | 1.333 | 5 | 5 | G1 | No |
| Patient 12 | Female | 60 | PHC | 5 | 3 | 1.667 | 26 | 8 | G2 | No |
| Patient 13 | Female | 55 | PTC | 6 | 3 | 2 | 7 | 6 | G1 | No |
| Patient 14 | Male | 63 | PTC | 8 | 2 | 4 | 25 | 7 | G2 | No |
| Patient 15 | Female | 40 | PTC | 6 | 5 | 1.2 | 6 | 8 | G1 | No |
| Patient 16 | Male | 27 | CP | 7 | 4 | 1.75 | 28 | 8 | G2 | No |
| Patient 17 | Female | 66 | PHC | 7 | 11 | 0.636 | 2 | 7 | G1 | No |
| Patient 18 | Male | 68 | PBC | 5 | 3 | 1.667 | 16 | 7 | G2 | No |
| Patient 19 | Male | 50 | PHC | 6 | 2 | 3 | 33 | 7 | G2 | Yes |
| Patient 20 | Female | 52 | PBC | 6 | 2 | 3 | 15 | 7 | G1 | Yes |
| Patient 21 | Male | 38 | CP | 7 | 1.5 | 4.667 | 38 | 7 | G3 | Yes |
| Patient 22 | Male | 58 | IgG4-P | 7 | 2 | 3.5 | 32 | 8 | G3 | Yes |
According to whether bleeding occurred within 2 wk, the patients were divided into a bleeding group (n = 8) and a nonbleeding group (n = 14). The Mann–Whitney U test was used to compare continuous variables between the bleeding and nonbleeding groups. A P value < 0.05 was considered statistically significant. All patients' continuous and categorical variables are represented as the mean ± SD and n (%), respectively. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic capability of CT, including calculation of the area under the curve (AUC) and 95% confidence interval.
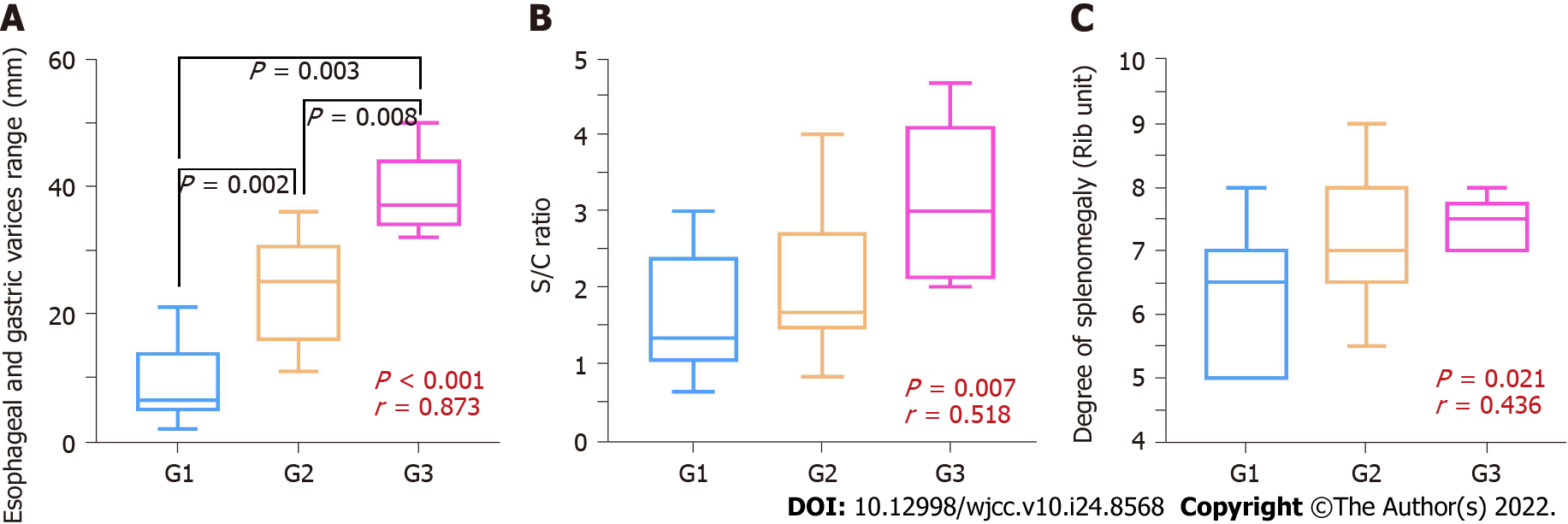
The results of Spearman's test are shown in Table 2. The parameters with the correlation coefficient from high to low were the range of varicose veins (correlation coefficient = 0.873, P < 0.001), S/C ratio (correlation coefficient = 0.518, P = 0.007), and number of rib units (correlation coefficient = 0.436, P = 0.021). The range of varicose veins showed the strongest correlation with the gastroscopic grade. In addition to the main measurement parameters, the width of the compression site also showed a statistically significant (P = 0.031) negative correlation; that is, the narrower the width, the higher the grade of esophageal varices. Figure 2 shows a box diagram with the three main measurement value distributions at different levels under gastroscopy.
| Sex | Age | Splenic portal vein width | Compression area vein width | S/C ratio | Varicose vein range | Rib unit | |
| Correlation coefficient | 0.757 | -0.296 | 0.330 | -0.405 | 0.518 | 0.873 | 0.436 |
| P value | < 0.001 | 0.091 | 0.067 | 0.031 | 0.007 | < 0.001 | 0.021 |
Surprisingly, the results also showed that the severity of esophageal varices in male patients with PSPH was usually higher than that in female patients, which had a statistically significant difference (P < 0.001). However, there was no statistically significant difference based on patient age.
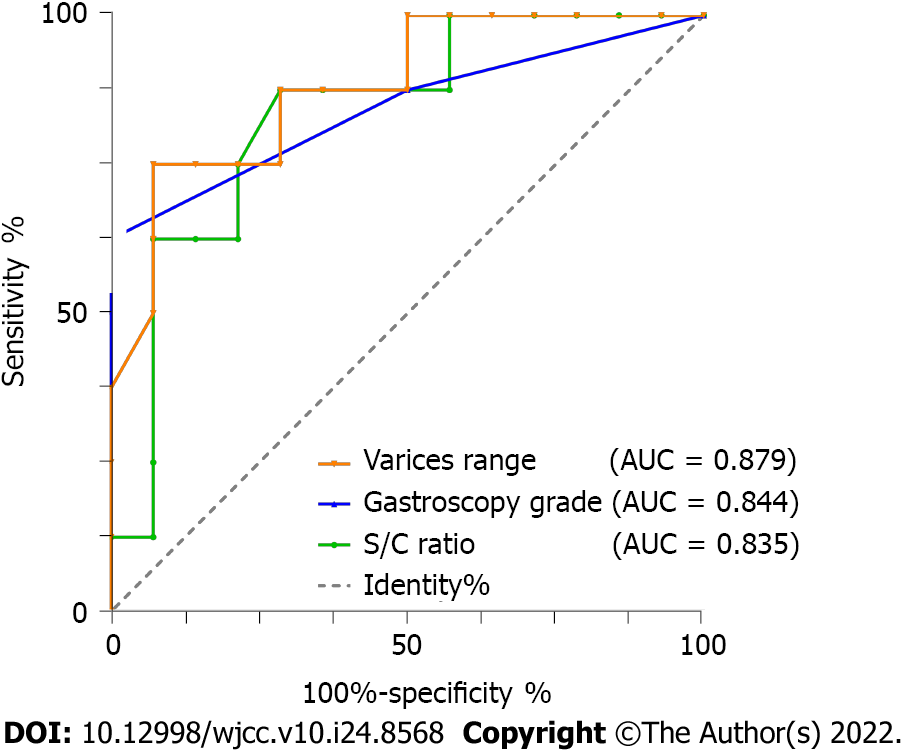
The Mann–Whitney U test showed that the S/C ratio, range of varicose veins, and gastroscopic grade were significant independent predictors of bleeding (Table 3). The ROC curve analysis showed excellent performance, with an AUC of 0.879 for the varicose vein range and 0.844 for the gastroscopic grade (Figure 3).
| Variable | Group | P value | |
| Bleeding (n = 8) | Non-bleeding (n = 14) | ||
| Clinical characteristics | |||
| Age, years | 46.000 ± 11.796 | 58.860 ± 11.890 | 0.024 |
| Gender | 0.079 | ||
| Male | 7 (50%) | 7 (50%) | |
| Female | 1 (12.5%) | 7 (87.5%) | |
| Gastroscopic grade | 0.003 | ||
| G1 | 1 (12.5%) | 7 (87.5%) | |
| G2 | 2 (22.2%) | 7 (77.8%) | |
| G3 | 5 (100%) | 0 (0%) | |
| CT measurement value | |||
| Splenic portal vein width (mm) | 6.571 ± 0.790 | 5.571 ± 1.223 | 0.245 |
| Compression area vein width (mm) | 2.375 ± 0.954 | 3.964 ± 2.308 | 0.074 |
| S/C ratio | 2.858 ± 0.989 | 1.701 ± 0.852 | 0.011 |
| Varicose vein range (mm) | 33.125 ± 10.316 | 15.286 ± 10.462 | 0.002 |
| Rib unit | 7.063 ± 0.729 | 6.857 ± 1.292 | 0.668 |
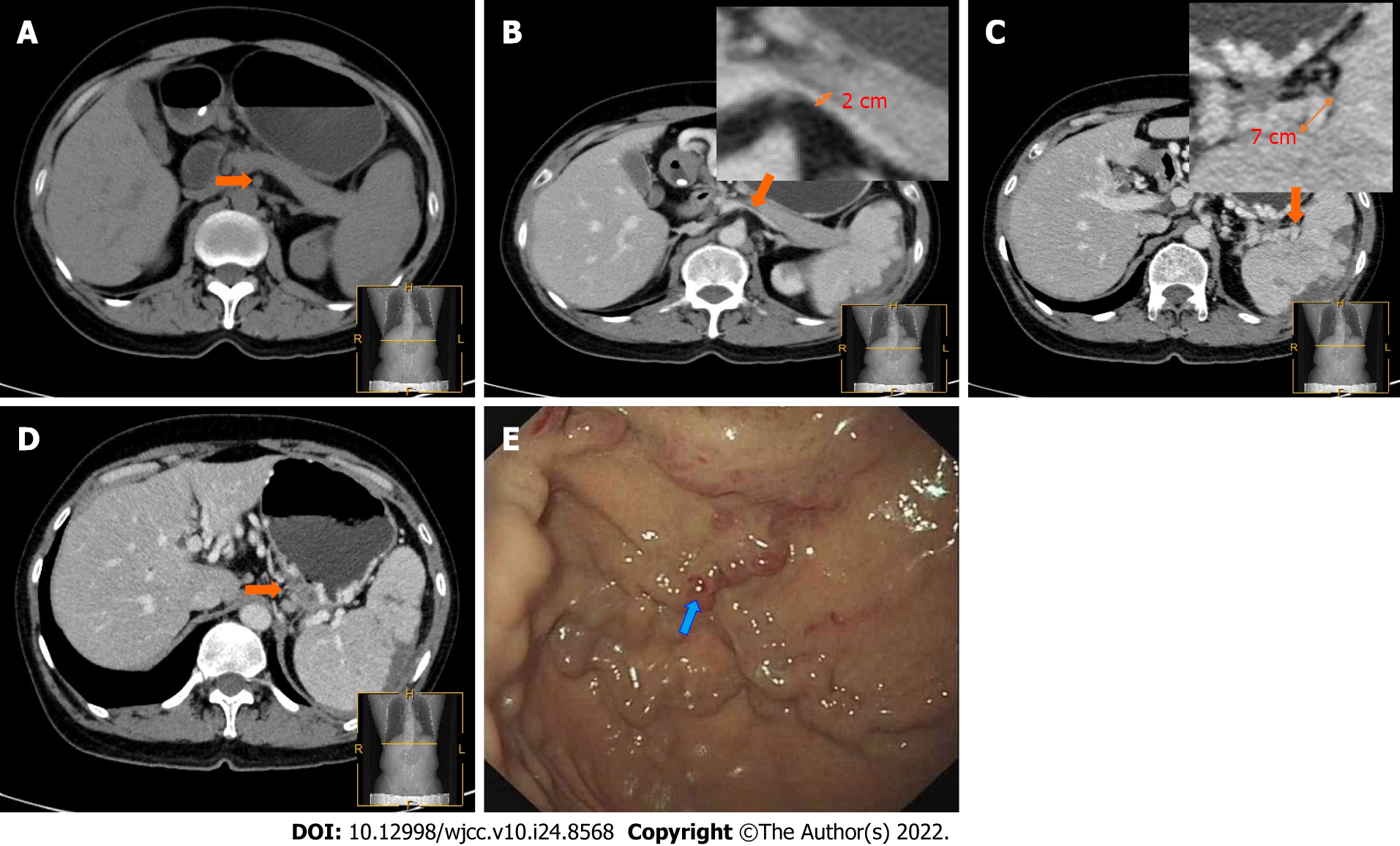
PSPH, as a rare type of portal hypertension, has vital clinical significance. Nevertheless, it is easy to ignore the existence of this disease because of the excessive emphasis on the original condition in diagnostic images[13]. However, gastroscopy usually delays the diagnosis of such diseases when the imaging findings are ignored, for example, as occurred in the patient (Figure 4) who had IgG4-related pancreatitis. Gastroscopy was only used 4 d after admission, and the typical "red sign" had already appeared. The patient developed massive gastrointestinal bleeding within 24 h. Although he was rescued in time, the prognosis was poor. While paying attention to the primary disease, physicians also need to evaluate whether the patient has excessive portal hypertension and the degree of splenic vein compression in the pancreatic area[14].
This study found that the degree of varicose veins under gastroscopy correlated with the maximum vertical measurement of esophageal varices under CT. The S/C ratio was also positively associated with the size of the spleen. Initially, we had various ideas for measuring the meridian, such as a second graded measurement of the varicose veins. In addition to calculating the vertical meridian of the largest plane, judgment of the density of varicose veins (through the number of cross-sections of the vessel on this plane) can be performed. However, such measurements have certain drawbacks. In some patients, although the degree of varicosity may be more significant, the blood vessel diameter may be smaller; other patients may show a more substantial degree of varicosity, but the vessel diameter may be larger. This results in the inability to perform practical grading measurements on patients, and the clinical operation is more complicated, requiring much more time for evaluation. Thus, this situation may be dangerous for emergency patients[15].
Compared with the range of varicose vessels, the S/C ratio shows a specific correlation. However, if the splenic vein is significantly compressed and interrupted, this measurement method will be difficult to perform. If enhanced CT can indicate the interruption point of the splenic vein in the portal phase, targeted diagnosis and treatment will be necessary to relieve the compression of the lesion. Although the size of the spleen is related to a certain degree, it is greatly affected by time. Splenic enlargement can also be resolved after relieving compression to avoid resection[16]. The early stage of PSPH usually manifests as esophageal and gastric varices. Additionally, this study found a higher grade of varicose veins in male patients than female patients. This may be related to men's alcohol consumption and women's sensitivity to pain, which allows early detection. This view has been confirmed in a study of acute pancreatitis[17,18].
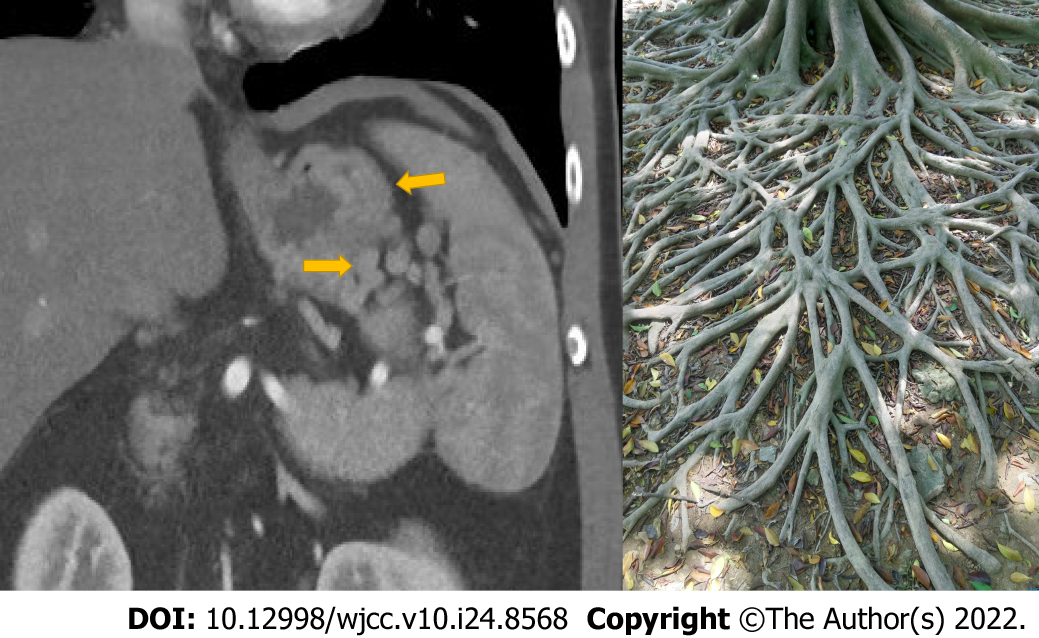
All patients in this study had underlying pancreatic disease. Vigilance regarding the occurrence of PSPH is required in such patients. Most studies regarding PSPH assessed by CT are rare case reports, and there has been no detailed research or consensus yet[19]. CT studies by Swan Specchi and Giovanna Bertolini in animal models have shown that esophageal variances often occur in the collateral circulation established by esophageal fundus veins, such as the left gastric vein and the left gastroepiploic vein, which CT can better visualize to show the collateral circulation in PSPH[20]. We recommend that for patients with underlying pancreatic disease, plain and enhanced CT scans should be used to make a preliminary judgment of the PSPH severity. We propose the use of the “banyan tree root” sign (Figure 5) to identify high-risk PSPH. When there is a banyan tree root-like change, clinicians should be highly vigilant regarding the risk of gastrointestinal bleeding; such patients need to undergo emergency gastroscopy to prevent a poor prognosis.
This study found that CT is a powerful supplement to gastroscopy. Although CT and gastroscopy alone provide excellent results when applied to detect PSPH-related bleeding, it is necessary to be alert to the occurrence of exceptional circumstances. For example, if the scope of gastric esophageal varices is enormous but the gastroscopic grade has not yet reached G3, the physician should still be alert for gastrointestinal bleeding. In patients with abdominal pain after pancreatic disease, even conventional CT scans can initially be used to determine whether there are esophageal varices in the fundus of the stomach. Enhanced CT can better assess the location and extent of stenosis. At the same time, gastroscopy is an invasive examination, and CT examination can be more convenient for judging the prognosis of pancreatic patients.
PSPH is relatively rare in clinical practice, and the number of patients included in this study is relatively small, which dramatically restricts the related statistical analysis. Although this study reached a somewhat preliminary conclusion, more cases are required to explore the role of imaging in this type of disease and reach a more accurate conclusion. The clinical judgment of PSPH is usually an exclusive diagnosis; that is, the regional portal hypertension of left-sided portal hypertension is caused only by compression of the pancreatic veins[21]. As PSPH is the only form of portal hypertension that can be cured entirely, excellent attention in clinical and imaging studies is required to better treat and improve the prognosis of PSPH patients.
This study has several limitations: (1) Since this was a small sample study, the results may be greatly influenced by individuals. The inclusion of more samples would further improve the credibility of this study; and (2) With a sufficient number of samples, a more reliable diagnostic model could be established, and a subset of patient data could be entered into the model to verify the model’s reliability.
Measuring gastric and esophageal varices and assessing the degree of splenic vein stenosis at the site of compression on CT combined with gastroscopy can effectively predict bleeding in patients with PSPH. CT is of great significance in the diagnosis and treatment of PSPH.
Pancreatic segmental portal hypertension (PSPH) is the only type of portal hypertension that can be completely cured.
PSPH can easily cause varicose veins in the esophagus and stomach and hemorrhage in the digestive tract.
To explore the application of computed tomography (CT) to examine the characteristics of PSPH and assess the risk level.
This was a retrospective analysis of CT images of 22 patients diagnosed with PSPH at our center. Spearman correlation analysis was performed using the range of esophageal and gastric varices (measured by the vertical gastric wall), the ratio of the width of the splenic portal vein to that of the compression site (S/C ratio), the degree of splenomegaly, and the stage determined by gastroscopy. The study examined whether patients experienced gastrointestinal bleeding within 2 wk and combined CT and gastroscopy to explore the connection between bleeding and CT findings.
The range of esophageal and gastric varices showed the best correlation in the diagnosis of PSPH (P < 0.001), and the S/C ratio (P = 0.007) was correlated with the degree of splenomegaly (P = 0.021) and PSPH (P < 0.05). The study revealed that male patients were more likely than females to progress to grade 2 or grade 3 as determined by gastroscopy. CT imaging demonstrated excellent performance, with an area under the curve of 0.879.
CT can be used to effectively analyze the imaging signs of PSPH, and CT combined with gastroscopy can effectively predict the risk level of gastrointestinal bleeding.
This was a detailed clinical imaging study (computed tomography, CT) of PSPH, the only curable form of portal hypertension. CT is of great significance in diagnosing and treating PSPH.
Thanks to Ms. Si-Ling Gu for her help in this research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Harada K, Japan; Virarkar M, United States S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Thompson RJ, Taylor MA, McKie LD, Diamond T. Sinistral portal hypertension. Ulster Med J. 2006;75:175-177. [PubMed] |
| 2. | Ren S, Zhang J, Chen J, Cui W, Zhao R, Qiu W, Duan S, Chen R, Chen X, Wang Z. Evaluation of Texture Analysis for the Differential Diagnosis of Mass-Forming Pancreatitis From Pancreatic Ductal Adenocarcinoma on Contrast-Enhanced CT Images. Front Oncol. 2019;9:1171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Hakim S, Bortman J, Orosey M, Cappell MS. Case report and systematic literature review of a novel etiology of sinistral portal hypertension presenting with UGI bleeding: Left gastric artery pseudoaneurysm compressing the splenic vein treated by embolization of the pseudoaneurysm. Medicine (Baltimore). 2017;96:e6413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Li ZY, Li B, Wu YL, Xie QP. Acute pancreatitis associated left-sided portal hypertension with severe gastrointestinal bleeding treated by transcatheter splenic artery embolization: a case report and literature review. J Zhejiang Univ Sci B. 2013;14:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Canbak T, Acar A, Kıvanç AE, Başak F, Kulalı F, Baş G. Sinistral Portal Hypertension Due to Pancreatic Hydatid Cyst. Turkiye Parazitol Derg. 2017;41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Shiihara M, Higuchi R, Izumo W, Yazawa T, Uemura S, Furukawa T, Yamamoto M. Retrospective evaluation of risk factors of postoperative varices after pancreaticoduodenectomy with combined portal vein resection. Pancreatology. 2020;20:522-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Strasberg SM, Bhalla S, Sanchez LA, Linehan DC. Pattern of venous collateral development after splenic vein occlusion in an extended Whipple procedure : comparison with collateral vein pattern in cases of sinistral portal hypertension. J Gastrointest Surg. 2011;15:2070-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Di Martino M, de la Hoz Rodríguez A, Real Martínez Y, Martín-Pérez E. Left-sided portal hypertension due to retroperitoneal fibrosis treated with an oesophagus preserving, modified Sugiura procedure. Ann R Coll Surg Engl. 2020;102:e48-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Reddy A, Sanniyasi S, George DJ, Narayanan CD. A rare case report of Solid Pseudopapillary Tumor of the pancreas with portal hypertension. Int J Surg Case Rep. 2016;22:35-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Fernandes A, Almeida N, Ferreira AM, Casela A, Gomes D, Portela F, Camacho E, Sofia C. Left-Sided Portal Hypertension: A Sinister Entity. GE Port J Gastroenterol. 2015;22:234-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Yu D, Li X, Gong J, Li J, Xie F, Hu J. Left-sided portal hypertension caused by peripancreatic lymph node tuberculosis misdiagnosed as pancreatic cancer: a case report and literature review. BMC Gastroenterol. 2020;20:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (16)] |
| 12. | Wang Q, Xiong B, Zheng C, Liang M, Han P. Splenic Arterial Embolization in the Treatment of Severe Portal Hypertension Due to Pancreatic Diseases: The Primary Experience in 14 Patients. Cardiovasc Intervent Radiol. 2016;39:353-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Xie CL, Wu CQ, Chen Y, Chen TW, Xue HD, Jin ZY, Zhang XM. Sinistral Portal Hypertension in Acute Pancreatitis: A Magnetic Resonance Imaging Study. Pancreas. 2019;48:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Mizuno S, Kato H, Yamaue H, Fujii T, Satoi S, Saiura A, Murakami Y, Sho M, Yamamoto M, Isaji S. Left-sided Portal Hypertension After Pancreaticoduodenectomy With Resection of the Portal Vein/Superior Mesenteric Vein Confluence in Patients With Pancreatic Cancer: A Project Study by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. Ann Surg. 2021;274:e36-e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Pereira P, Peixoto A. Left-Sided Portal Hypertension: A Clinical Challenge. GE Port J Gastroenterol. 2015;22:231-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Hayashi H, Shimizu A, Motoyama H, Kubota K, Notake T, Ikehara T, Yasukawa K, Kobayashi A, Soejima Y. Left-sided portal hypertension caused by idiopathic splenic vein stenosis improved by splenectomy: a case report. Surg Case Rep. 2020;6:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Li H, Yang Z, Tian F. Clinical Characteristics and Risk Factors for Sinistral Portal Hypertension Associated with Moderate and Severe Acute Pancreatitis: A Seven-Year Single-Center Retrospective Study. Med Sci Monit. 2019;25:5969-5976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Petrucciani N, Debs T, Rosso E, Addeo P, Antolino L, Magistri P, Gugenheim J, Ben Amor I, Aurello P, D'Angelo F, Nigri G, Di Benedetto F, Iannelli A, Ramacciato G. Left-sided portal hypertension after pancreatoduodenectomy with resection of the portal/superior mesenteric vein confluence. Results of a systematic review. Surgery. 2020;168:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 19. | Patrono D, Benvenga R, Moro F, Rossato D, Romagnoli R, Salizzoni M. Left-sided portal hypertension: Successful management by laparoscopic splenectomy following splenic artery embolization. Int J Surg Case Rep. 2014;5:652-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Specchi S, Bertolini G. CT angiography identifies collaterals in dogs with splenic vein obstruction and presumed regional splenic vein hypertension. Vet Radiol Ultrasound. 2020;61:636-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Beksac K, Karakoc D. Multiple Giant Splenic Artery Aneurysms Causing Sinistral (Left-Sided) Portal Hypertension. Case Rep Gastrointest Med. 2016;2016:6278452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |