Published online Aug 16, 2022. doi: 10.12998/wjcc.v10.i23.8141
Peer-review started: March 11, 2022
First decision: May 11, 2022
Revised: May 21, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: August 16, 2022
Processing time: 142 Days and 21.9 Hours
Sarcoidosis is a multisystem disorder with unknown etiology, and it predominantly affects the lungs and intrathoracic lymph nodes. For patients with atypical clinical manifestations, the diagnosis of sarcoidosis is difficult and specific biomarkers may play an important role in assisting diagnosis. Previous research has demonstrated a correlation between sarcoidosis and increased carbohydrate antigen 125 (CA125), but remains a lack of large cohort studies to validate this observation.
To compare serum CA125 levels in sarcoidosis patients and healthy controls, and explore whether CA125 can be used as a biomarker for the diagnosis of sarcoidosis.
In this study, the serum CA125 levels were measured by enzyme-linked immunosorbent assay in 108 consecutive sarcoidosis patients between June 2016 and December 2020 (31 males, 77 females; age at diagnosis 49.69 ± 9.10 years) and 112 healthy subjects. Data on the C-reactive protein, erythrocyte sedimentation rate, and angiotensin-converting enzyme were also collected. The association of serum CA125 levels with clinical, radiological, and respiratory functional characteristics was analyzed between patient groups with CA125 ≤ 35 U/mL or CA125 > 35 U/mL.
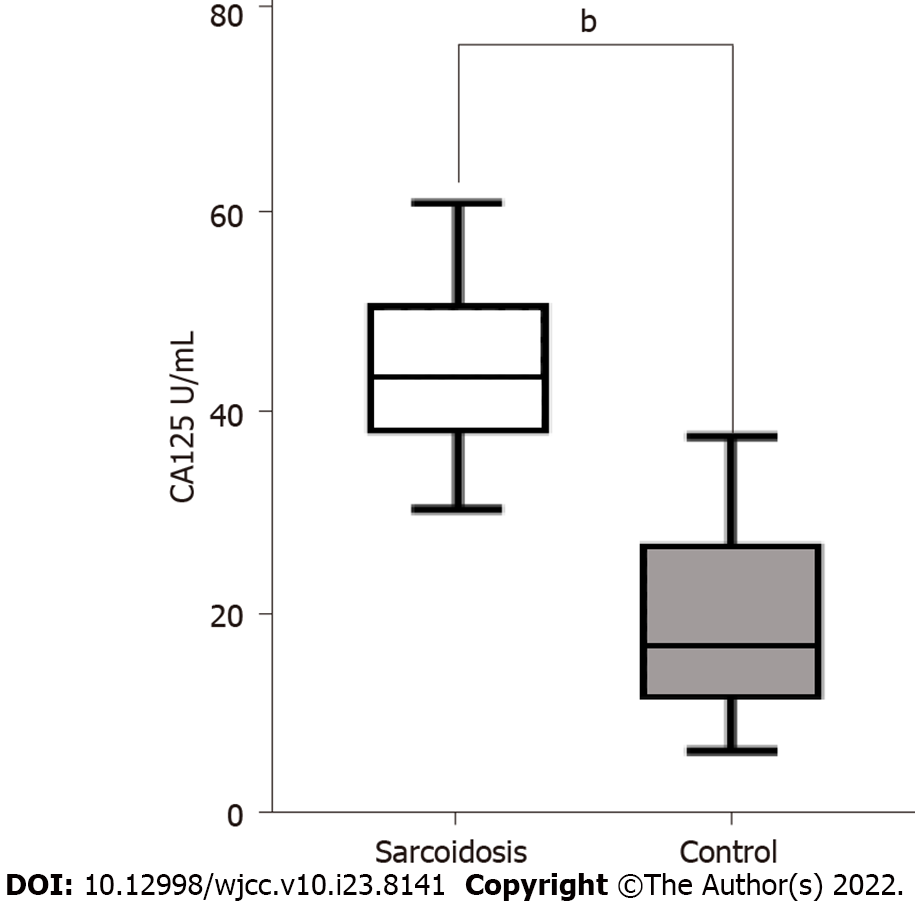
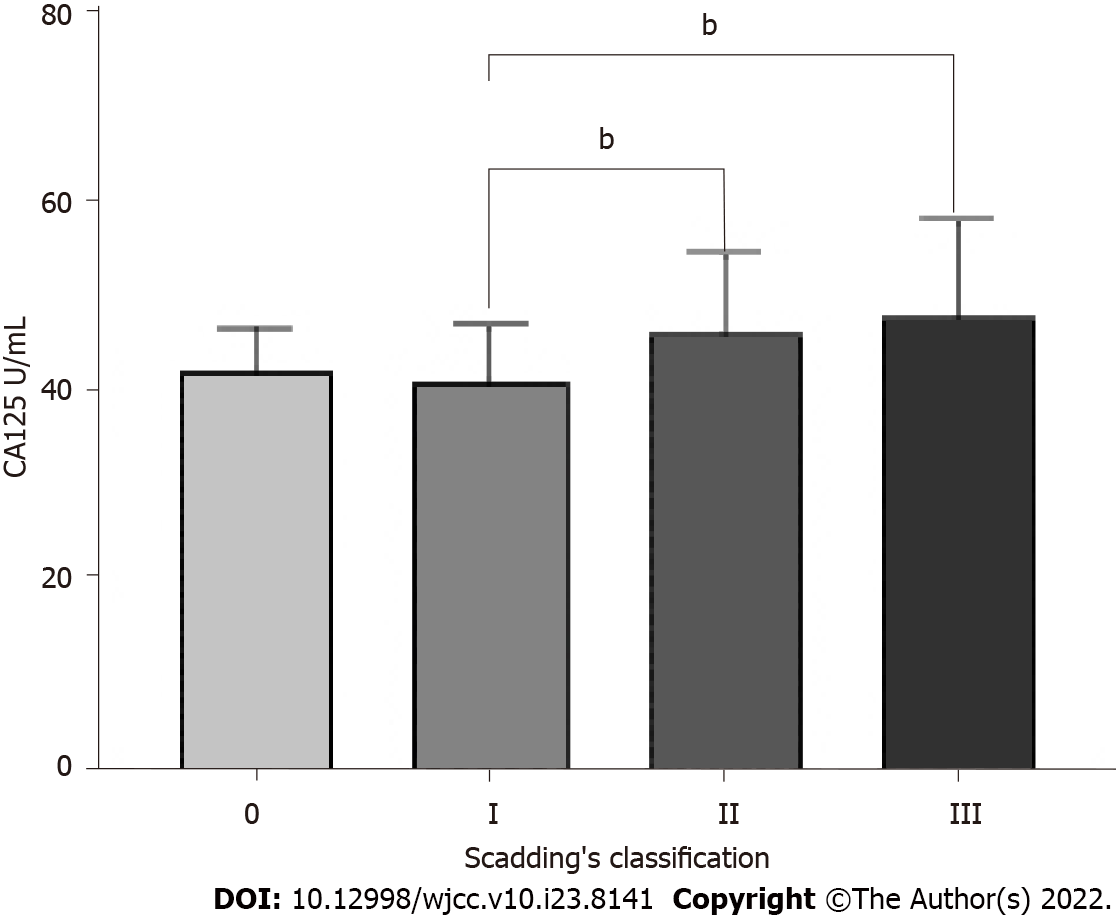
We found that serum CA125 levels were higher in sarcoidosis patients compared to healthy controls (median: 44.78 vs 19.11 U/mL, P < 0.001). The area under the receiver operator characteristic was 0.9833 (95%CI: 0.9717-0.9949), and the best cutoff point was 32.33 U/mL. The elevated serum CA125 was notably associated with the percentage of predicted forced vital capacity (FVC%) and neutrophil-to-lymphocyte ratio (P = 0.043 and P = 0.038, respectively) in sarcoidosis patients. Multivariate analysis revealed that FVC% was a statistically notable predictor of elevated serum CA125 (P = 0.029). Also, our research revealed that compared to patients with Stage I of radiology classification, patients with Stage II and III showed a higher concentration of serum CA125 (46.16 ± 8.32 vs 41.00 ± 6.04 U/mL, P = 0.005, and 47.92 ± 10.10 vs 41.00 ± 6.04 U/mL, P = 0.002, respectively).
Serum CA125 was highly increased in sarcoidosis patients and showed high efficiency for noninvasive diagnosis of the disease. In addition, abnormally elevated serum CA125 was correlated with pulmonary function and radiological Scadding’s classification of sarcoidosis.
Core Tip: This study revealed the overexpression of carbohydrate antigen 125 in sarcoidosis patients, and a close association between the expression level with the percentage of predicted of forced vital capacity measured in pulmonary function test and radiological Scadding’s classification of sarcoidosis. Carbohydrate antigen 125 may be a noninvasive diagnostic biomarker for sarcoidosis patients and may serve as a biomarker in the clinical prediction of sarcoidosis.
- Citation: Zhang Q, Jing XY, Yang XY, Xu ZJ. Diagnostic value of elevated serum carbohydrate antigen 125 level in sarcoidosis. World J Clin Cases 2022; 10(23): 8141-8151
- URL: https://www.wjgnet.com/2307-8960/full/v10/i23/8141.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i23.8141
Sarcoidosis is one of the most common interstitial lung diseases, characterized by non-caseating granulomas and involving almost every organ system in the body. The mechanism of granuloma formation consists of the activation of type 1 T helper cells, leading to the formation of sarcoid granulomas comprised of T cells, macrophages, and epithelioid and giant cells[1]. Most patients with sarcoidosis show a subacute or chronic course of the disease; the less frequent cases of acute onset include manifestations of bilateral hilar lymph node enlargement, arthritis, and nodular erythema, accompanied by fever and myalgia, also known as Löfgren’s syndrome[2]. Although imaging examination can assist physicians in the diagnosis of sarcoidosis, it is difficult to make a clear diagnosis for those patients with atypical clinical symptoms, and an invasive tissue biopsy will be required. Thus, it is critical to develop a useful non-invasive predictive biomarker for disease diagnosis, which would also be helpful for disease treatment and monitoring[3]. Few biomarkers have been proposed as associated with sarcoidosis, and the most well-known of these is serum angiotensin-converting enzyme (ACE). However, due to low sensitivity and specificity, no widely accepted biomarker has been proposed for sarcoidosis diagnosis or predicting disease course[4].
Carbohydrate antigen (CA) 125 is a high molecular weight transmembrane glycoprotein that cleaves from the cell membrane and secretes into serum as circulating CA125. CA125 can be detected on mesothelial cells of the coelomic epithelium, including the pleura, peritoneum and pericardium, and on epithelial cells of the fallopian tubes, endometrium and endocervix. It has been reported that CA125 expression is pronounced in areas of inflammation and adhesion[5]. Elevated serum CA125 has been found in both malignant and nonmalignant diseases, including serous ovarian carcinoma, endometrial carcinoma, adenocarcinoma of the lungs, lymphoma, tuberculosis, collagen vascular diseases, etc.[6-8]. The previous publications reported that elevated serum CA125 levels were correlated with sarcoidosis[9,10], suggesting its potential as a diagnostic biomarker for the disease.
We retrospectively screened 108 consecutive sarcoidosis patients who had been treated in the outpatient clinic of Peking Union Medical College Hospital between June 2016 and December 2020, and compared their levels of serum CA125 with those from a cohort of 112 healthy controls. The sarcoidosis diagnosis had been made by the pathology service following the American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and Other Granulomatous Disorders diagnostic criteria. Patients who had a history of sarcoidosis based on pathological, clinical, and laboratory abnormalities, and without another identified cause of granulomatous disease, were included in the cohort. Exclusion criteria included coexistent diagnosis of cancer, tuberculosis, or any other rheumatologic diseases, or missing clinical data.
Patients were followed up every 3-6 mo. The final follow-up point was June 2021. Follow-up information was obtained from outpatient follow-up records or telephone interviews. Classification of the sarcoidosis prognosis was based on the duration of the clinical disease course, as follows: (1) Asymptomatic or having acute symptoms with spontaneous resolution; and (2) Relapse or progressive course[11]. Clinical relapse of sarcoidosis was determined by the return of clinical manifestations severe enough to warrant treatment after withdrawal of an effective therapy[12]. We also recruited a cohort of healthy controls (n = 112) with no significant medical background.
Clinical and demographic characteristics were gathered from hospital medical records and physician questionnaires. Blood samples of the included sarcoidosis patients and healthy controls were obtained. Meanwhile, data from pulmonary function tests and high-resolution computed tomography (CT) scans were obtained. The stages of sarcoidosis were determined following the “Scadding” classification for sarcoidosis patients. C-reactive protein (commonly referred to as CRP), erythrocyte sedimentation rate (ESR), and ACE levels were also recorded. The high-resolution CT findings of sarcoidosis were independently reviewed by a single radiologist and a single respiratory physician, both blinded to the other’s interpretation. For study purposes, all patients were accessible to collect the information to identify individual participants during or after data collection.
Plasma samples from each patient were subjected to analysis of CA125 concentration via enzyme-linked immunosorbent assay, with 35 U/mL set as the cutoff value. Serum CA125 was measured by the Mlbioâ CA125 reagents assay (Shanghai Enzyme-linked Biotechnology Co. Ltd., Shanghai, China), which was based on the agglutination of antigen with a CA125 monoclonal antibody and absorbance detection. CA125 concentrations were expressed in U/mL.
Statistical analyses were performed using the SPSS statistical package software (version 26.0.0.0; IBM Corp., Armonk, NY, United States). Graphic representations of data were generated using GraphPad Prism (GraphPad Software Inc., San Diego, CA, United States). As the data had asymmetrical distribution and a non-parametrical test was used, variables were presented as median values and interquartile ranges. Categorical data were summarized using absolute values and percentages. Between-group comparisons were carried out by non-parametrical Mann-Whitney U test and one-way analysis of variance. Differences between proportions of categorical data were compared using Pearson’s χ2 test or Fisher’s exact test, where appropriate. Binary logistic regression analysis was performed to evaluate predictors that may affect elevated levels of CA125 in patients with sarcoidosis. Pearson’s or Spearman’s rank correlation coefficients were used to explore the relationship between CA125 levels and clinical parameters of sarcoidosis patients. P values of less than 0.05 were considered statistically significant.
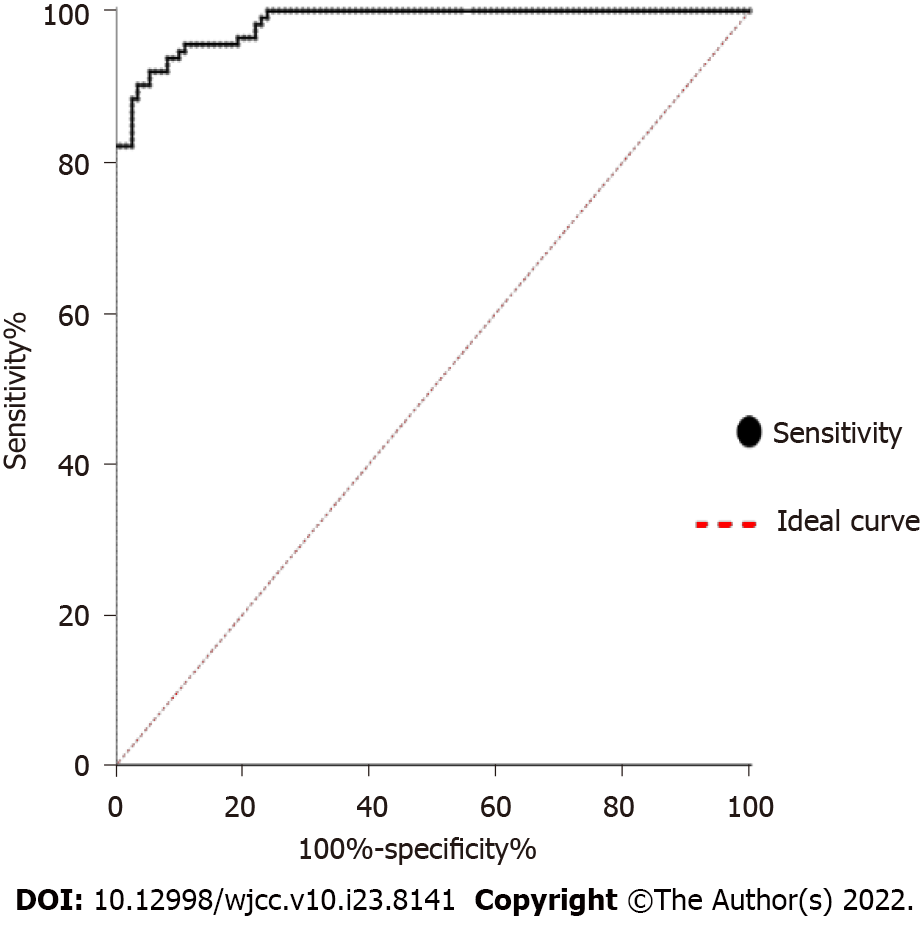
To evaluate the potential role of CA125 in sarcoidosis, we examined the expression of CA125 in the serum of 108 sarcoidosis patients and 112 healthy controls. As shown in Figure 1, the expression level of CA125 was notably upregulated in the serum of sarcoidosis patients (P < 0.001). Specifically, the median level was 44.78 U/mL in the sarcoidosis group compared to 19.11 U/mL in the healthy control group, suggesting that detection of serum CA125 may serve as a diagnostic test for sarcoidosis. Using the receiver operator characteristic, the optimal cutoff value to differentiate sarcoidosis from healthy controls was 32.33 U/mL, which yielded a specificity of 90.2% and sensitivity of 96.3%. The area under the curve was 0.9833 (95%CI: 0.9717-0.9949) (Figure 2).
The demographic data, baseline pulmonary function test values, serum CA125 and ACE levels, and laboratory test findings of patients with sarcoidosis are reported in Table 1. Among the total 108 sarcoidosis patients, 31 were male and 77 were female, with a median age of 51.00 years (interquartile range: 44.00-55.75 years). In addition, 75 (69.44%) presented as asymptomatic or with acute symptoms and spontaneous resolution, and 33 (30.56%) relapsed or showed a progressive course. In addition, 92 (85.19%) showed an elevated serum level of CA125 (≤ 35 U/mL).
| Characteristics | Total patients | CA125 ≤ 35 U/mL | CA125 > 35 U/mL | χ2/Z value | P value | |
| Total, n | 108 | 16 | 92 | |||
| Age at diagnosis in yr | 51.00 (44.00, 55.75) | 53.50 (48.25, 56.50) | 50.00 (43.00, 55.75) | -1.108 | 0.268 | |
| Sex | 1.000 | |||||
| Male | 31 (28.7) | 5 (31.3) | 26 (28.3) | |||
| Female | 77 (71.3) | 11 (68.8) | 66 (71.7) | |||
| Clinical presentation | ||||||
| Dyspnea | 76 (70.4) | 10 (62.5) | 66 (71.7) | 0.203 | 0.652 | |
| Cough | 58 (53.7) | 8 (50.0) | 50 (54.3) | 0.104 | 0.748 | |
| Erythema nodosum | 27 (25.0) | 7 (43.8) | 20 (21.7) | 2.446 | 0.118 | |
| Fever | 37 (34.3) | 6 (37.5) | 31 (33.7) | 0.088 | 0.767 | |
| Arthralgia/arthritis | 21 (19.4) | 4 (25.0) | 17 (18.5) | 0.071 | 0.790 | |
| Fatigue | 25 (23.1) | 6 (37.5) | 19 (20.7) | 1.331 | 0.249 | |
| Organ involvement | ||||||
| Pulmonary | 96 (88.9) | 12 (75) | 59 (64.1) | 0.715 | 0.398 | |
| Extra thoracic lymph nodes | 30 (27.8) | 4 (25.0) | 26 (28.3) | 0.000 | 1.000 | |
| Ocular | 12 (11.1) | 2 (12.5) | 10 (10.9) | 0.000 | 1.000 | |
| Liver | 9 (8.3) | 1 (6.3) | 7 (7.6) | 0.000 | 1.000 | |
| Cardiac | 8 (7.4) | 0 (0) | 9 (9.8) | 0.667 | 0.414 | |
| Pleural effusion | 11 (10.2) | 2 (12.5) | 9 (9.8) | 0.000 | 1.000 | |
| Scadding classification of chest radiography | 4.603 | 0.171 | ||||
| 0 | 2 (1.9) | 0 (0) | 2 (2.2) | |||
| I | 35 (32.4) | 2 (12.5) | 33 (35.9) | |||
| II | 51 (47.2) | 9 (56.3) | 42 (45.7) | |||
| III | 20 (18.5) | 5 (31.3) | 15 (16.3) | |||
| Natural history | 2.357 | 0.125 | ||||
| Asymptomatic or have acute symptoms with spontaneous resolution | 75 (69.4) | 8 (50) | 67 (72.8) | |||
| Relapse or progressive course | 33 (30.6) | 8 (50) | 25 (27.2) | |||
| Treatment | 2.760 | 0.314 | ||||
| Oral steroids | 34 (31.5) | 5 (31.3) | 29 (31.5) | |||
| ICS | 43 (39.8) | 3 (18.8) | 23 (25) | |||
| Oral steroids and methotrexate | 26 (24.1) | 6 (37.5) | 37 (40.2) | |||
| Others | 5 (4.6) | 2 (12.5) | 3 (3.3) | |||
| Pulmonary function tests % of predicted | ||||||
| TLC | 86.45 (80.15, 93.70) | 85.70 (79.23, 95.38) | 86.50 (80.05, 93.55) | -0.061 | 0.952 | |
| RV | 86.45 (80.05, 93.90) | 91.30 (71.50, 97.58) | 85.00 (63.00, 100.10) | -0.208 | 0.835 | |
| FEV1 | 85.40 (79.70, 98.33) | 85.85 (78.30, 92.08) | 85.40 (79.73, 98.60) | -0.511 | 0.610 | |
| FVC | 78.25 (73.30, 86.60) | 84.05 (76.98, 89.83) | 77.80 (73.03, 84.48) | -2.028 | 0.043a | |
| FEV1/FVC | 78.91 (73.68, 85.13) | 78.44 (70.54, 95.28) | 78.91 (73.68, 85.12) | -0.476 | 0.634 | |
| DLCO | 80.60 (73.58, 96.35) | 78.20 (71.30, 90.98) | 81.40 (74.10, 97.00) | -0.697 | 0.486 | |
| Peripheral blood counts | ||||||
| WBC ×109/L | 6.29 (5.35, 7.62) | 6.15 (5.52, 7.56) | 6.29 (5.16, 7.62) | -0.203 | 0.839 | |
| NLR | 2.76 (2.15, 4.01) | 3.74 (2.76, 4.55) | 2.74 (2.03, 3.85) | -2.076 | 0.038a | |
| Serum chemistries | ||||||
| ALT | 19.0 (15.0, 30.0) | 17.5 (15.0, 25.5) | 19.0 (15.0, 30.0) | -0.628 | 0.530 | |
| AST | 23.00 (17.00, 32.00) | 22.50 (17.00, 36.25) | 23.00 (17.00, 28.75) | -0.412 | 0.680 | |
| Cr | 65.00 (53.23, 76.00) | 66.00 (56.75, 81.50) | 65.00 (53.00, 75.00) | -0.978 | 0.328 | |
| ESR | 8.00 (5.00, 17.00) | 6.50 (4.25, 11.50) | 9.00 (5.25, 17.75) | -1.469 | 0.142 | |
| CRP | 1.28 (0.57, 4.60) | 1.58 (0.38, 2.60) | 1.28 (0.59, 4.98) | -1.086 | 0.277 | |
| ACE | 27.50 (15.25, 39.00) | 25.50 (15.25, 37.25) | 28.00 (15.25, 47.25) | -0.620 | 0.535 | |
| BAL % cells | ||||||
| BAL % macrophage | 76.0 (65.5, 88.5) | 76.0 (65.0, 89.0) | 77.5 (65.5, 88.5) | -0.281 | 0.778 | |
| BAL % lymphocyte | 21.00 (8.00, 34.00) | 22.00 (7.25, 34.00) | 20.00 (8.00, 33.75) | -0.407 | 0.684 | |
| CD4+T% | 75.20 (61.90, 84.73) | 72.85 (60.85, 89.00) | 75.20 (62.63, 83.60) | -0.351 | 0.726 | |
| CD4/CD8 ratio | 5.05 (3.30, 9.50) | 7.05 (2.78, 9.50) | 4.90 (3.30, 9.50) | -0.476 | 0.634 | |
The observed upregulation of CA125 in the serum of sarcoidosis patients suggested that CA125 might be involved in the disease development. In particular, we analyzed the correlation between CA125 concentrations and clinical phenotypes using patient groups with CA125 levels of ≤ 35 U/mL (n = 16) or CA125 > 35 U/mL (n = 92). Univariate analysis revealed no remarkable differences between the two groups for sex, age, clinical presentation, radiological classification, natural history, treatment, and bronchoalveolar lavage fluid characteristics. The results showed the percentage of predicted of forced vital capacity (FVC% predicted) and neutrophil-to-lymphocyte ratio to be associated with elevated serum CA125 (P = 0.043 and P = 0.038, respectively). With the exceptions of pulmonary function test and peripheral blood counts, other variables assessed did not differ notably between the two groups (Table 1).
Erythema nodosum, natural history, FVC% predicted, and ESR were entered into the binary logistic regression analysis, and the results demonstrated FVC% predicted as a risk factor for elevated CA125 levels in sarcoidosis patients (P = 0.029) (Table 2).
| Characteristics | β | SE | Wald value | P value | OR | 95%CI | |
| Erythema nodosum | 0.983 | 0.607 | 2.621 | 0.105 | 2.672 | 0.813 | 8.785 |
| Scadding classification of chest radiography | -0.654 | 0.439 | 2.22 | 0.136 | 0.52 | 0.22 | 1.229 |
| Natural history | -1.068 | 0.597 | 3.194 | 0.074 | 0.344 | 0.107 | 1.109 |
| Forced vital capacity | -0.055 | 0.025 | 4.788 | 0.029a | 0.946 | 0.9 | 0.994 |
| ESR | 0.028 | 0.036 | 0.613 | 0.434 | 1.029 | 0.958 | 1.105 |
| NLR | -0.145 | 0.099 | 2.116 | 0.164 | 0.865 | 0.712 | 1.052 |
Scadding’s classification for the total 108 patients led to 2 patients being classified as Stage 0, 35 as Stage I, 49 as Stage II, 22 as Stage III, and 0 as Stage IV. Statistically significant differences in CA125 were found between Stage II and Stage I (46.16 ± 8.32 vs 41.00 ± 6.04 U/mL, P = 0.005), also between Stage III and Stage I (47.92 ± 10.10 vs 41.00 ± 6.04 U/mL, P = 0.002) (Figure 3).
As shown in Supplementary Table 1, Spearman correlation analysis of the sarcoidosis patients revealed no correlation between CA125 vs CRP (r = -0.013, P = 0.897), CA125 vs ACE (r = -0.045, P = 0.642), nor CA125 vs ESR (r = 0.049, P = 0.614).
In this study, we found CA125 to be correlated with the clinical presentation of sarcoidosis, lung function (FVC% predicted), and the radiological Scadding’s classification. Our collective findings also supported the potential of CA125 as a diagnostic biomarker; specifically, the higher area under the curve value suggested that CA125 is a diagnostic indicator for sarcoidosis in particular. To the best of our knowledge, this study is the most extensive prognostic study of the correlation between CA125 and the clinical characteristics of sarcoidosis to be published to date.
In general, CA125 is the most commonly used tumor marker for detecting epithelial ovarian cancer. However, elevated CA125 is also seen in many other diseases besides cancers. A multicenter study demonstrated CA125 as a biomarker of epithelial injury in patients with idiopathic pulmonary fibrosis[13]. Also, many organs express CA125 in the non-pathologic condition, including the peritoneum, pericardium epithelia, pleura, and mature airway epithelial cells[14,15]. CA125 expressed on coelomic epithelium is an O-glycosylated protein of the mucin family and serves to protect the epithelial cells from particles and infectious agents in the mucous barrier[16]. Furthermore, it can occur in forms that are membrane-anchored or soluble, with the latter resulting from cleavage of the former[17].
Davies et al[18] used immunohistochemistry to determine the localization of CA125 in human tracheal tissue by LUM16-2 anti-serum, and they detected CA125 Localized around the cilia. It was also recently reported that interstitial lung disease patients have a remarkably higher level of serum CA125 compared to healthy controls, which suggests a specific correlation between interstitial lung disease and CA125[19,20]. Maher et al[13] showed that CA125 could be a potential theranostic marker of response to anti-fibrotic therapy in idiopathic pulmonary fibrosis patients, with the benefit of being a minimally invasive marker. Those authors also reported having detected CA125 in normal lungs exclusively in the apical aspect of the bronchial epithelium; however, in fibrotic lung tissue, they found CA125 to be overexpressed in the metaplasic epithelium of fibrotic lesions, and the production to be related to mucus secretion.
Current serum biomarkers produced by inflammatory cells and involved in granuloma formation include serum ACE, serum amyloid A, chitotriosidase, serum soluble interleukin-2 receptor, and B-cell activating factor, among others[21-25]. ACE, as a most well-known serum biomarker of sarcoidosis, was identified as a diagnostic marker of active disease in 1975[21], and since then has been shown to be involved in the pathogenesis of sarcoidosis as an essential modulator of granulomatous formation[26]. However, the low sensitivity among sarcoidosis patients (roughly 22%-86%) has left the diagnostic value of serum ACE levels debatable[3]. The ideal biomarker for sarcoidosis has not yet been discovered. In our study, we found the best cutoff point of CA125 to be 32.33 U/mL, which yielded a sensitivity of 96.3% and a specificity of 90.2%. Our research showed the sensitivity of CA125 for diagnosing sarcoidosis is higher than the sensitivity of the conventional biomarker of ACE.
According to these results, CA125 has high sensitivity and specificity for sarcoidosis and is a valuable parameter in the determination of disease diagnosis. Our results showed that high CA125 levels (> 32.33 U/mL) were predictive of diagnosing sarcoidosis. Although our study found that the threshold of CA125 Level with diagnostic value of sarcoidosis was 32.33 U/mL, due to the small sample size of this study, we still used the standard diagnostic cut-off value of CA125 (35 U/mL) to complete the further analysis.
In our study of sarcoidosis patients, we found that the increase of CA125 was obvious in patients with Stages II and III Scadding’s classification; both stages showed sarcoidosis lung involvement. This could be related to the infiltration of pulmonary lesions in patients with Stage II and Stage III sarcoidosis, resulting in lung mesothelial lining cell injury. When CA125 enters the blood from the lung, an increased level of serum CA125 results. However, none of the patients included in this study were defined as stage IV of Scadding’s classification, which may be related to the lower incidence of sarcoidosis with pulmonary fibrosis features on chest imaging[27,28]. In the future, more patients with Scadding stage IV need to be further included to explore the differences in CA125 levels with patients with other stages.
Our results revealed inverse correlations between serum CA125 levels and FVC% predicted at the baseline time point. As noted previously, patients with FVC and diffusing capacity of the lung for carbon monoxide reduction have a higher risk of disability, loss of life due to pulmonary involvement, loss of quality of life, comorbidities, and loss of quality of life due to glucocorticoid treatment[29]. However, our results demonstrated that no significant differences were found between the elevated serum CA125 levels and active or chronic prognosis of sarcoidosis.
Limited publications, including case reports and literature reviews, have focused on the association between pulmonary sarcoidosis and CA125 levels so far. Those previous studies have indicated that elevated levels of CA125 in sarcoidosis patients are related to pleural effusion, peritoneal involvement, liver, spleen, and pulmonary abnormalities, and pericardial effusion[10,30]. In particular, Kudaiberdiev et al[30] reported a case of cardiac sarcoidosis with severe pericardial effusion and increased CA125 levels; histological examination of the pericardial lymph node specimen validated the diagnosis. In addition, elevated serum CA125 has been reported in several peritoneal sarcoidosis cases, and other benign diseases and gynecologic and non-gynecologic carcinomas were excluded from the patients[31,32]. Nicolini et al[33] reported on a rare case of peritoneal sarcoidosis, in which the patient presented with average CA125 levels and pulmonary abnormalities. In our study population, we observed elevated serum CA125 levels in 9 cases of pleural effusion, 7 cases with cardiac involvement, 9 cases with liver involvement, and 81 cases with pulmonary sarcoidosis, which is consistent with the previously published research. However, the relationship between the serum CA125 levels and the various affected organs in sarcoidosis patient needs more evidence.
The study we presented herein is the first analysis of the association between serum CA125 levels and disease characteristics in pulmonary sarcoidosis. The main limitations of the present study are its retrospective and monocentric nature, and the absence of a validation cohort. Second, the number of patients included in this study was relatively small, which limited our power to explore the statistical differences in different disease prognosis groups. However, this is the largest study ever conducted on CA125 in pulmonary sarcoidosis and the results clearly show its potential as a diagnostic biomarker correlating with several clinical and radiological parameters. Future prospective studies should be designed to focus on evaluating the role of CA125 in clinical decision-making in sarcoidosis patients.
In conclusion, this study aimed to identify a new noninvasive biomarker of sarcoidosis that could facilitate assisting disease diagnosis and treatment. Furthermore, a strong correlation was found between serum CA125 with the FVC% predicted in the pulmonary function test and radiological Scadding’s classification of sarcoidosis patients, which suggests that these may be useful biomarkers that may be employed in clinical management to avoid the aggravation of sarcoidosis.
Sarcoidosis is a multisystem disorder with unknown etiology that predominantly affects the lungs and intrathoracic lymph nodes. At present, diagnostic biomarkers for sarcoidosis remain controversial. Carbohydrate antigen (CA) 125 is an O-glycosylated protein of the mucin family that serves a protective function in the mucus barrier on the surfaces of epithelial cells and has a potential role in the inflammatory pathogenesis of sarcoidosis.
CA125 is a tumor marker for many cancers; however, elevated CA125 is found in the sera of patients afflicted with many other diseases. The expression levels of CA125 and their clinical significance are still unknown for sarcoidosis patients.
The aim of this study was to assess the applicability of CA125 for sarcoidosis diagnosis and analyze the association between CA125 and clinical characteristics of sarcoidosis patients.
In this study, serum CA125 Level was measured by an enzyme-linked immunosorbent assay in 108 consecutive sarcoidosis patients treated between June 2016 to December 2020 (31 males, 77 females; age at diagnosis 49.69 ± 9.10 years) and compared with that in 112 healthy subjects. Data on C-reactive protein level, erythrocyte sedimentation rate, and angiotensin-converting enzyme level were also collected. The association of serum CA125 levels with clinical, radiological, and respiratory functional characteristics was analyzed between sarcoidosis patient groups with CA125 ≤ 35 U/mL or CA125 > 35 U/mL.
The authors found that serum CA125 levels were higher in sarcoidosis patients compared to healthy controls (median: 44.78 U/mL vs 19.11 U/mL, P < 0.001). The area under the receiver operator characteristic curve was 0.9833 (95%CI: 0.9717-0.9949), and the best cutoff point was 32.33 U/mL. The elevated serum CA125 was notably associated with FVC % predicted and neutrophil-to-lymphocyte ratio (P = 0.043 and P = 0.038, respectively) of the sarcoidosis patients. Multivariate analysis revealed that forced vital capacity % predicted was a statistically significant predictor of elevated serum CA125. Also, our research revealed that compared to patients with Stage I radiology classification, sarcoidosis patients with Stages II and III showed higher concentrations of serum CA125.
Abnormally elevated serum CA125 is important in the noninvasive diagnosis of sarcoidosis and strongly associated with the clinical characteristics among sarcoidosis patients.
Abnormally elevated serum CA125 Level may be a potentially useful biomarker for sarcoidosis diagnosis and has an association with disease characteristics.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Garcia-Pola M; Karavaş E, Turkey S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 347] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Drent M, Crouser ED, Grunewald J. Challenges of Sarcoidosis and Its Management. N Engl J Med. 2021;385:1018-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 214] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 3. | Ramos-Casals M, Retamozo S, Sisó-Almirall A, Pérez-Alvarez R, Pallarés L, Brito-Zerón P. Clinically-useful serum biomarkers for diagnosis and prognosis of sarcoidosis. Expert Rev Clin Immunol. 2019;15:391-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Gerke AK. Treatment of Sarcoidosis: A Multidisciplinary Approach. Front Immunol. 2020;11:545413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 5. | Núñez J, de la Espriella R, Miñana G, Santas E, Llácer P, Núñez E, Palau P, Bodí V, Chorro FJ, Sanchis J, Lupón J, Bayés-Genís A. Antigen carbohydrate 125 as a biomarker in heart failure: a narrative review. Eur J Heart Fail. 2021;23:1445-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 6. | Takeshima N, Shimizu Y, Umezawa S, Hirai Y, Chen JT, Fujimoto I, Yamauchi K, Hasumi K. Combined assay of serum levels of CA125 and CA19-9 in endometrial carcinoma. Gynecol Oncol. 1994;54:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Wei G, Yuping Z, Jun W, Bing Y, Qiaohua Z. CA125 expression in patients with non-Hodgkin's lymphoma. Leuk Lymphoma. 2006;47:1322-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Park M, Dhawan R, Whittaker E, Kon OM. CA 125 and TB. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Herati RS, Koh JM, Gorospe EC. Localized hepatosplenic sarcoidosis with an elevated serum CA-125 level. ScientificWorldJournal. 2010;10:298-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Lee IS, Kim SB, Moon CS, Jung SM, Kim SY, Kim EY, Jung JY, Kang YA, Kim YS, Kim SK, Chang J, Park MS. Sarcoidosis presenting with massive pleural effusion and elevated serum and pleural fluid carbohydrate antigen-125 levels. Tuberc Respir Dis (Seoul). 2012;73:320-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383:1155-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 762] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 12. | Gottlieb JE, Israel HL, Steiner RM, Triolo J, Patrick H. Outcome in sarcoidosis. The relationship of relapse to corticosteroid therapy. Chest. 1997;111:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 187] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Maher TM, Oballa E, Simpson JK, Porte J, Habgood A, Fahy WA, Flynn A, Molyneaux PL, Braybrooke R, Divyateja H, Parfrey H, Rassl D, Russell AM, Saini G, Renzoni EA, Duggan AM, Hubbard R, Wells AU, Lukey PT, Marshall RP, Jenkins RG. An epithelial biomarker signature for idiopathic pulmonary fibrosis: an analysis from the multicentre PROFILE cohort study. Lancet Respir Med. 2017;5:946-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 14. | Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol. 2012;30:876-882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 309] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 15. | Sevinc A, Buyukberber S, Sari R, Kiroglu Y, Turk HM, Ates M. Elevated serum CA-125 levels in hemodialysis patients with peritoneal, pleural, or pericardial fluids. Gynecol Oncol. 2000;77:254-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Ma J, Rubin BK, Voynow JA. Mucins, Mucus, and Goblet Cells. Chest. 2018;154:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 276] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 17. | Moniaux N, Escande F, Porchet N, Aubert JP, Batra SK. Structural organization and classification of the human mucin genes. Front Biosci. 2001;6:D1192-D1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 272] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Davies JR, Kirkham S, Svitacheva N, Thornton DJ, Carlstedt I. MUC16 is produced in tracheal surface epithelium and submucosal glands and is present in secretions from normal human airway and cultured bronchial epithelial cells. Int J Biochem Cell Biol. 2007;39:1943-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Amigues I, Ramadurai D, Swigris JJ. Current Perspectives On Emerging Biomarkers For Rheumatoid Arthritis-Associated Interstitial Lung Disease. Open Access Rheumatol. 2019;11:229-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Wang T, Zheng XJ, Ji YL, Liang ZA, Liang BM. Tumour markers in rheumatoid arthritis-associated interstitial lung disease. Clin Exp Rheumatol. 2016;34:587-591. [PubMed] |
| 21. | Lieberman J. Elevation of serum angiotensin-converting-enzyme (ACE) level in sarcoidosis. Am J Med. 1975;59:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 608] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 22. | Bargagli E, Magi B, Olivieri C, Bianchi N, Landi C, Rottoli P. Analysis of serum amyloid A in sarcoidosis patients. Respir Med. 2011;105:775-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Popević S, Šumarac Z, Jovanović D, Babić D, Stjepanović M, Jovičić S, Šobić-Šaranović D, Filipović S, Gvozdenović B, Omčikus M, Milovanović A, Videnović-Ivanov J, Radović A, Žugić V, Mihailović-Vučinić V. Verifying Sarcoidosis Activity: Chitotriosidase versus ACE in Sarcoidosis - a Case-control Study. J Med Biochem. 2016;35:390-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Sakthivel P, Bruder D. Mechanism of granuloma formation in sarcoidosis. Curr Opin Hematol. 2017;24:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 25. | Ando M, Goto A, Takeno Y, Yamasue M, Komiya K, Umeki K, Nureki SI, Miyazaki E, Kadota JI. Significant elevation of the levels of B-cell activating factor (BAFF) in patients with sarcoidosis. Clin Rheumatol. 2018;37:2833-2838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Chopra A, Kalkanis A, Judson MA. Biomarkers in sarcoidosis. Expert Rev Clin Immunol. 2016;12:1191-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | SCADDING JG. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years' observation. Br Med J. 1961;2:1165-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 420] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 28. | Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1469] [Cited by in RCA: 1425] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 29. | Baughman RP, Valeyre D, Korsten P, Mathioudakis AG, Wuyts WA, Wells A, Rottoli P, Nunes H, Lower EE, Judson MA, Israel-Biet D, Grutters JC, Drent M, Culver DA, Bonella F, Antoniou K, Martone F, Quadder B, Spitzer G, Nagavci B, Tonia T, Rigau D, Ouellette DR. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. 2021;58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 278] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 30. | Kudaiberdiev T, Tukusheva E, Gaibyldaev Z, Tursunbekova G, Kadyraliev Z, Akhmedova I, Tulopbergenov N, Muraliev E. Massive pericardial effusion causing cardiac tamponade accompanied by elevated CA-125 and thoracic lymphadenopathy in sarcoidosis: a case report. Int J Surg Case Rep. 2020;72:355-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Kalluri M, Judson MA. Sarcoidosis associated with an elevated serum CA 125 level: description of a case and a review of the literature. Am J Med Sci. 2007;334:441-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Chubineh S, Katona K. A rare case of peritoneal sarcoidosis in a 36-year-old construction worker. Case Rep Gastroenterol. 2008;2:369-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Nicolini A, Vita M, Lanata S. Peritoneal sarcoidosis: an unusual presentation and a brief review of the literature. Monaldi Arch Chest Dis. 2011;75:132-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |