Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8525
Peer-review started: February 19, 2022
First decision: April 19, 2022
Revised: May 10, 2022
Accepted: July 16, 2022
Article in press: July 16, 2022
Published online: August 26, 2022
Processing time: 177 Days and 20.7 Hours
Hepatocellular carcinoma (HCC) is the most common type of primary liver malignancy. Contrast-enhanced ultrasound (CEUS) uses contrast microbubbles during ultrasound, allowing the detection and characterization of malignant focal liver lesions with much higher diagnostic accuracy than conventional ultrasound; however, there are few reports focusing on the pattern of enhancement of CEUS for the diagnosis of HCC smaller than 2 cm.
To investigate the clinical value of CEUS in the early detection of small HCC with high risk factors.
A total of 395 patients with 632 nodules at high risk of HCC, who underwent regular follow-up at Xuhui Dahua Hospital from January 2007 to December 2021, were retrospectively examined. Conventional ultrasonography combined with CEUS was adopted to analyze the echo, size, location, and enhancement characteristics of benign and malignant nodules, as well as the enhancement methods for HCC with different diameters.
The follow-up rate and duration were 92.15% (364/395) and 51.28 ± 45.09 mo, respectively. Conventional ultrasonography combined with CEUS revealed 65 (11.80%) nodules with a follow-up diagnosis of HCC, 19 (3.45%) dysplastic nodules, and 467 (84.75%) benign cirrhotic hyperplastic nodules. Among 65 cases of confirmed HCC, 40 (61.54%) were transformed from hypoechoic nodules, 9 (13.85%) from hyperechoic nodules, and the remaining 16 (24.62%) from isoechoic nodules. Significant differences in CEUS characteristics were found among cirrhotic nodules, dysplastic nodules, and HCC nodules at each phase. Significant differences in the enhancement mode were observed between nodules ≤ 1 cm and those 1–2 cm. The smaller the HCC nodule, the later the contrast agent began to flush and the longer the duration of contrast enhancement.
Conventional ultrasonography combined with CEUS could identify small HCC and help monitor patients with an early diagnosis of HCC. Significant differences in the enhancement mode are noted between nodules ≤ 1 cm and those 1–2 cm.
Core Tip: Conventional ultrasonography combined with contrast-enhanced ultrasound is a feasible way to identify small hepatocellular carcinoma (HCC) and help monitor patients with small HCC. There are significant differences between the liver nodules ≤ 1 cm and those in the range 1-2 cm regarding enhancement mode.
- Citation: Mei Q, Yu M, Chen Q. Clinical value of contrast-enhanced ultrasound in early diagnosis of small hepatocellular carcinoma (≤ 2 cm). World J Clin Cases 2022; 10(24): 8525-8534
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8525.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8525
Liver cancer is the sixth most common cancer worldwide, but the third most common cause of cancer-related death[1]. Hepatocellular carcinoma (HCC) is the most common type of primary liver malignancy. Approximately 548000 people are diagnosed with HCC every year, and almost as many people die as a result of it[2]. Developing countries have a two to three times higher incidence of HCC compared with Western countries[3]. It is estimated that the incidence of HCC will continue to increase until 2030[4].
Imaging, involving multiphase computed tomography (CT) or magnetic resonance imaging (MRI), forms the basis for the diagnosis of HCC[5]. The development and progression of HCC are accompanied by a series of changes in hemodynamics in the lesion. During the transformation of a cirrhotic nodule to a dysplastic nodule and small HCC, the blood supply of the nutrient arteries in the nodule gradually increases, while that of the original portal vein gradually decreases. Ultrasound is a safe, commonly available, and cost-effective tool for screening patients with cirrhosis for HCC, but its diagnostic use is limited by its low specificity due to the variable appearances of HCC. The diagnostic accuracy of conventional ultrasonography for hepatic space-occupying lesions is only 53%–77%[6]. Contrast-enhanced ultrasound (CEUS) uses contrast microbubbles during ultrasound, allowing the detection and characterization of malignant focal liver lesions with much higher diagnostic accuracy[7]. The main advantage of CEUS is its ability to display microcirculatory blood perfusion of tissues in real time. The differences in the blood perfusion of benign and malignant tumors allow sonographers to make a qualitative and quantitative diagnosis[8]. It greatly increases the detection rate and diagnostic accuracy of early-stage tumors and satellite lesions of malignant tumors[9].
The diagnosis of small HCC relies solely on imaging because patients usually have no clinical signs[10]. However, these small nodules rarely display the radiological hallmarks of HCC[11]. Delaying diagnosis until the development of < 2 cm nodules results in increased treatment failure or recurrence[12]. The use of CEUS to monitor and diagnose small HCC may improve patient outcomes.
We hypothesized that CEUS would be safe and effective in detecting small HCC. Therefore, the present study was conducted to investigate the clinical value of CEUS in the early detection of small HCC in patients at high risk of HCC. We analyzed the data of regular conventional color Doppler ultrasonography, follow-up, and CEUS of the liver performed in patients at high risk for HCC in our department followed for more than 10 years.
From January 2007 to December 2021, the clinical data of patients at high risk of HCC who underwent regular follow-up at Xuhui Dahua Hospital were retrospectively collected. The inclusion criteria were as follows: (1) Patients with chronic liver disease and those with cirrhosis diagnosed by clinical and related imaging examinations; (2) Patients with cirrhosis complicated by intrahepatic space-occupying lesions, but undetermined nature of the lesions, or increase in one of the combined indicators of HCC [alpha-fetoprotein (AFP) or the new HCC marker glypican-3], but imaging examination showing no space-occupying lesions; (3) Family history of HCC; and (4) Status post HCC surgery. The exclusion criteria were as follows: (1) Hepatic tumor greater than 2 cm in diameter, or patients with advanced tumors complicated by a portal vein tumor thrombus; and (2) Patients with metastatic liver cancer. This study was approved by the Dahua Hospital of Xuhui District Institutional Review Board (No. 201607). The patients gave consent for inclusion in the study.
The whole dynamic process of liver CEUS included arterial phase (15–30 s), portal phase (31–120 s), and delayed phase (121–360 s). A differential diagnosis was performed based on the hemodynamic characteristics of the intrahepatic nodules on dynamic CEUS depending on the time of wash-in and wash-out. According to the American College of Radiology (ACR) CEUS Liver Imaging Reporting and Data System (CEUS LI-RADS), the CEUS features of HCC included homogeneous or inhomogeneous hyperechoic enhancement in the arterial phase and hypoechoic enhancement in the portal or delayed phases, which was called the "fast-in and fast-out" type and was a typical enhancement pattern of HCC[13,14]. Specifically, if CEUS showed rapid wash-in and no significant wash-out in the portal and delayed phases, namely, a "fast-in and isochronous-out" type, it was considered an atypical enhancement pattern of HCC (mostly manifestation patterns of small HCC). If CEUS showed that the lesion area had slower wash-in, and the portal and delayed phases showed isoechoic enhancement and wash-out isochronously as with the liver parenchyma, namely, the "slow-in and isochronous-out" type, it suggested a dysplastic nodule. If CEUS showed isochronous enhancement for the intrahepatic lesion area and the peripheral liver parenchyma and the portal and delayed phases showed isoechoic enhancement and wash-out isochronously as with the liver parenchyma, it suggested a benign liver nodule.
All patients were clinically diagnosed with small HCC according to the HCC treatment guidelines issued by the European Association for the Study of the Liver. Common imaging tests included four-phase multidetector CT and dynamic contrast-enhanced MRI. Clinical diagnosis of small HCC was comprehensively based on the patient’s history of chronic liver disease, AFP (> 400 μg/L for 1 mo or > 200 μg/L for over 2 mo), and typical HCC manifestations revealed by imaging examinations (hypervascular liver lesions in the arterial phase with washout in the portal veins or in the delayed phase)[15]. Patients with a high suspicion of HCC were treated by puncture and then surgery.
Color ultrasound instruments (Siemens Sequoia 512 and Siemens S2000, Siemens Ltd., Munich, Germany; Philips iU22, Royal Dutch Philips Electronics Ltd., Amsterdam, Netherlands, and LOGIQ E9, General Electric Co., Fairfield, Connecticut, United States) were used with an abdominal convex array probe, at a frequency of 1–5 MHz. The contrast agent was SonoVue containing 59 mg sulfur hexafluoride gas, and the microbubble suspension was prepared using 5 mL of 0.9% NaCl solution. According to the weight of each patient, a microbubble suspension of 2 mL/50 kg was taken for Siemens Sequoia 512 and S2000 ultrasound instruments, and 0.1 mL was added for each additional 5 kg. For Philips iU22 and GE GLOGIQ E9, 1 mL/50 kg of the suspension was used and 0.1 mL was added for each additional 5 kg. The microbubble suspension was added via bolus injection through the peripheral vein, and 5 mL of 0.9% NaCl solution was intravenously injected immediately. The CEUS information was stored in the form of real-time dynamic video and qualitative and quantitative analyses of the contrast enhancement mode were conducted.
CEUS was performed by associate chief physicians and reviewed by physicians with the same qualification or superior physicians.
Statistical analyses were performed using SPSS software V. 22.0 (IBM Corp., Armonk, NY, United States). Categorical data are presented as n (%). Continuous data with a normal distribution are presented as the mean ± SD. Measurement data in the two groups were compared using a two-sample unpaired-sample t test. Categorical data were compared using the chi-square test. Simple logistic regression was used to analyze and weigh various ultrasound indicators and overall scores in predicting the odds ratio of HCC. P < 0.05 indicated a statistically significant difference.
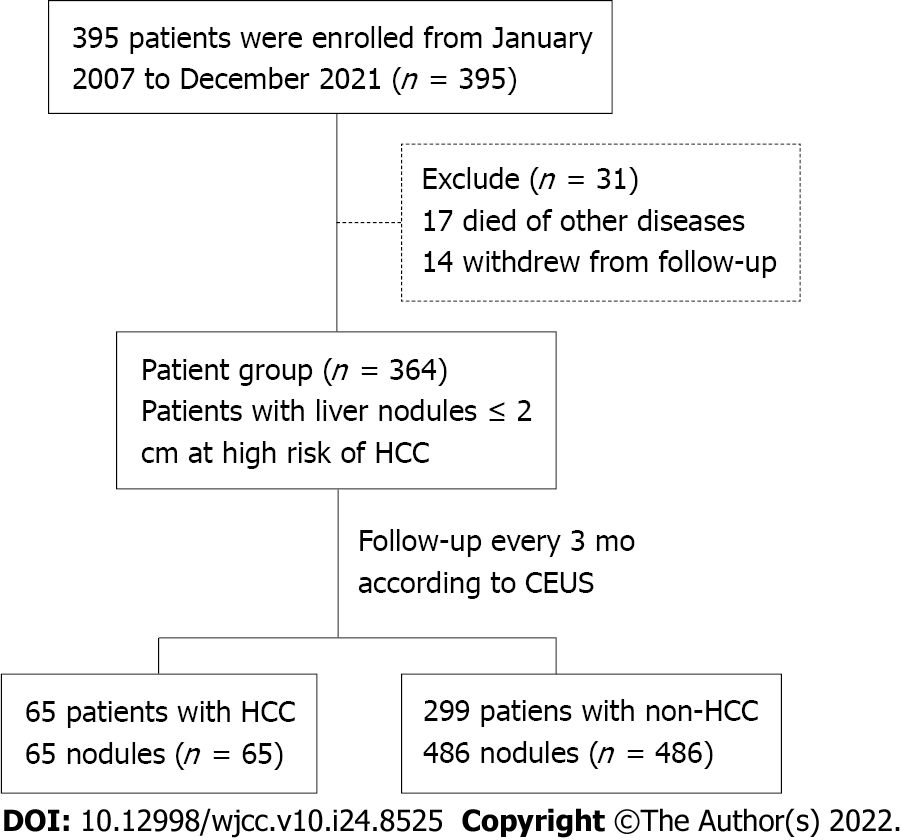
A total of 395 patients were enrolled in the 14 years of follow-up, including 230 men and 165 women. Their age range was 11–85 years, with a mean of 54.67 ± 12.84 years. In these patients, 632 nodules were detected. The follow-up rate was 92.15% (364/395), and the rate of loss to follow-up was 7.85% (31/395); 14 died of decompensated cirrhosis or other cardiovascular diseases, and 17 withdrew from the chronic disease management group. A total of 551 nodules in 364 patients were selected for follow-up. During the regular follow-up every 3 mo, according to CEUS, 65 (11.80%) were diagnosed with HCC, 19 (3.45%) with dysplastic nodules, and 467 (84.75%) with benign cirrhotic hyperplastic nodules (Figure 1 and Table 1). In 65 patients diagnosed with HCC based on CEUS, 55 (84.6%) were pathologically confirmed by further needle biopsy. Of the 93 patients who underwent surgery and needle biopsy, 1 had missed diagnosis (1 patient had 2 HCC nodules in the liver, but only 1 was found by CEUS). This might be related to the scanning section selected by physicians or the blind area of the scanning section of the liver using CEUS. No significant error was found between the HCC nodule size in surgical results and that found using CEUS (error ≤ 5 mm).
| Category | Patients (n = 364) | P value | |
| HCC (n = 65) | Non-HCC (n = 299) | ||
| Age (yr), mean ± SD | 49.86 ± 0.84 | 60.40 ± 1.72 | < 0.001 |
| Male, n (%) | 45 (69.23) | 218 (72.90) | NS |
| Family history of HCC, n (%) | 8 (12.31) | 11 (3.67) | NS |
| BMI (kg/m2), n (%) | NS | ||
| > 28 | 4 (6.15) | 28 (9.36) | |
| ≤ 28 | 61 (93.85) | 211 (90.94) | |
| HBV, n (%) | 65 (100) | 219 (73.24) | < 0.01 |
| HCV, n (%) | 2 (3.08) | 24 (8.03) | NS |
| Alcohol cirrhosis, n (%) | 2 (3.08) | 5 (1.67) | NS |
| Diabetes, n (%) | 8 (12.3) | 46 (15.38) | NS |
The ultrasound characteristics of the nodules classified into HCC and non-HCC groups are shown in Table 2. Significant differences were found between the groups, except for nodule location. A total of 40 nodules (61.54%) were transformed from hypoechoic nodules to HCC, 9 (13.85%) from hyperechoic nodules to HCC, and 16 (24.62%) from isoechoic nodules to HCC. A summary of the CEUS characteristics in different phases is shown in Table 3. Significant differences were observed between the cirrhotic nodules, dysplastic nodules, and HCC nodules in each phase.
| Category | Nodules (n = 551) | P value | |
| HCC (n = 65) | Non-HCC (n = 486) | ||
| Ultrasonographic characteristics, n (%) | < 0.05 | ||
| Hyperecho | 13 (20) | 175 (36.01) | |
| Isoecho | 5 (7.69) | 12 (2.47) | |
| Hypoecho | 47 (72.31) | 299 (61.52) | |
| Nodule size (mm) | 14.6 ± 0.48 | 12.2 ± 10.27 | < 0.05 |
| Location, n (%) | NS | ||
| Left liver | 9 (13.85) | 77 (15.84) | |
| Right liver | 56 (86.15) | 409 (84.16) | |
| CEUS characteristic, n (%) | Nodules (n = 551) | P value | ||
| CN (n = 467) | DN (n = 19) | HCC (n = 65) | ||
| Arterial phase | < 0.05 | |||
| Hyperecho | 0 | 1 (5.26) | 56 (83.60) | |
| Isoecho | 438 (93.79) | 8 (42.11) | 9 (16.40) | |
| Hypoecho | 29 (6.21) | 10 (52.63) | 0 | |
| Portal phase | < 0.05 | |||
| Hyperecho | 0 | 0 | 0 | |
| Isoecho | 464 (99.36) | 17 (89.47) | 50 (72.71) | |
| Hypoecho | 3 (0.64) | 2 (10.53) | 15 (27.29) | |
| Delayed phase | < 0.05 | |||
| Hyperecho | 0 | 0 | 0 | |
| Isoecho | 464 (99.36) | 17 (89.47) | 27 (41.54) | |
| Hypoecho | 3 (0.64) | 2 (10.53) | 38 (58.46) | |
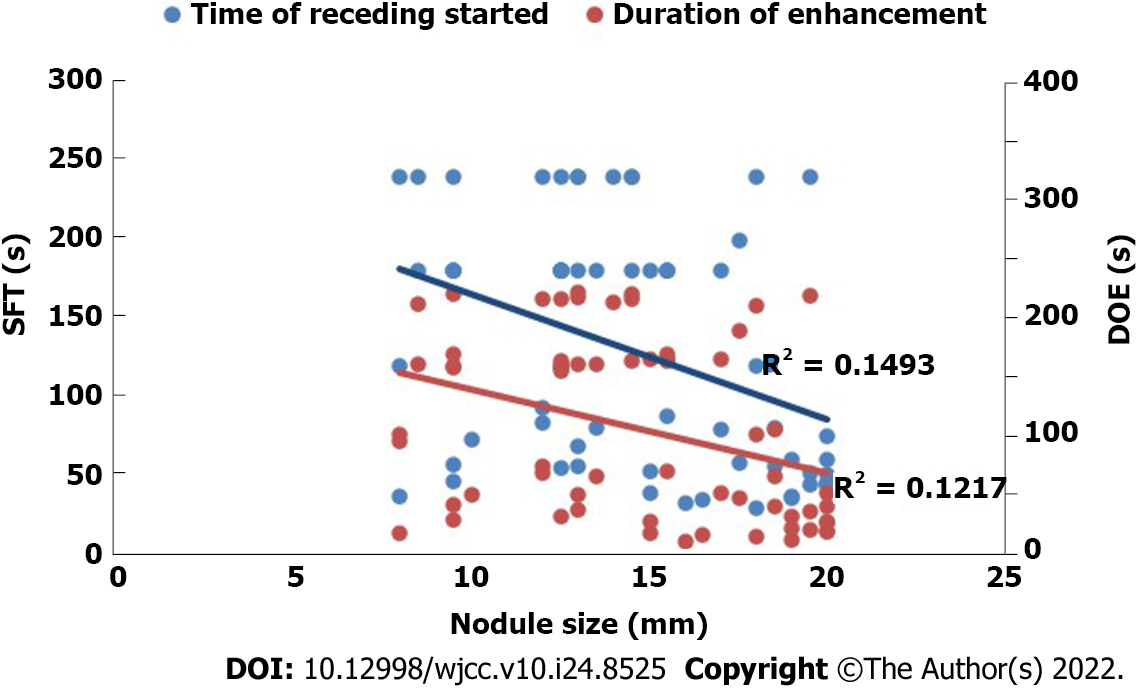
As shown in Table 4, a comparison of CEUS findings according to the size of the HCC nodules revealed significant differences between the nodules ≤ 1 cm and those 1–2 cm in enhancement time and pattern (P < 0.05). Figure 2 shows that the HCC nodule size negatively correlated with the start time of wash-out of the contrast agent, with a correlation coefficient (r) of –0.386. The smaller the HCC nodule, the later the contrast agent began to wash out (Figures 3 and 4). The HCC nodule size negatively correlated with the duration of enhancement of the contrast agent (r = –0.349). The smaller the HCC nodule, the longer the duration of enhancement of the contrast agent.
| Category | Nodule size | P value | |
| ≤ 1 cm | 1-2 cm | ||
| No. | 13 | 52 | |
| Type of echo, n (%) | NS | ||
| Hyperecho | 1 (7.69) | 8 (15.38) | |
| Isoecho | 2 (15.38) | 14 (26.92) | |
| Hypoecho | 10 (76.92) | 30 (57.69) | |
| Enhancement time (s), mean ± SD | 15.12 ± 1.44 | 19.90 ± 0.61 | < 0.05 |
| Wash-out time (s), mean ± SD | 115.20 ± 10.25 | 80.13 ± 13.18 | NS |
| Arterial phase (0-30 s), n (%) | 0 | 1 (1.92%) | |
| Portal phase (31-120 s), n (%) | 4 (30.77) | 28 (53.85) | |
| Delayed phase (121-360s), n (%) | 1 (7.69) | 4 (7.69) | |
| > 360 s, n (%) | 8 (61.54) | 19 (36.54) | |
| CEUS pattern, n (%) | < 0.05 | ||
| Fast-in and fast-out | 5 (38.46) | 33 (63.46) | |
| Fast-in and isochronous-out | 8 (61.54) | 19 (36.54) | |
This study aimed to diagnose patients with chronic liver disease using convenient, safe, inexpensive, and real-time dynamic ultrasonic examinations. Conventional ultrasound and CEUS were used for screening, grouping, and monitoring of patients with chronic liver disease, so as to achieve an early diagnosis of small HCC. A total of 65 patients with HCC were diagnosed in early monitoring during the 11-year study involving 395 patients with chronic liver disease in different stages. The confirmation rate was 99.6% (64/65), with one missed diagnosis because the patient had two HCC nodules on the liver and the second nodule was found on a preoperative MRI scan. The use of CEUS in this study allowed stratification of the small hepatic nodules into HCC and non-HCC groups to identify patients in need of comprehensive prevention and treatment.
In this study, early and regular evaluation by CEUS revealed hyperechoic enhancement in the arterial phase of the cirrhotic nodules. HCC could be confirmed regardless of whether it had a "fast-in and fast-out" or "fast-in and isochronous-out" pattern. A change in the contrast enhancement mode suggested pathological changes in the nodules. The development of the ACR CEUS LI-RADS has been welcomed for its standardization of CEUS information, allowing for a more accurate diagnosis of hepatic nodules[16]. The LI-RADS uses the size of a lesion, the type and degree of arterial phase enhancement, the presence of wash-out, and the timing and degree of wash-out as the major features used for categorizing CEUS images[13]. These features were also significant in the present study in differentiating between cirrhotic nodules, dysplastic nodules, and HCC.
CEUS for small nodules is important for patients at risk of HCC because the diagnosis of a single small HCC comes with a good prognosis and provides a potential for cure[17]. This study showed a high rate of diagnosis of small HCCs, which were all later confirmed to be HCCs. These results compared well with the findings of other studies that aimed to distinguish HCC from benign nodules when they were smaller than 2 cm. The use of gadoxetic acid–enhanced and diffusion-weighted MRI resulted in a sensitivity and accuracy of 91% and 89%, respectively[18]. Another study using LI-RADS with CT achieved a sensitivity and specificity of 72.7% and 90%, respectively[19]. However, one study used CEUS to identify hypervascularity in 190 nodules (95.5%) of 199 histologically confirmed HCC nodules[20]. The advantage of CEUS over other methods with similar results is that it is a modification of the standard ultrasound screening. However, the technique does have some limitations. As shown by the missed diagnosis of one HCC in a patient who already had one HCC, the ultrasonic examination of the liver showed blind areas and interference by gas, or the ribs might result in a missed diagnosis. To address this, it is necessary to select different sections and change positions to observe various sections of the liver. CEUS is also a technique that requires experience, proficiency in section examinations, and understanding of nodules. Insufficient proficiency or understanding may lead to missed diagnosis or misdiagnosis.
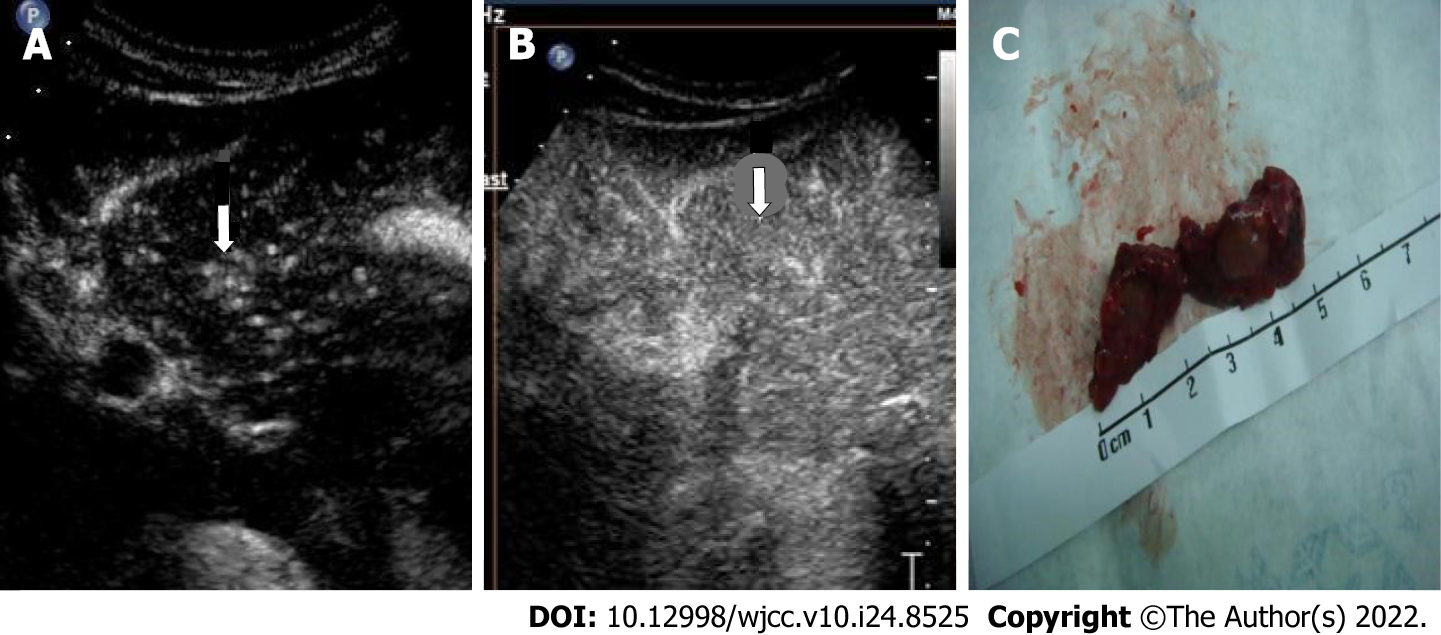
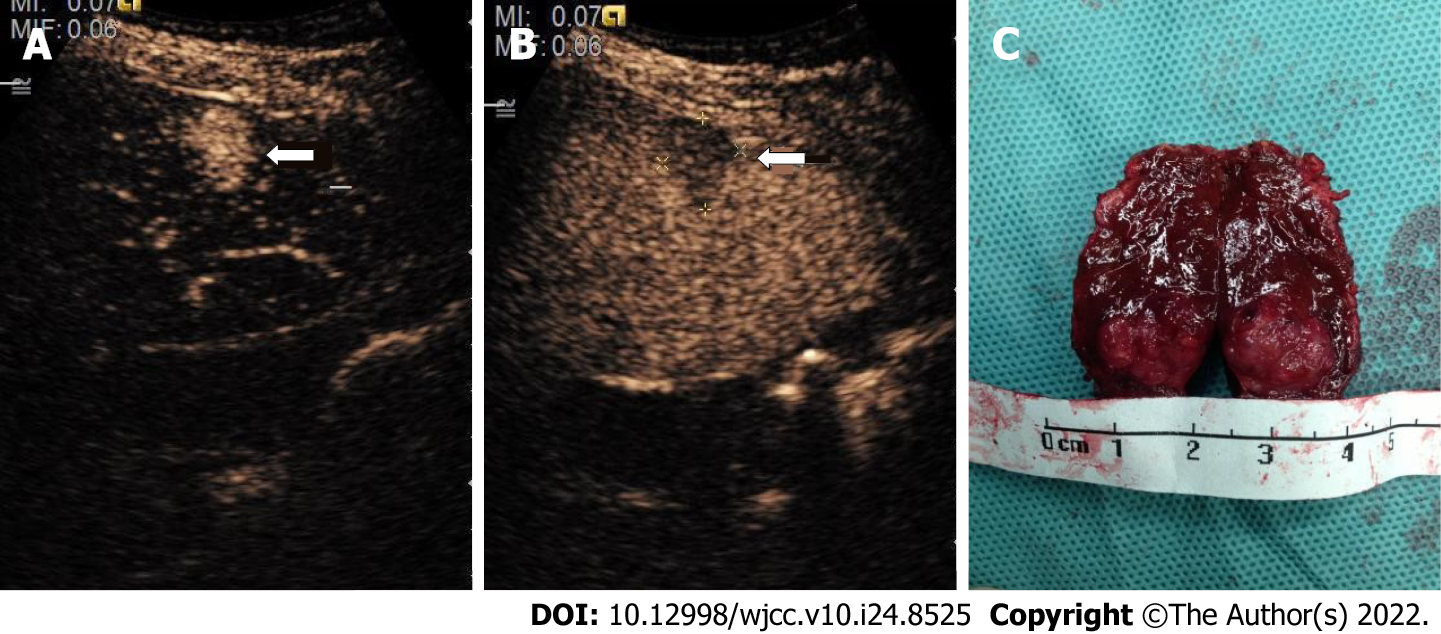
In this study, patients with a high suspicion of HCC, such as LR-3, LR-4, and LR-5 according to CEUS LI-RADS, were treated by puncture and then surgery. Generally, the biopsy was performed by physicians in Ultrasound and Intervention Department. Once HCC was confirmed by pathological methods, the patients should undergo surgery. In order to reduce the possibility of tumor implantation and recurrence, the resection margin should be obviously larger than the diameter of tumor nodules (Figures 3 and 4). Many studies have explored the CEUS images of HCC larger than 20 mm[15,20,21], but comparative studies between HCC nodules ≤ 1 cm and those 1–2 cm are few. However, this study compared the enhancement mode of CEUS for small HCC nodules ≤ 2 cm of different sizes. The results showed that the smaller the HCC nodule, the longer the duration of enhancement of the contrast agent. This might be related to the differentiation of HCC and the blood supply of the hepatic artery and portal vein for HCC. The mechanism of why this enhancement mode is formed needs further exploration.
The study had some limitations. The patients were enrolled from one medical center, and hence the number of small HCC was lower. A larger study from more centers would provide more evidence for these results. No direct comparison with alternative MRI or CT methods was found. So, we cannot directly infer that CEUS is superior to CT or MRI for the diagnosis of small HCC.
Conventional ultrasonography combined with CEUS could identify small HCC and help monitor patients with early diagnosis of HCC. Significant differences are found in the enhancement of cirrhotic nodule, dysplasia, and HCC using CEUS, and also between the HCC nodules ≤ 1 cm and those 1–2 cm in the enhancement mode.
Contrast-enhanced ultrasound (CEUS) is challenging in the diagnosis of small hepatocellular carcinoma (HCC) with a diameter of less than 2 cm.
Many studies have explored the CEUS images of HCC larger than 20 mm, but comparative studies between HCC nodules ≤ 1 cm and those 1–2 cm are rare. However, this study compared the enhancement mode of CEUS for small HCC nodules ≤ 2 cm of different sizes.
To investigate the clinical value of CEUS in the early detection of small HCC with high risk factors, especially to compare the enhancement mode of CEUS for small HCC nodules ≤ 2 cm of different sizes.
Conventional ultrasonography combined with CEUS was adopted to analyze the echo, size, location, and enhancement characteristics of benign and malignant nodules, as well as the enhancement methods for HCC with different diameters.
Conventional ultrasonography combined with CEUS revealed 65 (11.80%) nodules with a follow-up diagnosis of HCC, 19 (3.45%) dysplastic nodules, and 467 (84.75%) benign cirrhotic hyperplastic nodules. There were 40 cases (61.54%) of HCC transformed from hypoechoic nodules, 9 (13.85%) from hyperechoic nodules, and the remaining 16 (24.62%) from isoechoic nodules. Significant differences in CEUS characteristics were found among cirrhotic nodules, dysplastic nodules, and HCC nodules in each phase. Significant differences in the enhancement mode were observed between nodules ≤ 1 cm and those 1–2 cm.
Conventional ultrasonography combined with CEUS could identify small HCCs and help monitor patients with an early diagnosis of HCC. Significant differences in the enhancement mode were noted between nodules ≤ 1 cm and those between 1–2 cm. The smaller the HCC nodule, the later the contrast agent began to flush and the longer the duration of contrast enhancement.
Small HCC ultrasound imaging enhancement pattern compared with other medical imaging enhancement pattern is the research direction in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: El-Nakeep S, Egypt; Papadopoulos N, Greece S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21354] [Article Influence: 2135.4] [Reference Citation Analysis (3)] |
| 2. | McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv Nutr. 2016;7:418-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 903] [Article Influence: 100.3] [Reference Citation Analysis (0)] |
| 3. | McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 648] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 4. | Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol. 2016;34:1787-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 357] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 5. | Rastogi A. Changing role of histopathology in the diagnosis and management of hepatocellular carcinoma. World J Gastroenterol. 2018;24:4000-4013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 6. | Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 570] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 7. | Zhang J, Yu Y, Li Y, Wei L. Diagnostic value of contrast-enhanced ultrasound in hepatocellular carcinoma: a meta-analysis with evidence from 1998 to 2016. Oncotarget. 2017;8:75418-75426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Tian H, Wang Q. Quantitative analysis of microcirculation blood perfusion in patients with hepatocellular carcinoma before and after transcatheter arterial chemoembolisation using contrast-enhanced ultrasound. Eur J Cancer. 2016;68:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Xu Y, Lu X, Mao Y, Sang X, Zhao H, Du S, Xu H, Sun Y, Yang H, Chi T, Yang Z, Zhong S, Huang J. Clinical diagnosis and treatment of alpha-fetoprotein-negative small hepatic lesions. Chin J Cancer Res. 2013;25:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Wang M, Wei C, Shi Z, Zhu J. Study on the diagnosis of small hepatocellular carcinoma caused by hepatitis B cirrhosis via multi-slice spiral CT and MRI. Oncol Lett. 2018;15:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Bolondi L, Gaiani S, Celli N, Golfieri R, Grigioni WF, Leoni S, Venturi AM, Piscaglia F. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology. 2005;42:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 309] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 12. | Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, Pinna AD. Cost-effectiveness of hepatic resection vs percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 310] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 13. | Wilson SR, Lyshchik A, Piscaglia F, Cosgrove D, Jang HJ, Sirlin C, Dietrich CF, Kim TK, Willmann JK, Kono Y. CEUS LI-RADS: algorithm, implementation, and key differences from CT/MRI. Abdom Radiol (NY). 2018;43:127-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 14. | Lyshchik A, Kono Y, Dietrich CF, Jang HJ, Kim TK, Piscaglia F, Vezeridis A, Willmann JK, Wilson SR. Contrast-enhanced ultrasound of the liver: technical and lexicon recommendations from the ACR CEUS LI-RADS working group. Abdom Radiol (NY). 2018;43:861-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 15. | Huang JY, Li JW, Lu Q, Luo Y, Lin L, Shi YJ, Li T, Liu JB, Lyshchik A. Diagnostic Accuracy of CEUS LI-RADS for the Characterization of Liver Nodules 20 mm or Smaller in Patients at Risk for Hepatocellular Carcinoma. Radiology. 2020;294:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 16. | Terzi E, Iavarone M, Pompili M, Veronese L, Cabibbo G, Fraquelli M, Riccardi L, De Bonis L, Sangiovanni A, Leoni S, Zocco MA, Rossi S, Alessi N, Wilson SR, Piscaglia F; CEUS LI-RADS Italy study group collaborators:. Contrast ultrasound LI-RADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1,006 nodules. J Hepatol. 2018;68:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 17. | Fateen W, Ryder SD. Screening for hepatocellular carcinoma: patient selection and perspectives. J Hepatocell Carcinoma. 2017;4:71-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Kwon HJ, Byun JH, Kim JY, Hong GS, Won HJ, Shin YM, Kim PN. Differentiation of small (≤2 cm) hepatocellular carcinomas from small benign nodules in cirrhotic liver on gadoxetic acid-enhanced and diffusion-weighted magnetic resonance images. Abdom Imaging. 2015;40:64-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Abd Alkhalik Basha M, Abd El Aziz El Sammak D, El Sammak AA. Diagnostic efficacy of the Liver Imaging-Reporting and Data System (LI-RADS) with CT imaging in categorising small nodules (10-20 mm) detected in the cirrhotic liver at screening ultrasound. Clin Radiol. 2017;72:901.e1-901.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Giorgio A, Montesarchio L, Gatti P, Amendola F, Matteucci P, Santoro B, Merola MG, Merola F, Coppola C, Giorgio V. Contrast-Enhanced Ultrasound: a Simple and Effective Tool in Defining a Rapid Diagnostic Work-up for Small Nodules Detected in Cirrhotic Patients during Surveillance. J Gastrointestin Liver Dis. 2016;25:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Pan JM, Chen W, Zheng YL, Cheng MQ, Zeng D, Huang H, Huang Y, Xie XY, Lu MD, Kuang M, Hu HT, Chen LD, Wang W. Tumor size-based validation of contrast-enhanced ultrasound liver imaging reporting and data system (CEUS LI-RADS) 2017 for hepatocellular carcinoma characterizing. Br J Radiol. 2021;94:20201359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |