Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.8034
Peer-review started: February 28, 2022
First decision: May 30, 2022
Revised: June 10, 2022
Accepted: June 24, 2022
Article in press: June 24, 2022
Published online: August 6, 2022
Processing time: 143 Days and 15.7 Hours
Microcystic adnexal carcinoma (MAC) is a rare malignant cutaneous adnexal neoplasm, often presenting as a flesh-colored and slow-growing indurated plaque or cystic nodule in the mid-facial region. Its characteristic indolent presentation usually leads to initial misdiagnosis, resulting in tumor mismanagement and added morbidity due to increased propensity for local invasion.
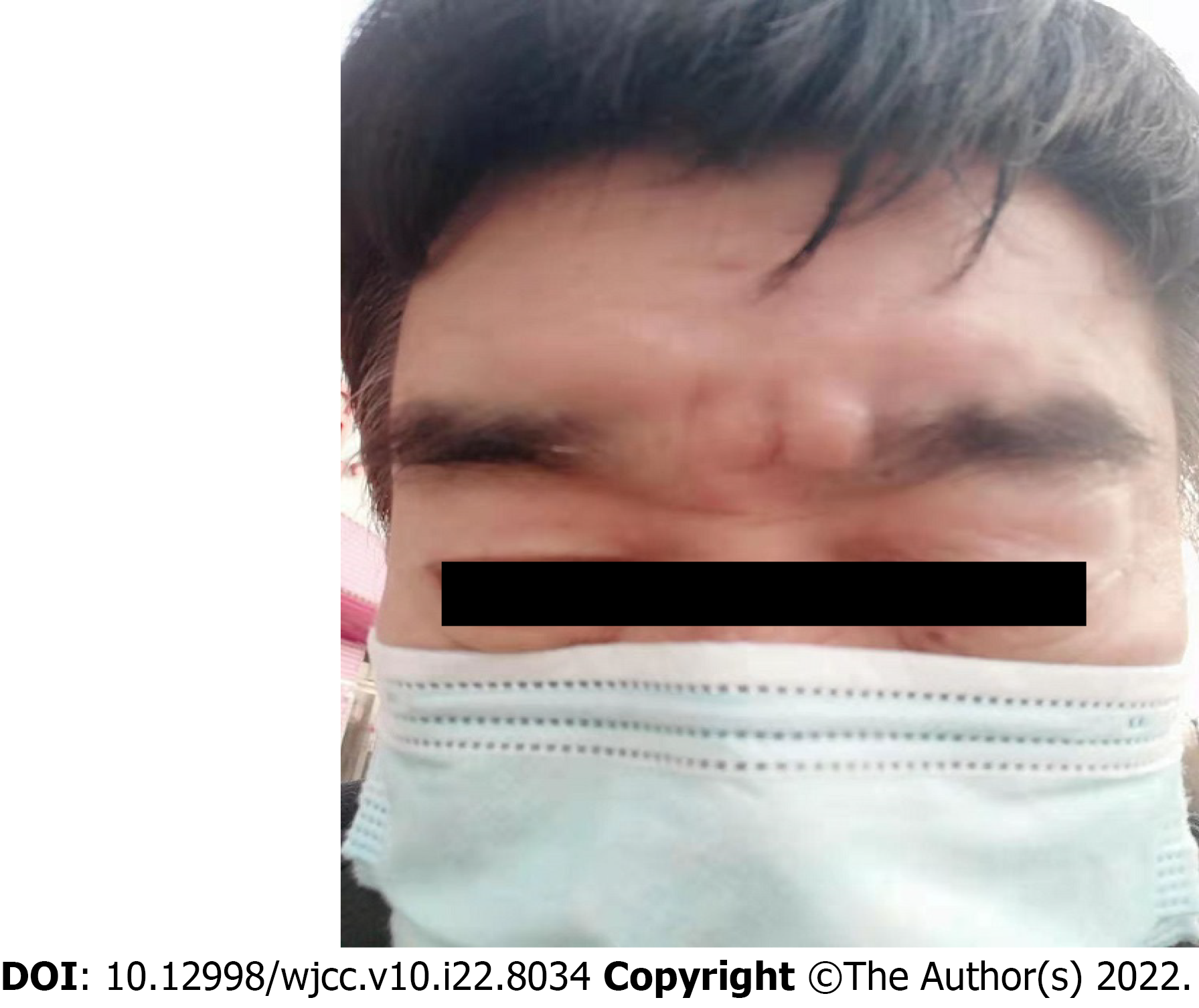
A 63-year-old Chinese male patient with a long-term history of excessive ultraviolet irradiation had received two surgeries for an “epidermal cyst” on his glabella and was presented to our hospital’s Dermatology Department for further diagnosis and therapy of the lesion on his glabella. One month ago, his two 7 mm × 7 mm subcutaneous nodules were diagnosed as "recurrent epidermal cysts", and he underwent local excision surgery. Additionally, he has post medical history of surgery for right clear cell renal carcinoma. According to his biopsy, the patient was diagnosed as MAC in our hospital, and a tumor remnant was found on his wound. He then underwent wide local excision to achieve negative margins and reconstruction of full-thickness flap transplantation for tissue coverage. He remained tumor-free after six months of follow-up.
This case highlights the importance of MAC’s possible pathogenic factor of excessive ultraviolet exposure, its differential diagnosis to avoid misdiagnosis and mismanagement to adverse prognosis, the patient’s particular medical history of clear cell renal carcinoma, the alert for any tumor recurrence in older patients, and his uncommon multiple nodules mess consisting of two 7 mm × 7 mm subcutaneous nodules, that will enrich the existing knowledge of MAC’s clinical features.
Core Tip: Microcystic adnexal carcinoma is an uncommon skin malignant tumor with a high misdiagnosis rate. We present a rare case of multiple nodules of microcystic adnexal carcinoma misdiagnosed as a “recurrent epidermal cyst.” The patient has a long-term history of excessive ultraviolet irradiation.
- Citation: Yang SX, Mou Y, Wang S, Hu X, Li FQ. Microcystic adnexal carcinoma misdiagnosed as a “recurrent epidermal cyst”: A case report. World J Clin Cases 2022; 10(22): 8034-8039
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/8034.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.8034
Microcystic adnexal carcinoma (MAC) is a rare cutaneous adnexal neoplasm that typically manifests as a single lesion in the mid-facial region[1,2]. It was first described by Goldstein et al[1] in 1982. Highly recurrent MAC often has deep, local infiltration and perineural invasion; however, regional lymph node or distant metastasis rarely occurs[5]. MAC has a high rate of misdiagnosis as an epidermal cyst due to its indolent features and lack of dermatologists’ familiarity[1,2]. We report a case of MAC misdiagnosed as a recurrent epidermal cyst occurring in a sun-exposed area, which was a mess consisting of two 7 mm × 7 mm subcutaneous nodules, rarely reported before. This study highlights the significance of the MAC’s differential diagnosis, its possible pathogenic factor of excessive ultraviolet exposure, and the patient’s particular medical history of right clear cell renal carcinoma. Besides, this case can help improve our wariness of seemingly innocent lesions that may suddenly appear in the head and neck region, especially with a history of excessive ultraviolet exposure or other malignant tumors[4].
A 63-year-old Chinese male patient was presented with complaints of a slowly growing mass on his glabella for 20 years. He had two excision surgeries, one ten years ago and one a month ago. The histopathological slice made in the local hospital a month ago reveals deep local infiltration and intramuscular invasion.
This lesion first appeared 20 years ago as a painless, skin-colored nodule on the glabella, with no signs of pruritus or numbness. Ten years ago, the lesion was diagnosed as an “epidermal cyst” and was removed in the local hospital without histopathological examination. Two new flesh-colored nodules emerged in the same place as the first lesion nine years ago. One month ago, the patient was diagnosed with a “recurrent epidermal cyst” in a local hospital (Figure 1). Its lesion was presented as a skin-colored mass on his glabella that was progressively growing, immovable and firm and comprised two 7 mm × 7 mm subcutaneous nodules. Subsequently, he underwent a local excision surgery. Biopsy revealed deep local infiltration and intramuscular invasion. He was then presented to our hospital for further diagnosis and therapy.
The patient had a medical history of right clear cell renal carcinoma, was surgically treated nine years ago, had not recurred, and denied any history of postoperative radiotherapy or chemotherapy.
The patient had a long-term history of excessive ultraviolet irradiation. He reported an occupational history of working as a truck driver for more than 40 years, frequently working in a sun-exposed environment for no less than six hours a day on average without any extra protection. Otherwise, there was nothing remarkable in his personal or family history.
Physical examination on admission showed an approximately 10 mm × 10 mm red papule on his glabella. No paresthesia of neck nodes was noted.
The histological and immunohistochemical patterns of the patient were consistent with MAC.
None of the imaging examinations revealed anomaly.
This patient was diagnosed as MAC on glabella with positive margins.
The patient underwent wide local excision to achieve negative margins and reconstruction of full-thickness flap transplantation for tissue coverage. Perineural invasion, regional lymph node, and distant metastasis were not discussed.
The operation and postoperative clinical course went uneventful, and the patient was discharged. Postoperative histological and immunohistochemical results confirmed MAC diagnosis and showed negative resection margins. Three months after the surgery, an examination in our hospital showed that the 30 mm × 35 mm surgical incision healed well without recurrence (Figure 2). Patient satisfaction with reconstruction was noted after three months on his next visit.
The MAC etiopathology remains unclear. Ultraviolet and therapeutic radiations have been implicated in their possible pathogenic factors[5-7]. The patient's occupation is unique to our case, coupled with his medical history of clear cell renal carcinoma[4]. He reported a history of excessive sun exposure, averaging no less than six hours a day for more than 40 years, without any extra protection. This resulted in dramatically elevated UV exposure levels, potentially posing an extra risk for MAC development[5]. Avoiding excessive UV irradiation may be one of the MAC's precautionary measures. Patients with predisposing factors such as irradiation may develop double cancers, including MAC[4]. The relation of MAC with renal cell carcinoma is still unclear. A thorough patient history, including occupation, malignant neoplasm illnesses, and heightened suspicion of this pathology, can aid in early identification and reduced morbidity[8].
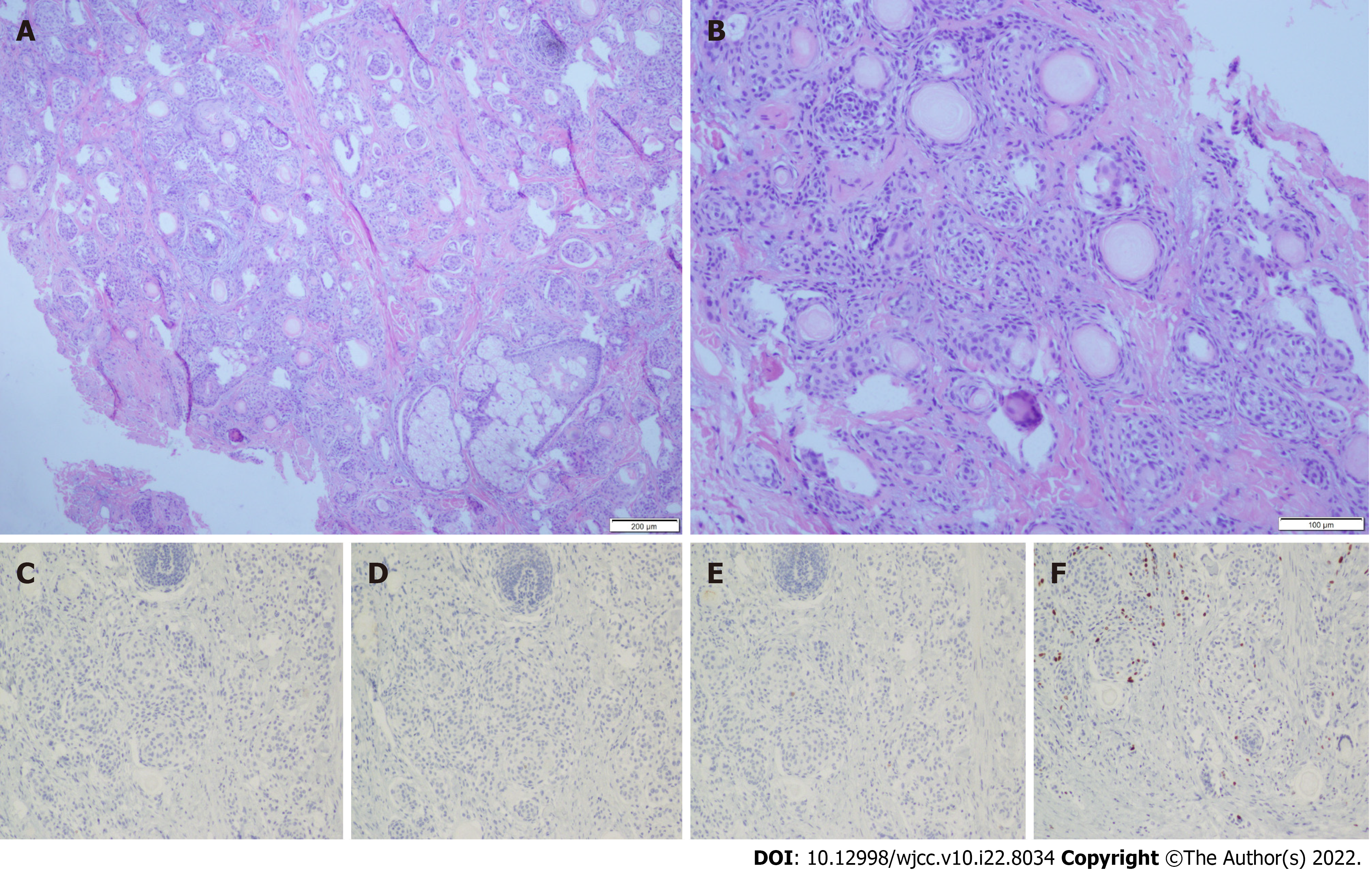
MAC is a rare cutaneous malignancy that often occurs in the mid-facial area, presenting as a slow-growing, flesh-colored, or whitish nodule[1]. Its characteristic indolent presentation usually leads to initial misdiagnosis, resulting in tumor mismanagement and added morbidity due to increased propensity for local invasion[3]. Its differential diagnoses include epidermal cyst, desmoplastic trichoepithelioma (DTE), morpheaform basal cell carcinoma (MBCC), squamous cell carcinoma, syringoma, and syringoid eccrine carcinoma (SEC)[1-3,6,9]. MAC's typical microscopic features are located inside the dermis in the histopathology examination. They are defined by a diffusely infiltrative growth with the invasion of the subcutis and deeper tissues. In a desmoplastic stroma, they develop in cords and strands. Keratocysts and dystrophic calcifications are common on the surface. Duct distinction is a bonus feature that comes in variable degrees. There is very little cytologic atypia. Perineural nerve and skeletal muscle invasion are almost always present (Figure 3A and B)[1,3,10]. The ductal differentiation of MAC can help distinguish it from other benign adnexal neoplasms such as epidermal cyst, DTE, and syringoma, which often require no treatment[11]; for example, the small keratin-filled cysts structure of MAC can help differentiate this from SEC[11]. A full-thickness biopsy, including incisional biopsies, punch biopsies, and excisional biopsies, is required to avoid missing its deep infiltrative nature[7, 9, 11]. We must appreciate the value of a prompt histological evaluation. A medical facility's standard practice is to perform histology on all biological materials (including benign skin lesions). The patient could have received better therapy if the skin samples had been evaluated histologically years ago.
Immunohistochemistry can serve as a supplement for distinguishing MAC from other neoplasms. However, none by itself is sensitive and specific enough for the diagnosis of MAC (Figure 3C-F)[7,11]. Carcinoembryonic antigen is a marker used to demonstrate ductal differentiation of MAC[3,9]. CK20 positivity has proven to be strongly predictive of DTE, whereas it is usually negative in MBCC and MAC[3,7]. CK19 positivity suggests MAC and not DTE. CD34 reveals focal stromal cell positivity in DTE, while the stromal cells in MAC and MBCC are often negative[3]. BerEP4 can be used to differentiate MAC (BerEP4 negative) from MBCC and DTE (both BerEP4 positive)[3,7,11]. Surgery is still the first-line treatment for this neoplasm, which mainly includes Mohs micrographic surgery (MMS) and wide local excision (WLE)[5,8,9,11]. MMS has been compared to routine surgical excision due to poorly defined clinical margins and a tendency for perineural invasion[7,8]. Although wide local excision was first accepted, recurrence rates have been found to be greater in patients who received MMS[9]. Our patient opted for WLE due to financial constraints, as MMS was much more expensive than WLE. Postoperative histological analysis showed he obtained negative margins. However, we will continue to follow up with our patient to observe his prognosis due to the indolent course of this tumor, with cases of recurrence reported up to 30 years after initial treatment[2, 9, 12].
Recently, some research indicated that distinct molecular markers for MAC might be potential diagnostic and therapeutic targets for the disease. Chen et al discovered TP53 mutations as well as chromosomal deletions of CDKN2A and CDKN2B in a 68-year-old man's metastatic MAC. CDK4 and CDK6 inhibitors have been discovered and are being researched for cancers that display these molecular markers. The Food and Drug Administration has authorized one for subgroups of breast cancer patients[13,14]. Yu et al[15] investigated four calcium signaling pathway genes, including CACNA1S, RYR1, ATP2A1, and MYLK3, that were elevated in MAC at the RNA level and expressed more in MAC than in normal sweat glands and histologic mimics of MAC at the protein level. C-kit has been found in a subgroup of MAC patients, boosting the prospect of future use of imatinib mesylate. The use of the anti-epidermal growth factor receptor (EGFR) antibody cetuximab and the multi-targeted tyrosine receptor kinase (MTRK) inhibitor sorafenib in the evidence-based clinical practice recommendations for MAC published in JAMA Dermatology[16].
MAC is an uncommon cutaneous adnexal malignancy, often showing as a flesh-colored and indolent lesion in the mid-facial area, leading to initial misdiagnosis and mismanagement to add morbidity. Dermatologists should raise the awareness of MAC and its differential diagnoses and be alert for any tumor recurrence or double cancers in elderly patients with predisposing factors. The link between renal cell carcinoma and MAC might be a coincidence that needs further investigation. Moreover, this study of rare multiple nodule lesions can enrich the knowledge of MAC's clinical features.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dermatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bairwa DBL, India; Covantsev S, Russia S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Xiao YD, Zhang MZ, Zeng A. Facial microcystic adnexal carcinoma - treatment with a "jigsaw puzzle" advancement flap and immediate esthetic reconstruction: A case report. World J Clin Cases. 2021;9:607-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Mamic M, Manojlovic L, Suton P, Luksic I. Microcystic adnexal carcinoma-diagnostic criteria and therapeutic methods: case report and review of the literature. Int J Oral Maxillofac Surg. 2018;47:1258-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Ohtsuka H, Nozawa R, Kushida Y. Synchronous microcystic adnexal carcinoma and gastric cancer with review of the literature. J Dermatol. 2005;32:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Waki Y, Kamiya K, Maekawa T, Komine M, Murata S, Ohtsuki M. Case of multiple microcystic adnexal carcinomas on the sun-exposed area. J Dermatol. 2018;45:e9-e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Stamey CR, Colegio OR, Book SE. Microcystic adnexal carcinoma of the glabella in a liver transplant recipient. JAAD Case Rep. 2021;10:126-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Worley B, Owen JL, Barker CA, Behshad R, Bichakjian CK, Bolotin D, Bordeaux JS, Bradshaw S, Cartee TV, Chandra S, Cho N, Choi J, Council ML, Eisen DB, Golda N, Huang CC, Ibrahim SF, Jiang SIB, Kim J, Lacutoure M, Lawrence N, Lee EH, Leitenberger JJ, Maher IA, Mann M, Minkis K, Mittal B, Nehal KS, Neuhaus I, Ozog DM, Petersen B, Samie F, Shin TM, Sobanko JF, Somani AK, Stebbins WG, Thomas JR, Thomas V, Tse D, Waldman A, Xu YG, Yu SS, Zeitouni NC, Ramsay T, Poon E, Alam M. Evidence-Based Clinical Practice Guidelines for Microcystic Adnexal Carcinoma: Informed by a Systematic Review. JAMA Dermatol. 2019;155:1059-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Liyanage SE, Saleh GM, Rose GE, Luthert PJ, Beaconsfield M, Verity DH. Delayed diagnosis of microcystic adnexal carcinoma in progressive eyelid distortion. Arch Ophthalmol. 2010;128:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Koh SH, Kang KR, Yang JH, Jung SW, Lee HJ. Microcystic Adnexal Carcinoma Misdiagnosed as Desmoplastic Trichoepithelioma on Preoperative Biopsy. Arch Craniofac Surg. 2015;16:43-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | van der Horst MPJ, Brenn T. Update on Malignant Sweat Gland Tumors. Surg Pathol Clin. 2017;10:383-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Aslam A. Microcystic Adnexal Carcinoma and a Summary of Other Rare Malignant Adnexal Tumours. Curr Treat Options Oncol. 2017;18:49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Lupton GP, McMarlin SL. Microcystic adnexal carcinoma. Report of a case with 30-year follow-up. Arch Dermatol. 1986;122:286-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov. 2016;6:353-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 712] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 14. | Chen MB, Laber DA. Metastatic Microcystic Adnexal Carcinoma with DNA Sequencing Results and Response to Systemic Antineoplastic Chemotherapy. Anticancer Res. 2017;37:5109-5111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Yu S, Wang Y, Tang B, Liu X, Song L, Xu G, Zhu H, Sun H. Four calcium signaling pathway-related genes were upregulated in microcystic adnexal carcinoma: transcriptome analysis and immunohistochemical validation. World J Surg Oncol. 2022;20:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Garcia A, Nelson K, Patel V. Emerging therapies for rare cutaneous cancers: A systematic review. Cancer Treat Rev. 2021;100:102266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |