Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8450
Peer-review started: March 22, 2022
First decision: June 7, 2022
Revised: June 20, 2022
Accepted: July 18, 2022
Article in press: July 18, 2022
Published online: August 26, 2022
Processing time: 146 Days and 22.3 Hours
Cerebral small vessel disease (CSVD) is a leading cause of age-related micro
Core Tip: Cerebral small vessel disease (CSVD) is a leading cause of age-related microvascular cognitive decline resulting in significant impairment. Despite the general acceptance of key terms from neuroimaging findings as observed on the magnetic resonance imaging (MRI), key questions on CSVD remain elusive. The MRI-based diffusion tensor imaging (DTI) offers non-invasive tool to quantitate brain white matter connections via fiber tracking that may inform the extent of CSVD-associated white matter damage. In this minireview, we highlight the advances in DTI pipeline processing and the prospect of DTI metrics as potential biomarker for CSVD amenable towards a routine clinical use.
- Citation: Safri AA, Nassir CMNCM, Iman IN, Mohd Taib NH, Achuthan A, Mustapha M. Diffusion tensor imaging pipeline measures of cerebral white matter integrity: An overview of recent advances and prospects. World J Clin Cases 2022; 10(24): 8450-8462
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8450.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8450
Cerebral small vessel disease (CSVD), in its prevalent sporadic form, refers to a syndrome of clinical and neuroimaging findings in ageing populations that is frequently related to vascular risk factors and onset of neurological impairments including stroke, dementia, parkinsonism, gait problems, and mood disturbances[1,2]. Sporadic CSVD pathologies are often heterogeneous, as evidenced by neuroimaging findings such as lacunes, white matter hyperintensities and enlarged perivascular spaces on T2-weighted magnetic resonance imaging (MRI)[3,4]. While conventional MRI and MRI-based diffusion-weighted imaging (DWI) provide a detailed picture of the overall severity of white matter involvement, it is only capable of measuring diffusion in a single direction[5]. Therefore, improved relationships and reliable lesion studies are desired to enhance the assessment of white matter architecture and connectivity such as white matter tractography using diffusion-based MRI (dMRI)[6,7].
Within dMRI, diffusion tensor imaging (DTI) offers data that can be used to explain brain white matter connections non-invasively through fiber tracking[8]. One of the more current improvements in DTI is the advancement of models of individual patient-specific white matter tracts, namely the diffusion tensor tractography, which is thought to be suitable to examine the effect of CSVD on white matter tracts[9]. Various sets of computerized software or image pipeline processing (i.e., from dMRI data capture to image processing and data interpretation) are used along the process due to a lack of standardization and variability in the reported findings[10]. As we strive to better understand this complex condition, this mini review will summarise the recent advances and prospects of DTI pipeline application for the clinical detection and assessment of the cerebral white matter integrity.
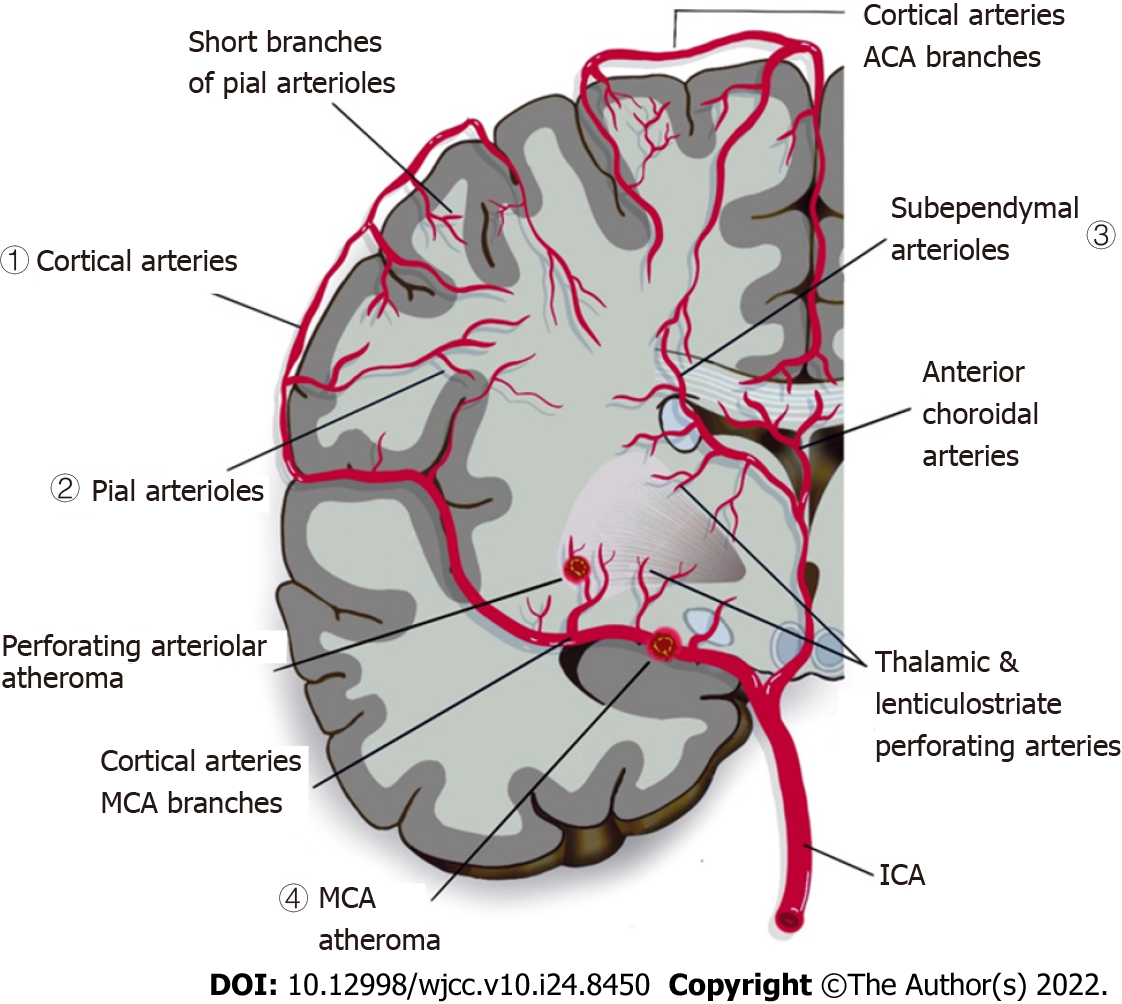
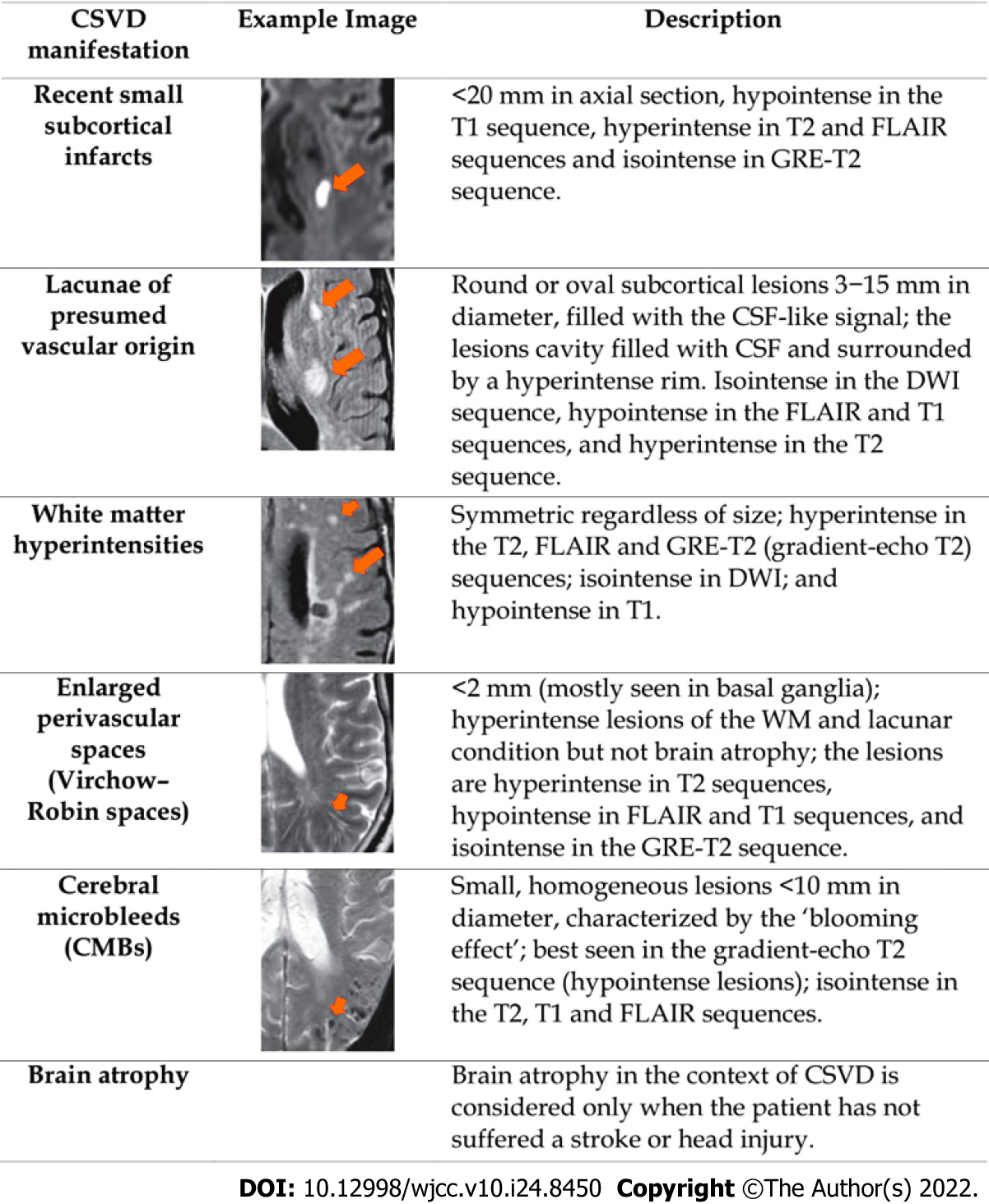
CSVD is widely recognised as a neurovascular syndrome featuring clinical, cognitive, neuroimaging, and neuropathological findings that arise from damage and/disruption involving a complex neurogliovascular unit in the brain[11-13]. Due to various vascular-pathologic developments that could disrupt the perforating cerebral capillaries and arteries that supply the brain subcortical region with restricted collaterals, parenchymal damage is seen in the grey and deep white matter of the subcortical area (as shown in Figure 1)[14-16]. Consequently, CSVD imposes a significant impact on neuropsychological function as well as common neuropathological processes, and contributes significantly to development of cognitive impairment, dementia, and stroke[17-20]. Moreover, conventional vascular risk factors such as hypertension, diabetes, hyperlipidaemia and smoking have been shown to increase the risk towards development and progression of CSVD[21]. There are two main forms of CSVD which includes amyloidal CSVD [sporadic and hereditary cerebral amyloid angiopathy] and non-amyloidal CSVD (age-related and vascular risk-factor-related small vessel, i.e., arteriolosclerosis)[22]. CSVD is commonly ascribed to a series of neuroimaging manifestations, consisting of recent small subcortical infarcts, lacunes, white matter hyperintensities (WMHs), cerebral microbleeds, prominent or enlarged perivascular spaces, cortical microinfarcts, and atrophy[3,23,24]. Figure 2 shows key manifestations of CSVD as streamlined for CSVD research/practical purposes by the standards for reporting and imaging of small vessel disease (STRIVE)[25]. Hence, DTI can provide a powerful insight into white matter integrity and damage found in CSVD, thus providing a possible marker for disease severity and relatable to CSVD symptoms and/or signs that would otherwise go undetected using conventional MRI[26].
DTI is a non-invasive dMRI-based method for visualizing tissue macro- and microstructures for pathological evaluation. Based on the microstructural features (e.g., fiber diameter, fiber density, and myelination) that limit perpendicular diffusion and restrict water movement in specific directions, DTI assesses the free movement of water molecules inside the white-matter tracts[27]. Isotropic diffusion occurs when there are no barriers in their path, such as in a beaker of water, where molecules bumping around due to thermal action will scatter in the same way. When molecules encounter oriented barriers, however, movement is no longer dispersed evenly along all paths, and diffusion becomes anisotropic[28].
The use of DTI as a visualization tool has helped to distinguish between large, oriented macro
The DTI parameter is reliant not entirely on static magnetic strength field, but rather on the signal-to-noise ratio (SNR) and impact of the artifact. The MRI platforms with 1.5T and 3T magnetic field strength are commonly used in routine DTI human brain scanning, although some centres have access to the 7T platform. It is acknowledged that scanning with a higher field strength improves the SNR, which equates to better results[30,31]. Aside from static magnetic fields, the number, power, and gradient coils also contribute significantly to the value of DTI data. The gradient task cycle verifies the methods for obtaining 2D images for each repetition time. The most recent scanners offer longer task cycles, allowing data to be stored in less time. Gradient force improvement allows for greater dispersion weighting and interpretation in a shorter time frame. This implies that the echo time can be reduced sequentially. Reducing the sensitivity effects will subsequently improve information conditions. Moreover, a high slew rate is desirable for DTI[6]. Nonetheless, the rapid growth and collapse of a strong gradient can cause picture-distorting eddy currents and mechanical vibrations, as well as peripheral nerve stimulation, which may result in patients' spontaneous muscle cramps. A clinical system operating for the maximum gradient amplitude is typically 40-80 milliTesla/meter (mT/m) plus 150-200 mT/m for each millisecond highest slew rate for safety reasons[32].
Non-standard apparatus offered by imaging manufacturers or built by and for investigation could improve DTI files. Using multichannel phased array coils instead of birdcage coils to improve SNR and allow equivalence scanning is a simple way to improve the quality of DTI files. However, an increase in the number of module coils may create a bias area that favors cortical exposure over deep white matter, making DTI in this region less ideal[32,33]. Without quantification, the data cannot be statistically analyzed, and visualization findings cannot be associated with scientific outcomes[34].
Visual evaluation of dMRI images can be validated by vague quantifiable measures taken from DTI files consisting of fractional anisotropy (FA), mean diffusivity (MD), and Trace (Tr). Tr of the diffusion tensor (D) indicates the total water content. Variations in Tr(D) can be recognized uniquely to variations in the formation of tissue[35]. FA calculates the relationship between the magnitude of the anisotropic element of D and the entire magnitude of D. FA values are in the limit of (0 or 1) and can be assessed in each voxel. FA is a commonly applied DTI measure that explains the intensity of diffusion anisotropy in a voxel[36,37].
DTI analyses are classified into three main groups: whole brain, regional and voxel-based methods. An increasing number of software tools are available to analyze DTI information which differ significantly in their purposes. The wide scope of analysis methods and diverse aims in software packages contributes to an absence of consistency that muddles the evaluation of DTI information and understanding the findings[38].
The whole-brain analysis technique is used to find a quantitative DTI measure from all the white matter voxels in the brain and used seed regions from all voxels in the brain[39,40]. Histogram analysis is used to summarize the DTI measured that has been obtained from the chosen voxels of interest which reveals the frequency distribution number of voxels with certain values of the diffusion amount[41,42]. Meanwhile, region-specific analysis techniques are region of interest (ROI) analysis and tractography analysis. ROI is a diffusion measure acquired from a region in a brain that is marked by manual or by automated segmentation or parcellation[43,44]. Whereas the voxel-based analysis (VBA) technique evaluates and compares DTI measurement in the tiniest imaging possible (i.e., the individual voxel)[45].
Fiber tracking or tractography is a post-processing method that can effectively analyze white matter fiber bundles in vivo. Tractography analysis describes the statistical rebuilding of the white matter fiber bundle interpretations by integration of the local diffusion tensor information from each voxel and associated with a riddle “connecting the dots”[46,47]. Tractography is reliant on user input to identify sites in the brain through which tracts are to be rebuilt and that reliant on options are recognized to be anatomically related[48].
DTI is applied in a range of clinical situations and is not restricted to neurological purposes. DTI is employed for variable measures in medical procedures even though its efficiency remains primarily a preclinical research device. Currently, the medical use of DTI by probable clinical value is for the preparation of neurosurgical and radiotherapeutic procedures. DTI application not only offers microstructural knowledge on biological location and structure, but also macrostructural information involving the white matter tracts and connections among vital cortical and subcortical functional regions in the brain[49]. Additionally, DTI can provide complementary knowledge about the essential subcortical structure and thus can be utilized in neurosurgical and radiotherapeutic planning. Moreover, DTI is also used in the identification and follow-up of brain tumors[50,51], multiple sclerosis[52], demyelinating disorder, dementia[53-55], psychiatry[56,57] and traumatic brain injury[58].
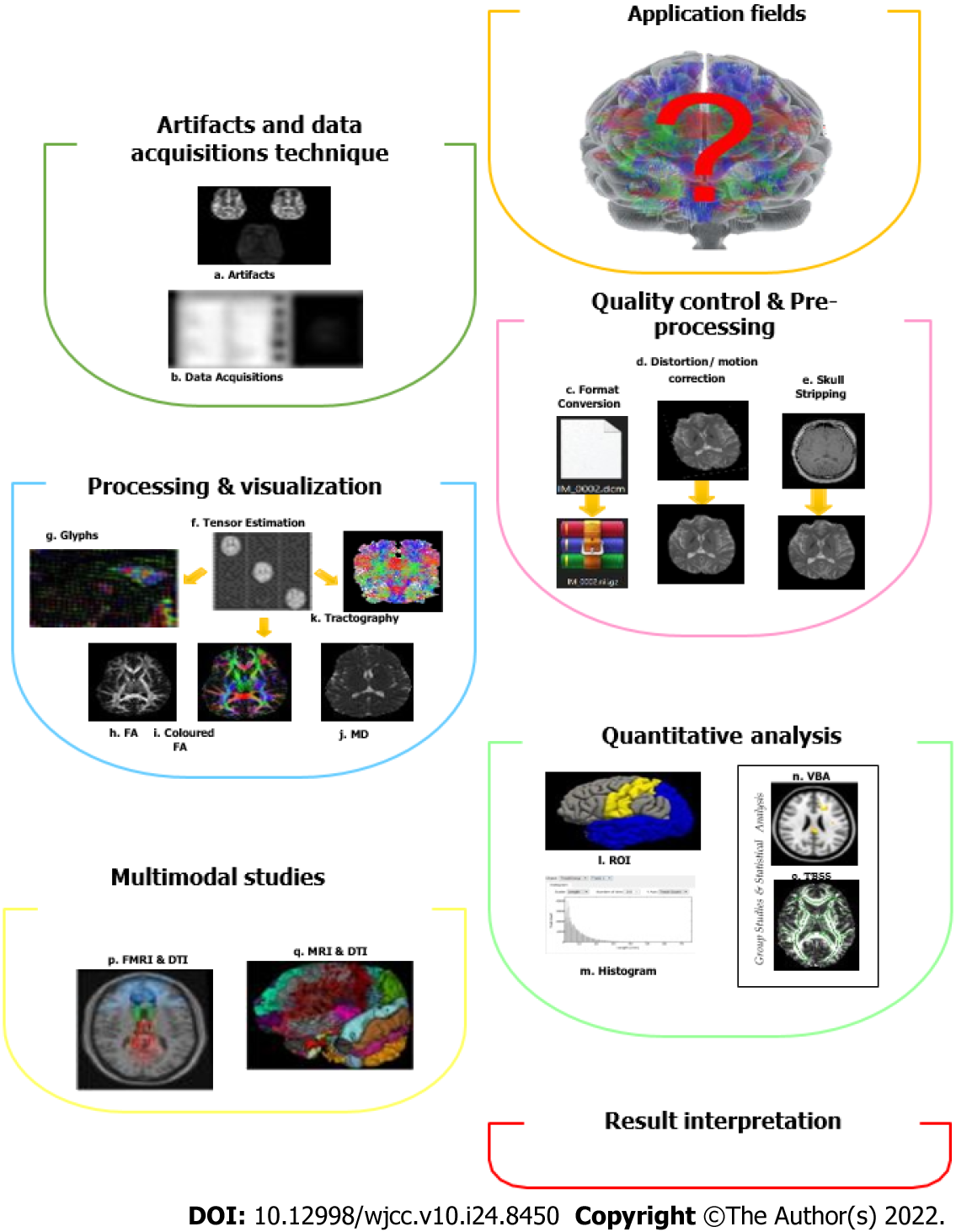
There are tons of computerized software tools available in the literature with various functionalities, varying from data import, basic image viewing and processing, image quality improvement, registration, automatic segmentation, and DTI tractography to higher-order diffusion modelling and enhanced tractography[38,59-61]. It can be classified into different functions or applications. The general technique in DTI pipeline processing (i.e., a connected series of image processing elements, in which the output becomes the input of the next image processing unit in a pipeline[62] and analysis involves a few steps, including artifacts and data acquisition techniques, quality control and pre-processing, processing and visualization, quantitative analysis, multimodal studies, and lastly the interpretation of results (Figure 3)[63].
Data acquisition is an essential step in DTI pipeline processing. Poor data acquisition can affect data quality and data analysis. Parameters that need to be considered in data acquisition include several diffusion directions, image resolution, b-value, b-value number, and average number. Table 1 shows the minimum required data acquisition parameters in DTI pipeline processing. The framework of the DTI pipeline is pre-processing, tensor estimation and fiber tracking. Due to the inadequate interoperability between the DTI analysis tools and the absence of standard DTI format[64], a lot of software bundles were developed and used to define their data format, such as Neuroimaging Informatics Technology Initiative (NIfTI)[65,66]. File formats such as dcm2nii, NIfTI tools, MRIcro and software converter package (e.g., Freesurfer, SPM, Splicer) are usually utilized to transform files from the original clinical-setting such as digital imaging and communications in medicine (DICOM) format[66,67]. In tensor estimation, there are three main methods used to estimate tensors which are Linear Least Square (very simple executed in a single-step process, but it depends on hardware, size, and several datasets)[68], Weighted Linear Least Squares (quick method, but it varies on the magnitude of the MRI files e.g., intensities of different DWI)[68] and Non-Linear Least Square (solved over the established system of nonlinear equations, but it also depends on the hardware and size of the dataset and may take minutes up to several hours to produce tensor estimation)[69]. Tools that can be used as tensor estimation are MedInria2[70], DTI Studio[71], Brain Voyager[72], and MRTrix[73].
| Data acquisition parameter | Minimum requirement | Comments |
| Field signal | 1.5 T or 3T | Give a higher visual score, larger number of fibres. 7T and above – enables identification of smaller anatomical structures |
| Number of diffusion direction | Approximate 21 | Lowest should be 6 diffusion direction, however it is advice to use more than 6 direction and can go up to 100 directions |
| Image resolution | depend on the FOV (usually 24 cm × 24 cm) | Large enough to prevent aliasing |
| B-value | 0-1000 s/mm2 | 500-5000+ for HARDI acquisition 2500 for q-space even higher, e.g., 5-8000 |
| Number of b-values (b = 0) images | 2 | About 1 per 6 diffusion images |
| Number of averages | NSA = 2 | Can improve the SNR |
Fiber tracking is the method for extraction of fiber pathways to quantify the white matter integrity[74]. The software used for fiber tracking includes DTITrack[63], Fiber navigator[70], MedInria2[70], and MRTrix[73]. The quantitative and correlation analysis consists of ROI analysis, VBA, and tract-based spatial statistics (TBSS) will be utilized to extract summary measures from either anatomical regions or the whole brain. In brief, ROI analysis is established on the manual delineation of prior certain regions of the brain or automated parcellations. VBA involved the registration of diffusion maps into a standard space to accomplish correspondences among individuals across voxel and consequently anatomical structures[75]. On the other hand, TBBS is a programmed technique for detecting group voxel-wise changes in the whole brain, established on the skeletonization of the group registered FA maps.
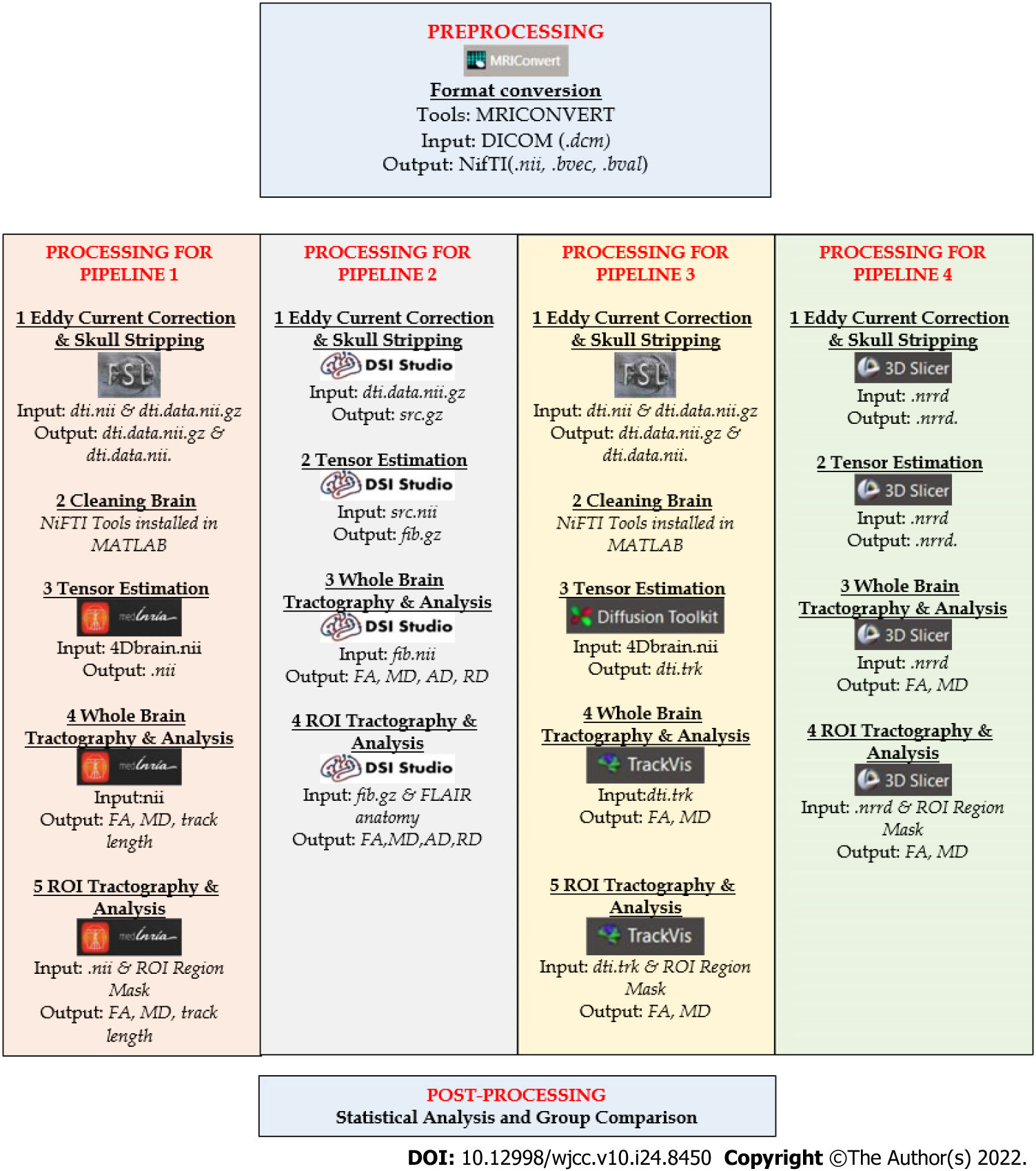
Whilst significant advancements over past two decades have been made in methodical technologies and breadth of applications, there remains no consensus on the ‘gold standard’ quantitative pipeline processing for CSVD studies – be it from diagnostic to prognostic implication. Most of the research teams used their own and different combinations in their pipelines[76,77] to assist with anatomical accuracy and interpreting results in research or clinical setting are done with caution. The four pipeline processing modalities described herein (Pipeline 1 to 4) are generally adopted in assessing white matter integrity in CSVD (as summarised in Figure 4).
Pipeline 1 (P1) consists of MRI Converter version 2.1.0, Brain Software Library or FSL Toolbox functional magnetic resonance imaging, and MedINRIA 2.2[70] as shown in Figure 4. MRI Convert is a medical image file conversion utility that can convert DICOM files to other formats including NIfTI format, FSL NIfTI format, analyze format, SPM99/Analyze format, Brain Voyager, and MetaImage volume formats. It creates a directory structure based on series and subjects. These directories and output files are given default names based on the subject, study, and series information and can be changed by the user.
FMRIB’s Diffusion Toolbox and BET-FSL are software tools for DWI analysis and are part of FMRIB’s Software Library (FSL) that can operate on Windows and Mac. It has a user-friendly graphical interface and command-line interface. It provides tools for data processing, local diffusion modelling, and tractography which work independently from each other. NIfTI Tools installed in MATLAB-Script is a tool that is used for motion and coordination correction or cleaning process. MedINRIA is an open-source software for medical image processing and visualization[78]. It offers database management and file import, 2D to 4D image visualization, diffusion image processing, segmentation of images, filtering of images and registration of images and has a strength in visualization aids.
The first step is the DICOM data of each participant is loaded into MRI Converter, converting the DICOM file format (.dcm) into NIfTI file format (.nii). Then, the DTI files are processed using the FSL toolbox that include eddy current correction (removal of artifact) and brain extraction tool (BET) (remove the skull and non-brain structure)[79]. The required data for eddy current correction is dti.nii, with corrected outputs being dti.data.nii.gz and dti.data.nii. It is assigned with such name to make further work easier. FSL toolbox is installed using a Virtual machine workstation that serves as a platform for Linux virtualization in Windows due to the incompatibility of the FSL toolbox to be installed directly to Windows. Then, the data from the FSL toolbox will undergo cleaning using NIFTI tools installed in MATLAB. Next, the clean DTI files are then uploaded on MedINRIA[70] to further the process of tensor estimation, whole brain, and ROI tractography analysis.
Pipeline 2 (P2) consists of MRIConverter version 2.1.0 and DSIStudio[71] software (http://dsi-studio.labsolver.org/). DSI Studio is a software for diffusion MRI analysis that provides functions including reconstruction, deterministic fiber tracking, and 3D visualization[70]. Additionally, DSI Studio can rotate to the source image to correct image orientation and present a real 3D tractography representation. DSI Studio's fiber tracking algorithm is a simplified variant of the deterministic tracking algorithm that uses measurable anisotropy as the final indicator[71]. A deterministic method is used as the main axis of the tensor. The tensor aligns with the main direction of the fiber that follows the optimal path. Most likely to suggest fiber orientation for each voxel. This approach aims to find the best trade-off between valid and invalid connections[80].
The first step is to import the DICOM data of each participant into the MRI converter and converted the DICOM file format (.dcm) to the NIfTI file format (.nii). The initial stage is to mask the brain to remove non-brain structures and the skull (skull striping). Eddy corrected DTI images with.nii/.bvec/.bval are primarily employed in this scenario. By default, all the settings are set to their default values. The data is then opened in DSI Studio, and the output of eddy corrected DTI generated a ".src" file. Next, the .src file is reconstructed, ".fib" data is retrieved to track the fibers and tractography, and the FA value is then recorded.
Pipeline 3 (P3) consists of MRI Converter version 2.1.0, FSL toolbox, Diffusion toolkit, and TrackVIs[70]. DTI-Toolkit is a spatial normalization and atlas construction toolkit optimized to examine white matter morphometry using DTI data[81]. It supports a standard-based IO file such as NIfTI. Users may need to know simple Unix command lines to conduct certain tasks. It also provides a chain of tools to manipulate tensor image weight such as resampling, smoothing, warping, registration, and visualization. It is free software under public license and easily used but does not support tensor reconstruction (pre-processing support) and probabilistic fiber tractography. It applies tensor-based registration using explicit optimization of tensor reorientation analytically to give the best performance for fiber tract analysis compared to other tools[82].
TrackVIs is a complete software package that does reconstruction, fiber tracking, analysis, and visualization. It can not only handle diffusion tensor data but can also process high angular resolution diffusion imaging (HARDI) data as well as diffusion spectrum imaging data and Q-Ball imaging data. It is stand-alone, cross-platform (works on all major platforms, including Windows XP, Mac OS X, and Linux), fast and efficient. TrackVis can read in the track data file and visualizes and analyses the tracks by user’s manipulation. TrackVis can read entire brain track information typically more noteworthy than 15 megabytes for a high-determination human output and permits the user to apply different track channels to choose and showcase fiber bundles[81].
The first step is to import DICOM data into MRI Converter and converted the DICOM file format (.dcm) to the NIfTI file format (.nii). Then, utilizing the diffusion toolkits, open the DTI file before uploading the processed data to TrackVis[71] to track the fibers and perform tractography. In the TrackVis program, track data from diffusion toolkits will be loaded either by File->Open Track or by drag-and-drop. Then, the brain image needs to be loaded for slice reference. In addition, only after the tract dataset is loaded, brain images can be loaded. TrackVis will automatically resample to match the track space from any image with different dimensions and/or voxel size. Lastly, when the tract data is loaded, acquisition of WBT and is followed by the ROI analysis value.
Pipeline 4 (P4) consists of MRI Converter version 2.1.0, and 3DSlicer[83]. 3D slicer is a software platform for the analysis and visualization including volume rendering, registration, and interactive segmentation of medical images and research in image-guided therapy[83]. It is free and open-source software that is extensible, with powerful plug-in capabilities for adding algorithms and applications. First is to load the DICOM data into the MRI Converter, converting the DICOM file format (.dcm) into NIfTI file format (.nii). Next is to upload the data through data loading, and then the data is converted into nearly raw raster data (.nrrd). Thus, this data can be used freely in this 3D Slicer. From this data, open the DTI file in (.nrrd) format, and choose the slicer DMRI program in the menu bar to undergo diffusion and to attain the tensor data. From the tensor data, we can convert the data to tract by using the draw effect tool. Then, the tract is converted into tractography by using tractography seeding in the 3D Slicer program and the tractography is then generated with the DTI parameter value. Finally, the same steps are repeated for ROI analysis in the 3D Slicer.
Table 2 summarises the relevant previous studies (apart from CSVD) where software in these four pipelines is utilised in image computing for quantitative imaging networks[83-94].
| Pipeline processing | Relevant applications |
| Pipeline 1 | Multiple sclerosis[52], Alzheimer's disease[53], cerebellar ataxia and cortical cerebellar atrophy[78], Krabbe disease[82], epilepsy[85], cubital tunnel syndrome[86], the white matter integrity of Alzheimer's disease[87], dementia[88] |
| Pipeline 2 | Cubital tunnel syndrome[86], the white matter integrity of Alzheimer’s disease[87], epilepsy[89], neurosurgery and brain tumour[90] |
| Pipeline 3 | Parkinson’s disease[91], Renal Failure[81,92], Epilepsy[93] |
| Pipeline 4 | Dementia[87], neurosurgery and brain tumour[94] |
Taken together, DTI is a specialized diagnostic imaging tool utilizing diffusion-weighted imaging of MRI. Further studies and improvements of the DTI are warranted in advancing its utility in everyday patient care. As with any modality or imaging tool, deliberation with referring physicians, physician assistants, and nurse practitioners to increase awareness of this new option is clinically beneficial. Continuing research on its growing capabilities offer a wider adoption among health professional, especially among radiologists in interpreting the clinical merits of DTI. As radiologists see the value in DTI, more precise recommendations can be made to acquire DTI that can aid in diagnoses and/or prognoses of numerous other diseases.
This short review highlights the potential role of DTI development and technology in our pursuit to understand the pathophysiology of CSVD. We summarise key aspects of DTI pipeline analysis and the clinical significance of pipeline processing that are pertinent for CSVD, in specific. As the interests in the field undoubtedly continue to grow, DTI metrics may serve as biomarker for routine clinical use to guide diagnosis, disease progress and prognosis for CSVD natural history, from it’s covert to symptomatic manifestations.
We wish to thank the Director of the Universiti Hospital of Universiti Sains Malaysia for the permission to use numerous anonymised imaging datasets in our on-going related research on CSVD.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neuroimaging
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ma C, China; Xu Y, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Lau AYL, Ip BYM, Ko H, Lam BYK, Shi L, Ma KKY, Au LWC, Soo YOY, Leung TWH, Wong A, Mok VCT. Pandemic of the aging society - sporadic cerebral small vessel disease. Chin Med J (Engl). 2021;134:143-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: A clinical review. Neurology. 2019;92:1146-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 445] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 3. | Shi Y, Wardlaw JM. Update on cerebral small vessel disease: a dynamic whole-brain disease. Stroke Vasc Neurol. 2016;1:83-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 322] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 4. | Caunca MR, De Leon-Benedetti A, Latour L, Leigh R, Wright CB. Neuroimaging of Cerebral Small Vessel Disease and Age-Related Cognitive Changes. Front Aging Neurosci. 2019;11:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Baliyan V, Das CJ, Sharma R, Gupta AK. Diffusion weighted imaging: Technique and applications. World J Radiol. 2016;8:785-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 166] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (3)] |
| 6. | Mueller BA, Lim KO, Hemmy L, Camchong J. Diffusion MRI and its Role in Neuropsychology. Neuropsychol Rev. 2015;25:250-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Rokem A, Takemura H, Bock AS, Scherf KS, Behrmann M, Wandell BA, Fine I, Bridge H, Pestilli F. The visual white matter: The application of diffusion MRI and fiber tractography to vision science. J Vis. 2017;17:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Qiu A, Mori S, Miller MI. Diffusion tensor imaging for understanding brain development in early life. Annu Rev Psychol. 2015;66:853-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 9. | Tae WS, Ham BJ, Pyun SB, Kang SH, Kim BJ. Current Clinical Applications of Diffusion-Tensor Imaging in Neurological Disorders. J Clin Neurol. 2018;14:129-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 10. | Tax CMW, Bastiani M, Veraart J, Garyfallidis E, Okan Irfanoglu M. What's new and what's next in diffusion MRI preprocessing. Neuroimage. 2022;249:118830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 11. | Li Q, Yang Y, Reis C, Tao T, Li W, Li X, Zhang JH. Cerebral Small Vessel Disease. Cell Transplant. 2018;27:1711-1722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 198] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 12. | D'Souza MM, Gorthi SP, Vadwala K, Trivedi R, Vijayakumar C, Kaur P, Khushu S. Diffusion tensor tractography in cerebral small vessel disease: correlation with cognitive function. Neuroradiol J. 2018;31:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Sorond FA, Cruz-Almeida Y, Clark DJ, Viswanathan A, Scherzer CR, De Jager P, Csiszar A, Laurienti PJ, Hausdorff JM, Chen WG, Ferrucci L, Rosano C, Studenski SA, Black SE, Lipsitz LA. Aging, the Central Nervous System, and Mobility in Older Adults: Neural Mechanisms of Mobility Impairment. J Gerontol A Biol Sci Med Sci. 2015;70:1526-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Benjamin P, Zeestraten E, Lambert C, Ster IC, Williams OA, Lawrence AJ, Patel B, MacKinnon AD, Barrick TR, Markus HS. Progression of MRI markers in cerebral small vessel disease: Sample size considerations for clinical trials. J Cereb Blood Flow Metab. 2016;36:228-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18:684-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 1029] [Article Influence: 171.5] [Reference Citation Analysis (0)] |
| 16. | Sudre CH, Smith L, Atkinson D, Chaturvedi N, Ourselin S, Barkhof F, Hughes AD, Jäger HR, Cardoso MJ. Cardiovascular Risk Factors and White Matter Hyperintensities: Difference in Susceptibility in South Asians Compared With Europeans. J Am Heart Assoc. 2018;7:e010533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Gong L, Liu XY, Fang M. Recent progress on small vessel disease with cognitive impairment. Int J Clin Exp Med. 2015;8:7701-7709. [PubMed] |
| 18. | Heye AK, Thrippleton MJ, Chappell FM, Hernández Mdel C, Armitage PA, Makin SD, Maniega SM, Sakka E, Flatman PW, Dennis MS, Wardlaw JM. Blood pressure and sodium: Association with MRI markers in cerebral small vessel disease. J Cereb Blood Flow Metab. 2016;36:264-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Lambert C, Sam Narean J, Benjamin P, Zeestraten E, Barrick TR, Markus HS. Characterising the grey matter correlates of leukoaraiosis in cerebral small vessel disease. Neuroimage Clin. 2015;9:194-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Du J, Zhu H, Yu L, Lu P, Qiu Y, Zhou Y, Cao W, Lu D, Zhao W, Yang J, Sun J, Xu Q. Multi-Dimensional Diffusion Tensor Imaging Biomarkers for Cognitive Decline From the Preclinical Stage: A Study of Post-stroke Small Vessel Disease. Front Neurol. 2021;12:687959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Wang Z, Chen Q, Chen J, Yang N, Zheng K. Risk factors of cerebral small vessel disease: A systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e28229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 22. | Osman O, De Guio F, Chabriat H, Jouvent E. Why Are Only Some Subcortical Ischemic Lesions on Diffusion Magnetic Resonance Imaging Associated With Stroke Symptoms in Small Vessel Disease? Stroke. 2018;49:1920-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Che Mohd Nassir CMN, Damodaran T, Yusof SR, Norazit A, Chilla G, Huen I, K N BP, Mohamed Ibrahim N, Mustapha M. Aberrant Neurogliovascular Unit Dynamics in Cerebral Small Vessel Disease: A Rheological Clue to Vascular Parkinsonism. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Litak J, Mazurek M, Kulesza B, Szmygin P, Litak J, Kamieniak P, Grochowski C. Cerebral Small Vessel Disease. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 25. | Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O'Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge Rv, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M; STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2813] [Cited by in RCA: 3984] [Article Influence: 332.0] [Reference Citation Analysis (0)] |
| 26. | Figley TD, Bhullar N, Courtney SM, Figley CR. Probabilistic atlases of default mode, executive control and salience network white matter tracts: an fMRI-guided diffusion tensor imaging and tractography study. Front Hum Neurosci. 2015;9:585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Taylor WD, Hsu E, Krishnan KR, MacFall JR. Diffusion tensor imaging: background, potential, and utility in psychiatric research. Biol Psychiatry. 2004;55:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Huisman TA. Diffusion-weighted and diffusion tensor imaging of the brain, made easy. Cancer Imaging. 2010;10 Spec no A:S163-S171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Sundgren PC, Dong Q, Gómez-Hassan D, Mukherji SK, Maly P, Welsh R. Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology. 2004;46:339-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 282] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 30. | Ladd ME, Bachert P, Meyerspeer M, Moser E, Nagel AM, Norris DG, Schmitter S, Speck O, Straub S, Zaiss M. Pros and cons of ultra-high-field MRI/MRS for human application. Prog Nucl Magn Reson Spectrosc. 2018;109:1-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 332] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 31. | Polders DL, Leemans A, Hendrikse J, Donahue MJ, Luijten PR, Hoogduin JM. Signal to noise ratio and uncertainty in diffusion tensor imaging at 1.5, 3.0, and 7.0 Tesla. J Magn Reson Imaging. 2011;33:1456-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Yousaf T, Dervenoulas G, Politis M. Advances in MRI Methodology. Int Rev Neurobiol. 2018;141:31-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 33. | Tocchio S, Kline-Fath B, Kanal E, Schmithorst VJ, Panigrahy A. MRI evaluation and safety in the developing brain. Semin Perinatol. 2015;39:73-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 34. | Brady LS. Assessing biomarkers for brain diseases: progress and gaps. Genome Med. 2013;5:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Wu TC, Wilde EA, Bigler ED, Li X, Merkley TL, Yallampalli R, McCauley SR, Schnelle KP, Vasquez AC, Chu Z, Hanten G, Hunter JV, Levin HS. Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Dev Neurosci. 2010;32:361-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 36. | Liu B, Zhu T, Zhong J. Comparison of quality control software tools for diffusion tensor imaging. Magn Reson Imaging. 2015;33:276-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Mukherjee P, Berman JI, Chung SW, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. AJNR Am J Neuroradiol. 2008;29:632-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 339] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 38. | Ressel V, van Hedel HJA, Scheer I, O'Gorman Tuura R. Comparison of DTI analysis methods for clinical research: influence of pre-processing and tract selection methods. Eur Radiol Exp. 2018;2:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Bernasconi N, Duchesne S, Janke A, Lerch J, Collins DL, Bernasconi A. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. Neuroimage. 2004;23:717-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 235] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 40. | Makropoulos A, Gousias IS, Ledig C, Aljabar P, Serag A, Hajnal JV, Edwards AD, Counsell SJ, Rueckert D. Automatic whole brain MRI segmentation of the developing neonatal brain. IEEE Trans Med Imaging. 2014;33:1818-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 41. | Ivkovic M, Liu B, Ahmed F, Moore D, Huang C, Raj A, Kovanlikaya I, Heier L, Relkin N. Differential diagnosis of normal pressure hydrocephalus by MRI mean diffusivity histogram analysis. AJNR Am J Neuroradiol. 2013;34:1168-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Jhaveri KS, Wong F, Ghai S, Haider MA. Comparison of CT histogram analysis and chemical shift MRI in the characterization of indeterminate adrenal nodules. AJR Am J Roentgenol. 2006;187:1303-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Gozzi A, Schwarz A, Reese T, Bertani S, Crestan V, Bifone A. Region-specific effects of nicotine on brain activity: a pharmacological MRI study in the drug-naïve rat. Neuropsychopharmacology. 2006;31:1690-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Wirth W, Buck R, Nevitt M, Le Graverand MP, Benichou O, Dreher D, Davies RY, Lee JH, Picha K, Gimona A, Maschek S, Hudelmaier M, Eckstein F; OAI Investigators. MRI-based extended ordered values more efficiently differentiate cartilage loss in knees with and without joint space narrowing than region-specific approaches using MRI or radiography--data from the OA initiative. Osteoarthritis Cartilage. 2011;19:689-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Dai H, Yin D, Hu C, Morelli JN, Hu S, Yan X, Xu D. Whole-brain voxel-based analysis of diffusion tensor MRI parameters in patients with primary open angle glaucoma and correlation with clinical glaucoma stage. Neuroradiology. 2013;55:233-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Campbell JS, Pike GB. Potential and limitations of diffusion MRI tractography for the study of language. Brain Lang. 2014;131:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 47. | Gutman DA, Keifer OP Jr, Magnuson ME, Choi DC, Majeed W, Keilholz S, Ressler KJ. A DTI tractography analysis of infralimbic and prelimbic connectivity in the mouse using high-throughput MRI. Neuroimage. 2012;63:800-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | Bucci M, Mandelli ML, Berman JI, Amirbekian B, Nguyen C, Berger MS, Henry RG. Quantifying diffusion MRI tractography of the corticospinal tract in brain tumors with deterministic and probabilistic methods. Neuroimage Clin. 2013;3:361-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 49. | Farquharson S, Tournier JD, Calamante F, Fabinyi G, Schneider-Kolsky M, Jackson GD, Connelly A. White matter fiber tractography: why we need to move beyond DTI. J Neurosurg. 2013;118:1367-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 322] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 50. | Soltaninejad M, Yang G, Lambrou T, Allinson N, Jones TL, Barrick TR, Howe FA, Ye X. Supervised learning based multimodal MRI brain tumour segmentation using texture features from supervoxels. Comput Methods Programs Biomed. 2018;157:69-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 51. | Wieshmann UC, Symms MR, Parker GJ, Clark CA, Lemieux L, Barker GJ, Shorvon SD. Diffusion tensor imaging demonstrates deviation of fibres in normal appearing white matter adjacent to a brain tumour. J Neurol Neurosurg Psychiatry. 2000;68:501-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Klistorner A, Wang C, Yiannikas C, Parratt J, Dwyer M, Barton J, Graham SL, You Y, Liu S, Barnett MH. Evidence of progressive tissue loss in the core of chronic MS lesions: A longitudinal DTI study. Neuroimage Clin. 2018;17:1028-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 53. | Brueggen K, Grothe MJ, Dyrba M, Fellgiebel A, Fischer F, Filippi M, Agosta F, Nestor P, Meisenzahl E, Blautzik J, Frölich L, Hausner L, Bokde ALW, Frisoni G, Pievani M, Klöppel S, Prvulovic D, Barkhof F, Pouwels PJW, Schröder J, Hampel H, Hauenstein K, Teipel S. The European DTI Study on Dementia - A multicenter DTI and MRI study on Alzheimer's disease and Mild Cognitive Impairment. Neuroimage. 2017;144:305-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Rensma SP, van Sloten TT, Launer LJ, Stehouwer CDA. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:164-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 229] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 55. | Wardlaw JM, Makin SJ, Valdés Hernández MC, Armitage PA, Heye AK, Chappell FM, Muñoz-Maniega S, Sakka E, Shuler K, Dennis MS, Thrippleton MJ. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimers Dement. 2017;13:634-643. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 56. | Lamar M, Charlton RA, Morris RG, Markus HS. The impact of subcortical white matter disease on mood in euthymic older adults: a diffusion tensor imaging study. Am J Geriatr Psychiatry. 2010;18:634-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Smagula SF, Aizenstein HJ. Brain structural connectivity in late-life major depressive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31:17-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 576] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 59. | Croall ID, Lohner V, Moynihan B, Khan U, Hassan A, O'Brien JT, Morris RG, Tozer DJ, Cambridge VC, Harkness K, Werring DJ, Blamire AM, Ford GA, Barrick TR, Markus HS. Using DTI to assess white matter microstructure in cerebral small vessel disease (SVD) in multicentre studies. Clin Sci (Lond). 2017;131:1361-1373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 60. | Lope-Piedrafita S. Diffusion Tensor Imaging (DTI). Methods Mol Biol. 2018;1718:103-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 61. | Wu YF, Wu WB, Liu QP, He WW, Ding H, Nedelska Z, Hort J, Zhang B, Xu Y. Presence of lacunar infarctions is associated with the spatial navigation impairment in patients with mild cognitive impairment: a DTI study. Oncotarget. 2016;7:78310-78319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Pandey AK, Dhiman S, ArunRaj ST, Patel C, Bal C, Kumar R. Designing and Comparing Performances of Image Processing Pipeline for Enhancement of I-131-metaiodobenzylguanidine Images. Indian J Nucl Med. 2021;36:125-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 63. | Soares JM, Marques P, Alves V, Sousa N. A hitchhiker's guide to diffusion tensor imaging. Front Neurosci. 2013;7:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 509] [Cited by in RCA: 567] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 64. | Patel V, Dinov ID, Van Horn JD, Thompson PM, Toga AW. LONI MiND: metadata in NIfTI for DWI. Neuroimage. 2010;51:665-676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Larobina M, Murino L. Medical image file formats. J Digit Imaging. 2014;27:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 66. | Li X, Morgan PS, Ashburner J, Smith J, Rorden C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J Neurosci Methods. 2016;264:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 601] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 67. | Bonilha L, Nesland T, Martz GU, Joseph JE, Spampinato MV, Edwards JC, Tabesh A. Medial temporal lobe epilepsy is associated with neuronal fibre loss and paradoxical increase in structural connectivity of limbic structures. J Neurol Neurosurg Psychiatry. 2012;83:903-909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 68. | Veraart J, Sijbers J, Sunaert S, Leemans A, Jeurissen B. Weighted linear least squares estimation of diffusion MRI parameters: strengths, limitations, and pitfalls. Neuroimage. 2013;81:335-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 365] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 69. | Kargar S, Borisch EA, Froemming AT, Kawashima A, Mynderse LA, Stinson EG, Trzasko JD, Riederer SJ. Robust and efficient pharmacokinetic parameter non-linear least squares estimation for dynamic contrast enhanced MRI of the prostate. Magn Reson Imaging. 2018;48:50-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Feigl GC, Hiergeist W, Fellner C, Schebesch KM, Doenitz C, Finkenzeller T, Brawanski A, Schlaier J. Magnetic resonance imaging diffusion tensor tractography: evaluation of anatomic accuracy of different fiber tracking software packages. World Neurosurg. 2014;81:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 71. | Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 834] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 72. | Liu J, Li W, Zhou S, Zhang L, Wang Z, Zhang Y, Jiang Y, Li L. Functional characteristics of the brain in college students with internet gaming disorder. Brain Imaging Behav. 2016;10:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Tristán-Vega A, Aja-Fernández S, Westin CF. Least squares for diffusion tensor estimation revisited: propagation of uncertainty with Rician and non-Rician signals. Neuroimage. 2012;59:4032-4043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Christidi F, Karavasilis E, Samiotis K, Bisdas S, Papanikolaou N. Fiber tracking: A qualitative and quantitative comparison between four different software tools on the reconstruction of major white matter tracts. Eur J Radiol Open. 2016;3:153-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 75. | Jones DK, Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 2010;23:803-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 626] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 76. | Du J, Zhu H, Zhou J, Lu P, Qiu Y, Yu L, Cao W, Zhi N, Yang J, Xu Q, Sun J, Zhou Y. Structural Brain Network Disruption at Preclinical Stage of Cognitive Impairment Due to Cerebral Small Vessel Disease. Neuroscience. 2020;449:99-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 77. | Huang L, Chen X, Sun W, Chen H, Ye Q, Yang D, Li M, Luo C, Ma J, Shao P, Xu H, Zhang B, Zhu X, Xu Y. Early Segmental White Matter Fascicle Microstructural Damage Predicts the Corresponding Cognitive Domain Impairment in Cerebral Small Vessel Disease Patients by Automated Fiber Quantification. Front Aging Neurosci. 2020;12:598242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 78. | Fukui Y, Hishikawa N, Sato K, Nakano Y, Morihara R, Ohta Y, Yamashita T, Abe K. Characteristic diffusion tensor tractography in multiple system atrophy with predominant cerebellar ataxia and cortical cerebellar atrophy. J Neurol. 2016;263:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 79. | Lützkendorf R, Bernarding J, Hertel F, Viezens F, Thiel A, Krefting D. Enabling of grid based diffusion tensor imaging using a workflow implementation of FSL. Stud Health Technol Inform. 2009;147:72-81. [PubMed] |
| 80. | Schilling KG, Janve V, Gao Y, Stepniewska I, Landman BA, Anderson AW. Histological validation of diffusion MRI fiber orientation distributions and dispersion. Neuroimage. 2018;165:200-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 81. | Serai SD, Otero HJ, Calle-Toro JS, Berman JI, Darge K, Hartung EA. Diffusion tensor imaging of the kidney in healthy controls and in children and young adults with autosomal recessive polycystic kidney disease. Abdom Radiol (NY). 2019;44:1867-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Wang Y, Gupta A, Liu Z, Zhang H, Escolar ML, Gilmore JH, Gouttard S, Fillard P, Maltbie E, Gerig G, Styner M. DTI registration in atlas based fiber analysis of infantile Krabbe disease. Neuroimage. 2011;55:1577-1586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 83. | Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30:1323-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4857] [Cited by in RCA: 5245] [Article Influence: 403.5] [Reference Citation Analysis (0)] |
| 84. | Laganà M, Rovaris M, Ceccarelli A, Venturelli C, Marini S, Baselli G. DTI parameter optimisation for acquisition at 1.5T: SNR analysis and clinical application. Comput Intell Neurosci. 2010;254032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 85. | Du H, Xie B, Lu P, Feng H, Wang J, Yuan S. Impaired white-matter integrity in photosensitive epilepsy: a DTI study using tract-based spatial statistics. J Neuroradiol. 2014;41:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Griffiths TT, Flather R, Teh I, Haroon HA, Shelley D, Plein S, Bourke G, Wade RG. Diffusion tensor imaging in cubital tunnel syndrome. Sci Rep. 2021;11:14982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 87. | Bergamino M, Keeling EG, Walsh RR, Stokes AM. Systematic Assessment of the Impact of DTI Methodology on Fractional Anisotropy Measures in Alzheimer's Disease. Tomography. 2021;7:20-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 88. | Mak E, Dounavi ME, Low A, Carter SF, McKiernan E, Williams GB, Jones PS, Carriere I, Muniz GT, Ritchie K, Ritchie C, Su L, O'Brien JT. Proximity to dementia onset and multi-modal neuroimaging changes: The prevent-dementia study. Neuroimage. 2021;229:117749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 89. | Houston JT, Nenert R, Allendorfer JB, Bebin EM, Gaston TE, Goodman AM, Szaflarski JP. White matter integrity after cannabidiol administration for treatment resistant epilepsy. Epilepsy Res. 2021;172:106603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Richards TJ, Anderson KL, Anderson JS. "Fully automated segmentation of the corticospinal tract using the TractSeg algorithm in patients with brain tumors". Clin Neurol Neurosurg. 2021;210:107001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 91. | Nigro P, Chiappiniello A, Simoni S, Paolini Paoletti F, Cappelletti G, Chiarini P, Filidei M, Eusebi P, Guercini G, Santangelo V, Tarducci R, Calabresi P, Parnetti L, Tambasco N. Changes of olfactory tract in Parkinson's disease: a DTI tractography study. Neuroradiology. 2021;63:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 92. | Otero HJ, Calle-Toro JS, Maya CL, Darge K, Serai SD. DTI of the kidney in children: comparison between normal kidneys and those with ureteropelvic junction (UPJ) obstruction. MAGMA. 2020;33:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 93. | Kreilkamp BAK, Lisanti L, Glenn GR, Wieshmann UC, Das K, Marson AG, Keller SS. Comparison of manual and automated fiber quantification tractography in patients with temporal lobe epilepsy. Neuroimage Clin. 2019;24:102024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 94. | Kikinis R, Pieper S. 3D Slicer as a tool for interactive brain tumor segmentation. Annu Int Conf IEEE Eng Med Biol Soc. 2011;2011:6982-6984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |