Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8436
Peer-review started: April 1, 2022
First decision: June 7, 2022
Revised: June 16, 2022
Accepted: July 16, 2022
Article in press: July 16, 2022
Published online: August 26, 2022
Processing time: 136 Days and 13 Hours
Although gastroesophageal reflux disease (GERD), a common chronic disease in clinical practice, has been widely studied, its potential adverse impact on patients is still a significant clinical concern. It is necessary to understand the pathogenesis of the disease and choose appropriate treatment according to its mechanism. The pathogenesis of GERD is diverse and complex. As the traditional treatment methods are expensive and ineffective in alleviating symptoms in some patients, new treatment options need to be explored. Our previous study suggested that the activation of nuclear factor-kappa beta (NF-κB) in esophageal mucosa may be related to the injury of epithelial barrier function caused by reflux. Based on the literature and our previous study results, it is speculated that inhibition of NF-κB activation may block the insult of GERD on the esophageal mucosal barrier. NF-κB may play an important role in the development of GERD. This article reviews the pathogenesis of GERD and the relationship between NF-κB and GERD, in order to provide new strategies for the treatment of GERD.
Core Tip: Gastroesophageal reflux disease (GERD) is one of the most common chronic diseases. Current treatments, including drugs and surgery, have significant side effects and some patients do not respond to treatment. This article reviews the pathogenesis of GERD, especially the relationship between the NF-κB pathway and GERD. We also assessed the latest studies on the effects of drugs inhibiting the NF-κB pathway in GERD, providing new possibilities for the treatment of GERD.
- Citation: Zhang ML, Ran LQ, Wu MJ, Jia QC, Qin ZM, Peng YG. NF-κB: A novel therapeutic pathway for gastroesophageal reflux disease? World J Clin Cases 2022; 10(24): 8436-8442
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8436.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8436
Gastroesophageal reflux disease (GERD) is a common clinical disorder in western countries[1]. Symptomatic GERD affects 10%-20% of the population in the western world and 5% of the population in Asia including China. GERD is a serious complication in patients who undergo esophagectomy and gastric tube reconstruction due to cancer. Approximately 50% of patients with esophageal cancer have GERD symptoms after surgery, including burning sensations in the pharynx and neck, obstruction when eating, cervical heartburn, belching, acid reflux, and retrosternal pain[2,3]. When reflux invades the mouth, this can cause soft tissue damage, erosive dental lesions[4], and exposure of dentin often causes painful symptoms[5]. GERD occurs not only after surgery, but also in the non-surgical population.
A large number of previous studies have confirmed that gastroesophageal reflux leads to the destruction of esophageal epithelial barrier function; however, the specific mechanism is still not completely clear. Gastroesophageal reflux leads to the destruction of esophageal epithelial barrier function by regulating the expression and distribution of tight junction proteins (such as Occludin, Cldn1, Cldn3 and Cldn4), reducing the number of desmosomes, and the direct hydrolysis of adhesive junction proteins (such as E-cadherin). This is manifested by the widening of intercellular spaces (ICS) and the reduction of trans-epithelial electrical resistance (TEER)[6-11]. In addition, this is accompanied by an inflammatory response in the mucosal epithelium[12].
Nuclear factor-kappa beta (NF-κB) is an important transcription factor associated with inflammation, which regulates apoptosis, viral replication, tumor formation and autoimmunity in addition to the inflammatory response. Reflux can directly stimulate the esophageal epithelium to recruit a large number of inflammatory cells, activate NF-κB and release inflammatory chemokines (such as interleukin (IL)-1β, IL-6 and IL-8). The up-regulated inflammatory factors and inflammatory cells in turn further activate NF-κB expression in esophageal epithelium[13]. Several clinical studies have shown that NF-κB and related inflammatory factors IL-1β and IL-8 are up-regulated in GERD esophageal mucosa[14-18]. Compared with traditional medications and surgical intervention, targeting NF-κB-mediated esophageal epithelial barrier injury may be a more effective treatment for GERD. It can not only effectively relieve symptoms, but also significantly reduce the side effects caused by medications. Unfortunately, there are few reports on this issue.
Our previous study suggested that the activation of NF-κB in esophageal mucosa may be responsible for the interruption of epithelial barrier function caused by reflux. NF-κB can be activated by different stimuli and is considered to be part of the systemic stress response. Based on the literature and our previous study results[19-28], it can be hypothesized that inhibition of NF-κB activation may block the damage to the esophageal mucosal barrier caused by GERD. To prove this theory, we plan in conjunction with in vitro experiments and an animal study to further elucidate the role of NF-κB in the mechanism of reflux-induced esophageal epithelial barrier dysfunction, and explore the effectiveness of specific inhibition of NF-κB activity on reflux-induced esophageal epithelial barrier dysfunction. This alternative therapeutic approach may be a superior intervention for GERD than traditional treatment. The completion of this study will not only further reveal the molecular pathogenesis of esophageal mucosal injury caused by GERD, but also provide a theoretical and experimental basis for the establishment of new treatment methods for GERD.
We conducted a descriptive review of the mechanism associated with GERD in relation to NF-κB. PubMed was searched for articles published from July 1966 to February 2022, using the following MeSH or free-text key words: GERD, NF-κB, pathogenesis, mechanism, inflammatory injury, and esophageal epithelial barrier. The search was limited to papers written in English, with no restrictions on the type of article.
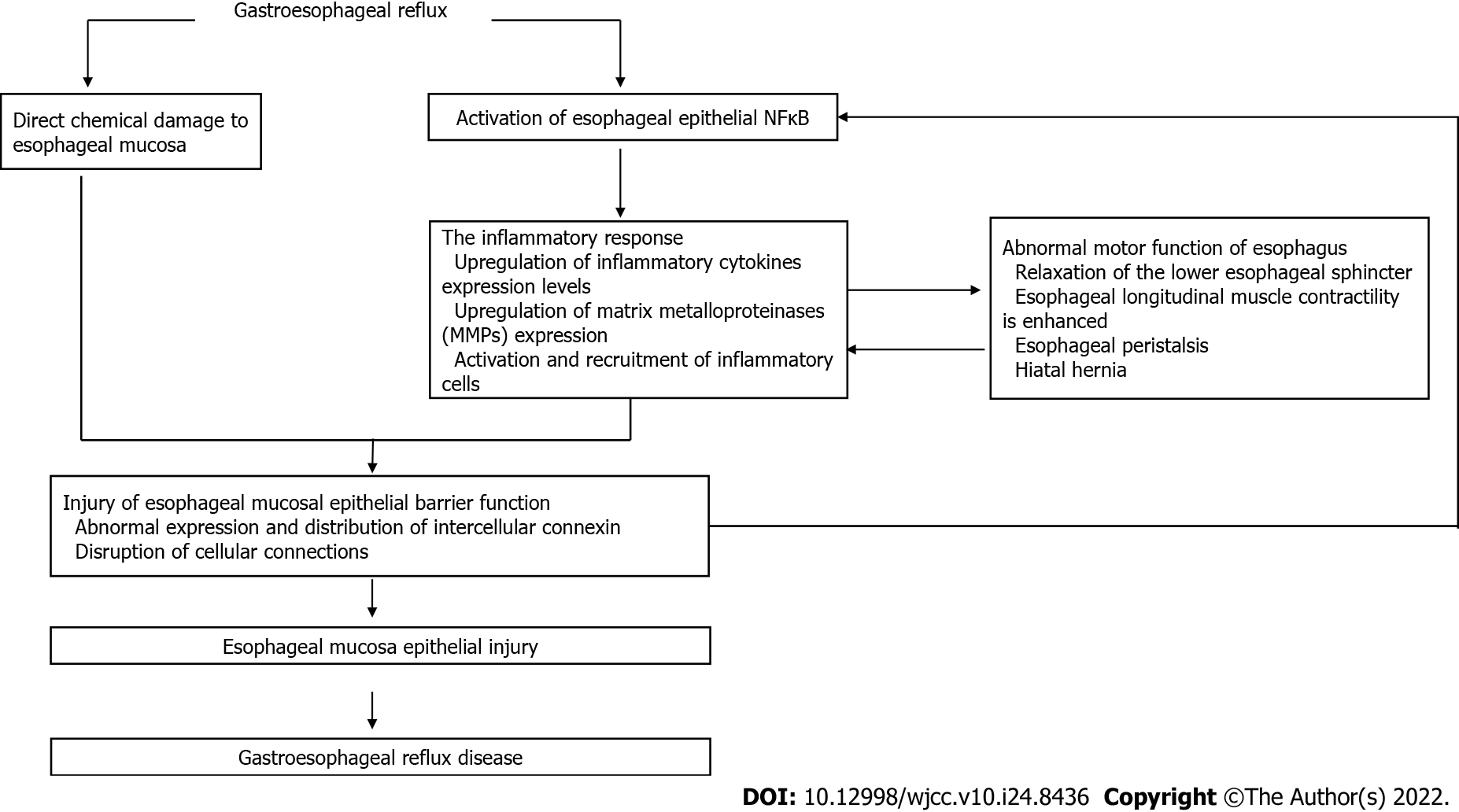
GERD is a disorder caused by the retrograde flow of reflux into the esophagus. The pathogenesis of GERD involves the interaction of chemical, mechanical, psychological and neural mechanisms (Figure 1).
The reflux insult to esophageal mucosa is the most important pathophysiological mechanism of GERD[29]. However, the components of refluxate are diverse, and include gastric acid, bile acid, and pepsin[30]. Each component has its unique destructive mechanism on the esophageal defense system and consequential impact. GERD is often thought of as acid reflux, where acid refers to hydrochloric acid (HCl)[30], which is a very destructive substance. At the cellular level, the damage caused by HCl to esophageal mucosa is partly due to its influence on the potential difference of esophageal mucosa, which leads to the loss of integrity of epithelial cells and degeneration and necrosis of these cells[31]. In the presence of an acid pocket and hiatal hernia, this increases the exposure time of the esophagus to acidic conditions and is more likely to lead to GERD[32]. However, some patients were found to have a transient elevation in pH up to 7.0 when esophageal pH was tested, indicating the possible presence of alkalinizing agents[33]. Some studies have shown that there is a correlation between bile acid concentration and elevated pH[34]. Under the action of acids, bile acids become lipophilic and can dissolve cell membranes, thus destroying the integrity of the cell after passing through the membrane. It has also been shown to increase the absorption of hydrogen ions in esophageal tissue[35], and the higher the bile concentration, the more esophageal epithelial cells are exposed to this environment, and severe injury can be expected[36]. Bile acid stimulates the release of various inflammatory factors, suggesting that it may have a direct insult on the esophagus[36]. Pepsin, as a peptidase, has a wide range of protein-substrate properties and its release into the esophagus and adjacent structures can cause injury to the surrounding tissues. Unlike the gastrointestinal tract, the esophagus lacks a layer of mucus to protect itself from pepsin digestion and cannot prevent digestion by raising its pH[37]. Thus, pepsin can be activated in the esophagus, leading to cell injury either directly or indirectly[38,39].
When the refluxate enters the esophagus, the esophageal mucosa cannot create the necessary biochemical environment to neutralize the reflux due to the lack of mucous secreting cells and bicarbonate production. In order to reduce the exposure time of the esophageal mucosa to reflux, the clearance mechanism is particularly important. Therefore, it can be speculated that a reduction in esophageal clearance rate will lead to GERD, which is supported by previous literature[40]. The factors affecting esophageal clearance include chemical and mechanical mechanisms, such as glandular secretion and esophageal motility pattern. Salivary secretion can affect esophageal clearance through neutralization of acid. It has been suggested in the literature that reduced salivary gland secretion due to other factors is associated with the development of GERD. The relationship between esophageal dysmotility and GERD is a bidirectional influence. Esophageal motor dysfunction and lower esophageal sphincter (LES) relaxation lead to prolonged indwelling of reflux in the esophagus and reduced clearance rate[41,42], subsequently leading to GERD.
Based on anatomy, the major portion of the esophagus is located in the thoracic cavity, and the pressure in the thoracic cavity is lower than that in the abdominal cavity. The maintenance of tension in the LES plays a crucial role in preventing reflux from entering the esophagus. The LES no longer maintains its tension due to external causes such as obesity, hiatal hernia, low tension in the LES itself, or elevated pressure in the abdominal cavity, resulting in reflux into the esophagus and the development of GERD[43,44]. As a related factor, shorter abdominal cavity length was found to cause more reflux[45], which may also be related to the formation of a pressure gradient.
The esophageal mucosal barrier is mainly composed of esophageal mucosal epithelial cells. The defensive barrier structure of esophageal epithelium is mainly composed of the apical junctional complexes (AJCs) of esophageal keratinocytes and epithelial cell membrane, which is responsible for preventing luminal ions (mainly hydrogen ions) and small molecules from entering the submucosa[46]. The cell AJCs consist of tight junctions, adherent junctions and desmosomes[47,48]. The esophageal epithelial barrier function mainly involves TEER, the permeability of mucosal epithelium to neutral small molecules and the ICS. A lower TEER value of the same type of epithelial tissue in the same area indicates that the mucosal permeability to ions is stronger, and the mucosal defense barrier function is weaker. GERD activates inflammation when the epithelial barrier is disrupted, and NF-κB is an important transcription factor associated with inflammation[49,50]. Reflux can directly stimulate the esophageal epithelium to produce inflammatory cytokines, up-regulate NF-κB expression, and release inflammatory chemokines such as IL-1β, IL-6 and IL-8. Changes in the microenvironment in turn activate NF-κB to form a positive feedback[11]. As previously discussed, a large number of clinical studies have shown that GERD esophageal epithelium NF-κB and related inflammatory factors are up-regulated. NF-κB can directly regulate tight junction protein expression and impair epithelial barrier function by relaxing tight junctions[51-53]. Previous animal studies have also shown that NF-κB pathway inhibitors can significantly prevent destruction of the reflux-induced esophageal mucosal barrier[44]. During reflux, TEER decreases, which can be offset by the use of inhibitors. Similarly, IL-1β and IL-6 were significantly reduced after the use of NF-κB inhibitors. In another animal model of GERD, a specific inhibitor of NF-κB was also used[20]. Compared with the control group, the inhibitor increased the pH of the distal esophagus, alleviated esophageal mucosal tissue injury and inhibited the inflammatory response, suggesting that NF-κB is a potential therapeutic target for GERD. In addition, in several animal studies using drugs that inhibit the NF-κB pathway, mucosal damage was significantly reduced compared with the control group, and the release of inflammatory factors was reduced as well as oxidation[21-24]. In an in vitro study, lipopolysaccharide (LPS)-induced inflammatory responses in RAW 264.7 cells were also found to be alleviated after treatment with drugs that inhibited the NF-κB pathway[25-28]. These studies suggest that drugs which inhibit the NF-κB pathway can relieve esophageal mucosal injury caused by GERD and down-regulate related inflammatory factors.
In our previous study, we established a mouse model of gastroesophageal reflux and found that injury of the epithelial barrier of reflux esophageal mucosa was associated with NF-κB-mediated inflammation. However, an esophageal perfusion model in rabbits (acid/bile salt was directly injected into the rabbit esophageal cavity through a catheter) suggested that damage to the epithelial barrier function of esophageal mucosa was related to direct chemical injury by reflux. Also, by comparing the above two studies, it was found that acid reflux did not cause obvious injury and inflammation to the esophageal mucous membrane epithelium in mice, but caused obvious damage and inflammation to the esophageal mucosa in rabbits[31]. These differences may be due to the fact that the esophageal mucosa of rodents (e.g., mice and rats) is coated with hyper-keratinized laminated squamous epithelium, which is highly resistant to acid. The esophageal mucosa of rabbit is similar to that of humans, and is covered with incomplete keratinized lamellar squamous epithelium and has poor resistance to acid. In conclusion, we propose that the reduction of esophageal mucosal barrier function induced by gastroesophageal reflux may be the result of a combination of direct chemical destruction and a NF-κB-mediated inflammation process.
The treatment of GERD has many challenges. First, the pathogenesis of GERD has not been completely clarified[54,55]. Although research has made progress in recent years, consensus results have not been established in the literature. However, the incidence of GERD is high and the impact on patients' quality of life is significant. Second, as mentioned above, traditional therapies are flawed and there is a lack of effective targets for treatment. Third, although the relationship between GERD and NF-κB is well documented and NF-κB inhibitors have only been shown to be effective in animal studies, more investigations are warranted to improve their clinical application.
As one of the most common chronic disorders, the symptoms of GERD can be variable, and include non-cardiogenic chest pain, chronic cough, hoarseness, globular and throat irritation[56]. NF-κB activation plays an important role in the development of GERD. However, there is limited information on the treatment of GERD via this pathway. NF-κB is a well-known transcription factor involved in inflammation and cell proliferation. If research is able to demonstrate the benefit of altering NF-κB level in the development of GERD, it would have an enormous impact on GERD treatment in clinical practice.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Buchaim RL, Brazil; Sumi Κ, Japan S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Ma YJ
| 1. | El-Serag HB. Time trends of gastroesophageal reflux disease: a systematic review. Clin Gastroenterol Hepatol. 2007;5:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 306] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 2. | Aly A, Jamieson GG. Reflux after oesophagectomy. Br J Surg. 2004;91:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Poghosyan T, Gaujoux S, Chirica M, Munoz-Bongrand N, Sarfati E, Cattan P. Functional disorders and quality of life after esophagectomy and gastric tube reconstruction for cancer. J Visc Surg. 2011;148:e327-e335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Ortiz AC, Fideles SOM, Pomini KT, Buchaim RL. Updates in association of gastroesophageal reflux disease and dental erosion: systematic review. Expert Rev Gastroenterol Hepatol. 2021;15:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Lussi A, Schlueter N, Rakhmatullina E, Ganss C. Dental erosion--an overview with emphasis on chemical and histopathological aspects. Caries Res. 2011;45 Suppl 1:2-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 265] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 6. | Tobey NA, Carson JL, Alkiek RA, Orlando RC. Dilated intercellular spaces: a morphological feature of acid reflux--damaged human esophageal epithelium. Gastroenterology. 1996;111:1200-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 328] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Jovov B, Que J, Tobey NA, Djukic Z, Hogan BL, Orlando RC. Role of E-cadherin in the pathogenesis of gastroesophageal reflux disease. Am J Gastroenterol. 2011;106:1039-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Oshima T, Koseki J, Chen X, Matsumoto T, Miwa H. Acid modulates the squamous epithelial barrier function by modulating the localization of claudins in the superficial layers. Lab Invest. 2012;92:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Chen X, Oshima T, Shan J, Fukui H, Watari J, Miwa H. Bile salts disrupt human esophageal squamous epithelial barrier function by modulating tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2012;303:G199-G208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Asaoka D, Miwa H, Hirai S, Ohkawa A, Kurosawa A, Kawabe M, Hojo M, Nagahara A, Minoo T, Ohkura R, Ohkusa T, Sato N. Altered localization and expression of tight-junction proteins in a rat model with chronic acid reflux esophagitis. J Gastroenterol. 2005;40:781-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Oguro M, Koike M, Ueno T, Asaoka D, Mori H, Nagahara A, Uchiyama Y, Watanabe S. Dissociation and dispersion of claudin-3 from the tight junction could be one of the most sensitive indicators of reflux esophagitis in a rat model of the disease. J Gastroenterol. 2011;46:629-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Yoshida N. Inflammation and oxidative stress in gastroesophageal reflux disease. J Clin Biochem Nutr. 2007;40:13-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Korkaya H, Liu S, Wicha MS. Regulation of cancer stem cells by cytokine networks: attacking cancer's inflammatory roots. Clin Cancer Res. 2011;17:6125-6129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 260] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 14. | Isomoto H, Nishi Y, Kohno S. CXC receptor 1 is overexpressed in endoscopy-negative gastroesophageal reflux disease. Scand J Gastroenterol. 2005;40:231-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Isomoto H, Saenko VA, Kanazawa Y, Nishi Y, Ohtsuru A, Inoue K, Akazawa Y, Takeshima F, Omagari K, Miyazaki M, Mizuta Y, Murata I, Yamashita S, Kohno S. Enhanced expression of interleukin-8 and activation of nuclear factor kappa-B in endoscopy-negative gastroesophageal reflux disease. Am J Gastroenterol. 2004;99:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Fitzgerald RC, Onwuegbusi BA, Bajaj-Elliott M, Saeed IT, Burnham WR, Farthing MJ. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut. 2002;50:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 211] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 17. | O'Riordan JM, Abdel-latif MM, Ravi N, McNamara D, Byrne PJ, McDonald GS, Κeeling PW, Κelleher D, Reynolds JV. Proinflammatory cytoκine and nuclear factor κappa-B expression along the inflammation-metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Gastroenterol. 2005;100(6):1257-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Cho SW, Hahm KB. Oxidative damages are critical in pathogenesis of reflux esophagitis: implication of antioxidants in its treatment. Free Radic Biol Med. 2001;30:905-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Fang Y, Chen H, Hu Y, Djukic Z, Tevebaugh W, Shaheen NJ, Orlando RC, Hu J, Chen X. Gastroesophageal reflux activates the NF-κB pathway and impairs esophageal barrier function in mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G58-G65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Yu HX, Wang XL, Zhang LN, Zhang J, Zhao W. Involvement of the TLR4/NF-κB Signaling Pathway in the Repair of Esophageal Mucosa Injury in Rats with Gastroesophageal Reflux Disease. Cell Physiol Biochem. 2018;51:1645-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Shin MR, Seo BI, Son CG, Roh SS, An HJ. Banhasasim-Tang Treatment Reduces the Severity of Esophageal Mucosal Ulcer on Chronic Acid Reflux Esophagitis in Rats. Biomed Res Int. 2017;2017:7157212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Huo X, Zhang X, Yu C, Zhang Q, Cheng E, Wang DH, Pham TH, Spechler SJ, Souza RF. In oesophageal squamous cells exposed to acidic bile salt medium, omeprazole inhibits IL-8 expression through effects on nuclear factor-κB and activator protein-1. Gut. 2014;63:1042-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Liu G, Jiang C, Li D, Yao L, Lin Y, Wang B, Qiu J, Wang W. Isorhamnetin alleviates esophageal mucosal injury in a chronic model of reflux esophagitis. Eur J Pharmacol. 2019;864:172720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Lee JA, Shin MR, Kim MJ, Lee JH, Park HJ, Roh SS. Protective Effects of Inflammation of Curcumae Longae Rhizoma 30% EtOH Extract on Acute Reflux Esophagitis Rats. Biomed Res Int. 2021;2021:8854945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Nam HH, Nan L, Choo BK. Dichloromethane Extracts of Geranium Koreanum Kom. Alleviates Esophagus Damage in Acute Reflux Esophagitis-Induced Rats by Anti-Inflammatory Activities. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Nan L, Nam HH, Park BY, Kim BT, Choo BK. Ameliorative effects of Magnolia sieboldii buds hexane extract on experimental reflux esophagitis. Phytother Res. 2020;34:2385-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Nan L, Nam HH, Choo BK, Park JC, Kim DG, Lee JH, Moon KH. An Ethanolic Extract of Allium hookeri Root Alleviates Reflux Esophagitis and Modulates NF-κB Signaling. Evid Based Complement Alternat Med. 2018;2018:1834681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Nam HH, Nan L, Choo BK. Anti-Inflammation and Protective Effects of Anethum graveolens L. (Dill Seeds) on Esophageal Mucosa Damages in Reflux Esophagitis-Induced Rats. Foods. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Sharma P, Yadlapati R. Pathophysiology and treatment options for gastroesophageal reflux disease: looking beyond acid. Ann N Y Acad Sci. 2021;1486:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 30. | Tack J, Pandolfino JE. Pathophysiology of Gastroesophageal Reflux Disease. Gastroenterology. 2018;154:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 219] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 31. | Orlando RC, Powell DW, Carney CN. Pathophysiology of acute acid injury in rabbit esophageal epithelium. J Clin Invest. 1981;68:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Mitchell DR, Derakhshan MH, Robertson EV, McColl KE. The Role of the Acid Pocket in Gastroesophageal Reflux Disease. J Clin Gastroenterol. 2016;50:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Lin KM, Ueda RK, Hinder RA, Stein HJ, DeMeester TR. Etiology and importance of alkaline esophageal reflux. Am J Surg. 1991;162:553-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Stein HJ, Feussner H, Kauer W, DeMeester TR, Siewert JR. Alkaline gastroesophageal reflux: assessment by ambulatory esophageal aspiration and pH monitoring. Am J Surg. 1994;167:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Siddiqui A, Rodriguez-Stanley S, Zubaidi S, Miner PB Jr. Esophageal visceral sensitivity to bile salts in patients with functional heartburn and in healthy control subjects. Dig Dis Sci. 2005;50:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Nehra D, Howell P, Williams CP, Pye JK, Beynon J. Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut. 1999;44:598-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 299] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 37. | Vaezi MF, Richter JE. Role of acid and duodenogastroesophageal reflux in gastroesophageal reflux disease. Gastroenterology. 1996;111:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 341] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 38. | Johnston N, Knight J, Dettmar PW, Lively MO, Koufman J. Pepsin and carbonic anhydrase isoenzyme III as diagnostic markers for laryngopharyngeal reflux disease. Laryngoscope. 2004;114:2129-2134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 149] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 39. | Tobey NA, Hosseini SS, Caymaz-Bor C, Wyatt HR, Orlando GS, Orlando RC. The role of pepsin in acid injury to esophageal epithelium. Am J Gastroenterol. 2001;96:3062-3070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Johnston N, Dettmar PW, Lively MO, Postma GN, Belafsky PC, Birchall M, Koufman JA. Effect of pepsin on laryngeal stress protein (Sep70, Sep53, and Hsp70) response: role in laryngopharyngeal reflux disease. Ann Otol Rhinol Laryngol. 2006;115:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Jones MP, Sloan SS, Jovanovic B, Kahrilas PJ. Impaired egress rather than increased access: an important independent predictor of erosive oesophagitis. Neurogastroenterol Motil. 2002;14:625-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Chrysos E, Prokopakis G, Athanasakis E, Pechlivanides G, Tsiaoussis J, Mantides A, Xynos E. Factors affecting esophageal motility in gastroesophageal reflux disease. Arch Surg. 2003;138:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Fuchs KH, Meining A. Current Insights in the Pathophysiology of Gastroesophageal Reflux Disease. Chirurgia (Bucur). 2021;116:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Wang YK, Hsu WH, Wang SS, Lu CY, Kuo FC, Su YC, Yang SF, Chen CY, Wu DC, Kuo CH. Current pharmacological management of gastroesophageal reflux disease. Gastroenterol Res Pract. 2013;2013:983653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | DeMeester TR, Wernly JA, Bryant GH, Little AG, Skinner DB. Clinical and in vitro analysis of determinants of gastroesophageal competence. A study of the principles of antireflux surgery. Am J Surg. 1979;137:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 123] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Orlando RC. The integrity of the esophageal mucosa. Balance between offensive and defensive mechanisms. Best Pract Res Clin Gastroenterol. 2010;24:873-882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 47. | Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 772] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 48. | Rodgers LS, Fanning AS. Regulation of epithelial permeability by the actin cytoskeleton. Cytoskeleton (Hoboken). 2011;68:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 49. | Shaw DE, Sousa AR, Fowler SJ, Fleming LJ, Roberts G, Corfield J, Pandis I, Bansal AT, Bel EH, Auffray C, Compton CH, Bisgaard H, Bucchioni E, Caruso M, Chanez P, Dahlén B, Dahlen SE, Dyson K, Frey U, Geiser T, Gerhardsson de Verdier M, Gibeon D, Guo YK, Hashimoto S, Hedlin G, Jeyasingham E, Hekking PP, Higenbottam T, Horváth I, Knox AJ, Krug N, Erpenbeck VJ, Larsson LX, Lazarinis N, Matthews JG, Middelveld R, Montuschi P, Musial J, Myles D, Pahus L, Sandström T, Seibold W, Singer F, Strandberg K, Vestbo J, Vissing N, von Garnier C, Adcock IM, Wagers S, Rowe A, Howarth P, Wagener AH, Djukanovic R, Sterk PJ, Chung KF; U-BIOPRED Study Group. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015;46:1308-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 408] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 50. | Rayet B, Gélinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938-6947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 873] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 51. | Al-Sadi R, Ye D, Dokladny K, Ma TY. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J Immunol. 2008;180:5653-5661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 334] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 52. | Al-Sadi R, Ye D, Said HM, Ma TY. IL-1beta-induced increase in intestinal epithelial tight junction permeability is mediated by MEKK-1 activation of canonical NF-kappaB pathway. Am J Pathol. 2010;177:2310-2322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 53. | Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641-4649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 464] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 54. | Richter JE. The many manifestations of gastroesophageal reflux disease: presentation, evaluation, and treatment. Gastroenterol Clin North Am. 2007;36:577-599, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Herregods TV, Bredenoord AJ, Smout AJ. Pathophysiology of gastroesophageal reflux disease: new understanding in a new era. Neurogastroenterol Motil. 2015;27:1202-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 56. | Patcharatrakul T, Gonlachanvit S. Gastroesophageal reflux symptoms in typical and atypical GERD: roles of gastroesophageal acid refluxes and esophageal motility. J Gastroenterol Hepatol. 2014;29:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |