Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.8009
Peer-review started: February 14, 2022
First decision: April 8, 2022
Revised: April 11, 2022
Accepted: June 26, 2022
Article in press: June 26, 2022
Published online: August 6, 2022
Processing time: 157 Days and 21.8 Hours
Acute aortic dissection (AAD) is a high mortality disease that can lead to acute ischemic strokes (AIS). Some of the patients with AAD combined with AIS initially present with neurological symptoms, which can easily lead to missed or delayed AAD diagnosis. This is attributed to the lack of physician awareness or the urgency of patient thrombolysis. Intravenous administration of thrombolytic therapy (IVT) for AAD is associated with poor prognostic outcomes. We report a patient with AIS combined with AAD who developed a massive cerebral infarction after receiving IVT for a missed AAD diagnosis.
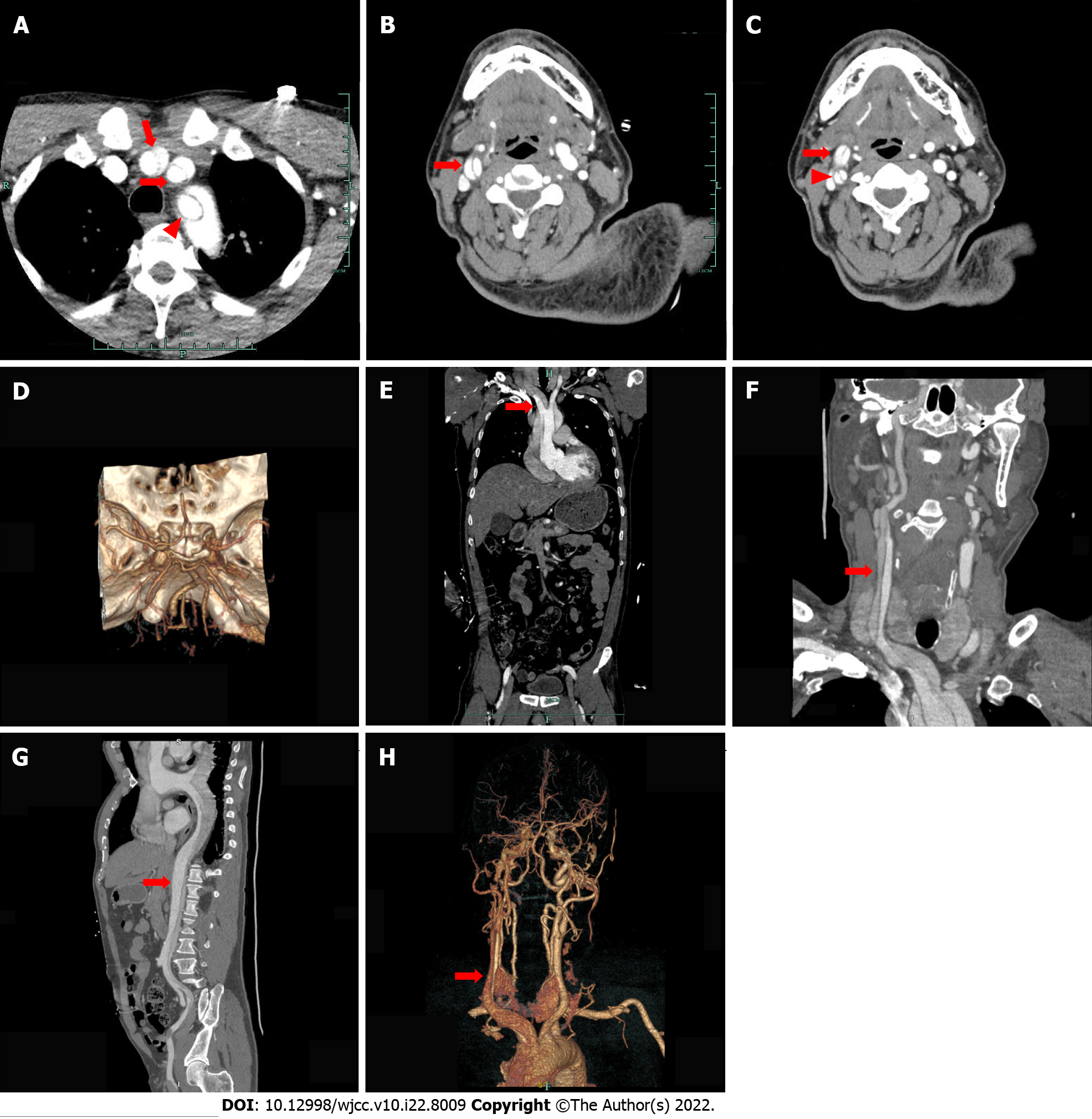
A 49-year-old man was admitted to a local hospital with an acute onset of left-sided limb weakness accompanied by slurred speech. The patient had a history of hypertension that was not regularly treated with medication. Physical examination revealed incomplete mixed aphasia and left limb hemiparesis. Cranial computed tomography (CT) scan showed bilateral basal ganglia and lateral ventricular paraventricular infarct lesions. The patient was diagnosed with AIS and was administered with IVT. After IVT, patient’s muscle strength and consciousness deteriorated. From the local hospital, he was referred to our hospital for further treatment. Emergency head and neck CT angiography (CTA) scans were performed. Results showed multiple cerebral infarctions, and aortic dissection in the ascending aorta, innominate artery, as well as in the right common carotid artery. Then, the CTA of thoracoabdominal aorta was performed, which revealed a Stanford type A aortic dissection and aortic dissection extending from the aortic root to the left external iliac artery. Laceration was located in the lesser curvature of the aortic arch. AAD complicated with AIS was considered, and the patient was immediately subjected to cardiovascular surgery for treatment. The next day, the patient underwent aortic arch and ascending aortic replacement and aortic valvuloplasty.
Clinical manifestations for AAD combined with AIS are diverse. Some patients may not exhibit typical chest or back pains. Therefore, patients should be carefully evaluated to exclude AAD before administering IVT in order to avoid adverse consequences.
Core Tip: Acute aortic dissection (AAD) is a high mortality condition that can lead to acute ischemic stroke (AIS). A patient was treated with thrombolytic therapy in a local hospital for AIS but his symptoms did not improve and progressed to a large cerebral infarction. The patient was eventually diagnosed with AAD. This is a rare case and we should rule out AAD before thrombolysis in patients with cerebral infarction.
- Citation: He ZY, Yao LP, Wang XK, Chen NY, Zhao JJ, Zhou Q, Yang XF. Acute ischemic Stroke combined with Stanford type A aortic dissection: A case report and literature review. World J Clin Cases 2022; 10(22): 8009-8017
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/8009.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.8009
For patients with acute ischemic stroke (AIS) presenting with moderate to severe neurological deficits, intravenous rt-PA within 4.5 h of symptom onset remains the standard treatment option in the absence of contraindications to thrombolytic therapy[1]. Some patients with acute ischemic cerebral infarction present themselves to hospitals with little time left for thrombolysis, however, patient care should involve ruling out conditions associated with increased risks of hemorrhagic complications, such as acute aortic dissection (AAD), before administering thrombolytic therapy.
Because of the extension of the dissection into the common carotid artery, the prevalence of cerebral infarction in patients with Stanford type A aortic dissection cases is approximately 6%[2]. The most common presentation of AAD is a sudden onset of severe chest or back pains, without evidence of myocardial ischemia. However, a subset of AAD patients initially present with neurologic symptoms, including transient or permanent central neurologic symptoms (such as syncope) and various spinal symptoms (such as paralysis or paraplegia)[3]. The AAD patients that present with neurological symptoms can easily be misdiagnosed. Because of the high risk of aortic arch or ascending aortic rupture, AAD is a contraindication for thrombolytic therapy[4].
A patient with AAD combined with cerebral infarction was diagnosed with cerebral infarction at a local hospital and administered with thrombolytic therapy. Then, the patient was referred to our hospital for further treatment. He was found to have cerebral infarction combined with AAD, and was surgically treated. We intend to use this case report and literature review to provide basis for clinical practice.
The main complaint was left-sided limb weakness and slurred speech.
A 49-year-old middle-aged man was admitted to a local hospital with a sudden onset of left-sided limb weakness and slurred speech for 1 h. The patient was conscious upon admission and had no discomforts, such as headaches or chest pain.
He had a previous history of hypertension and was not on any oral medications to control blood pressure.
He denied any family history of brain diseases.
Clinical examination upon admission revealed consciousness, incomplete mixed aphasia, grade 2 muscle strength in the left upper extremity, grade 0 muscle strength in the left lower extremity, and normal muscle strength in the right limb.
Blood tests were performed. Routine blood tests: leukocyte counts, 15.8 × 109/L; platelet counts, 119 × 109/L; erythrocytes, 4.95 × 1012/L; and calcitoninogen, 4.49 ng/mL. Cardiac enzyme profiling revealed: lactate dehydrogenase, 768 U/L; creatine kinase, 1743 U/L; creatine kinase isoenzyme, 74 U/L. Blood gas analysis revealed: lactate, 2.5 mmol/L With regards to coagulation function, prothrombin time was 15.2 s, with an international standardized ratio of 1.27, prothrombin time was 18.4 s, fibrinogen was 1.07 g/L, and D-dimer was 7.190 mg/L.
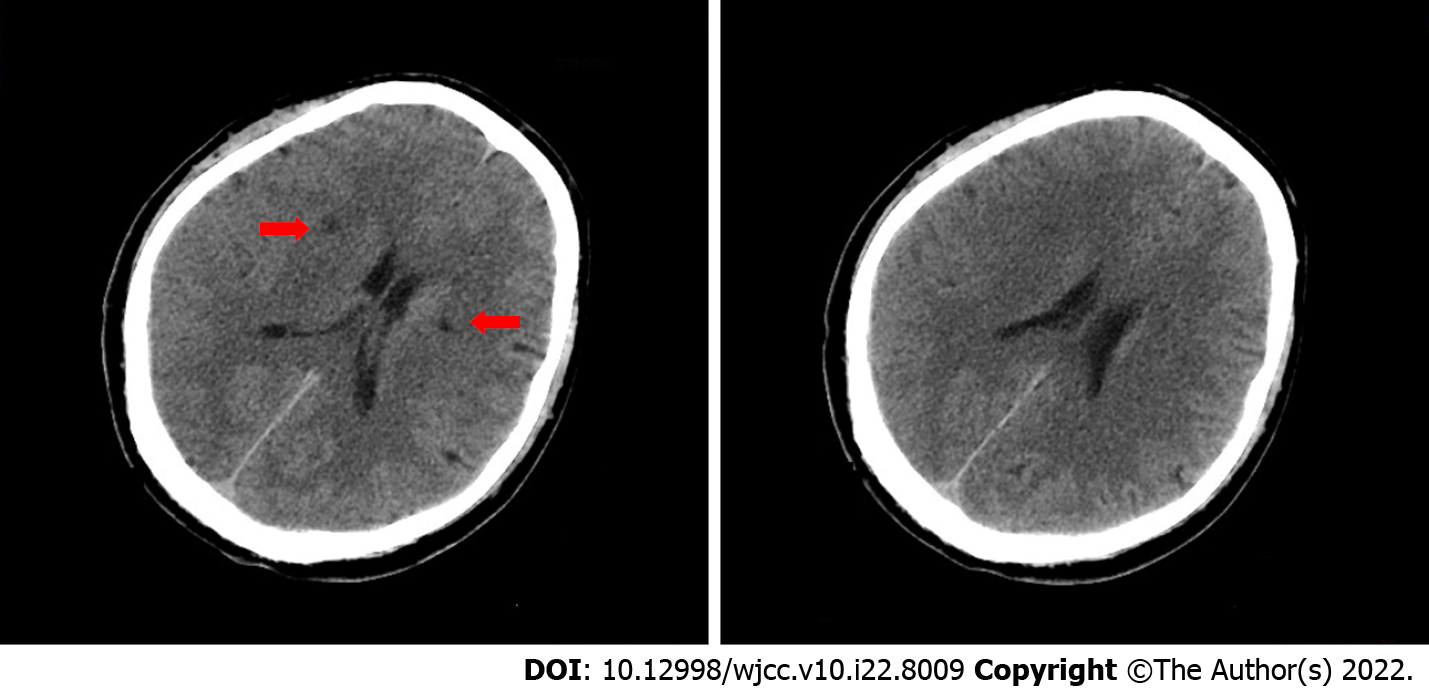
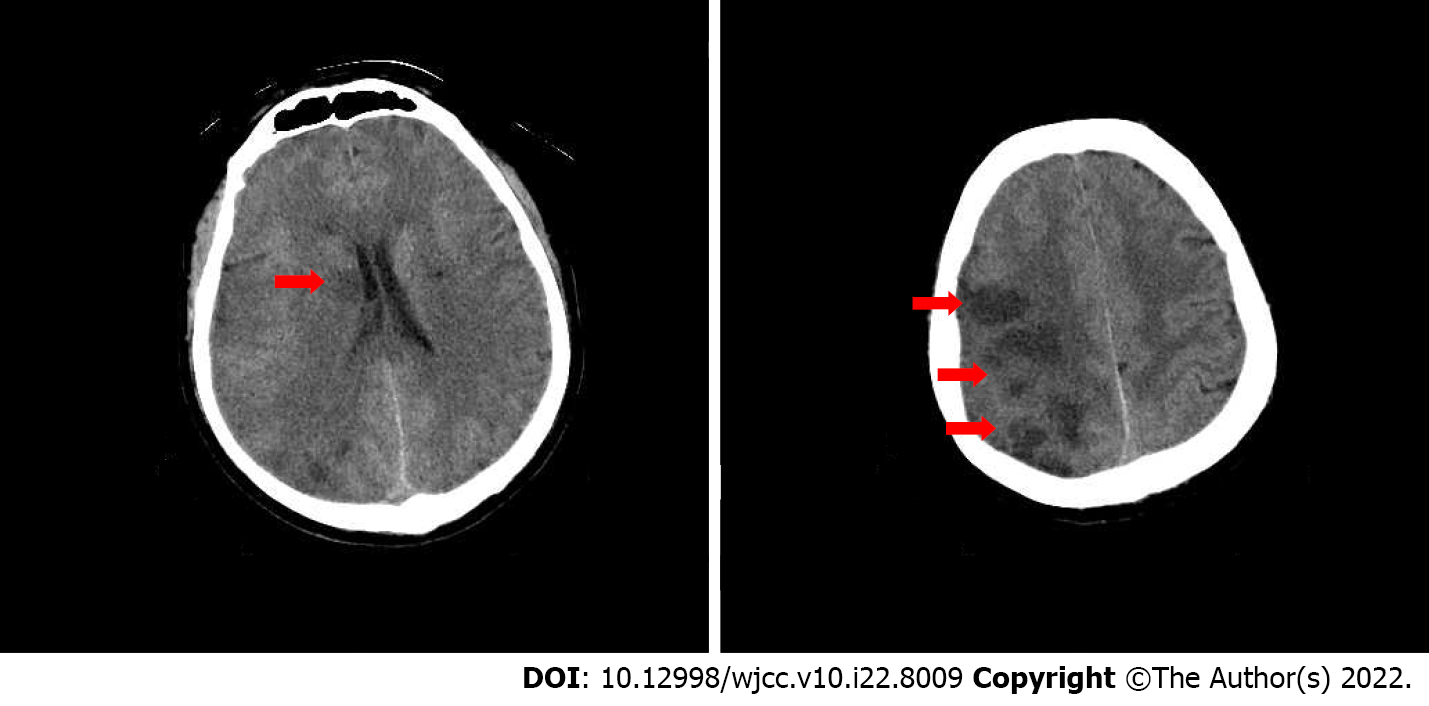
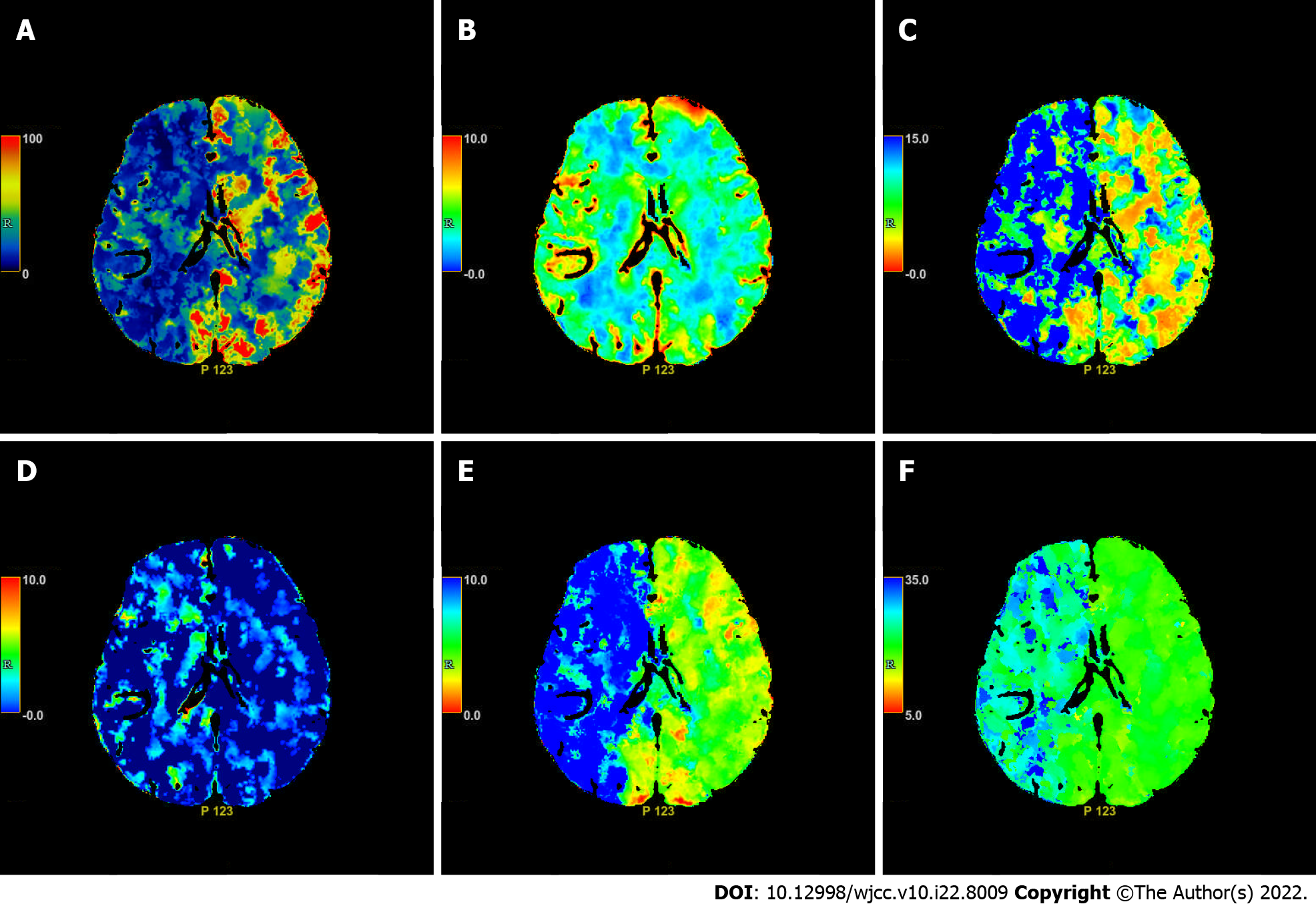
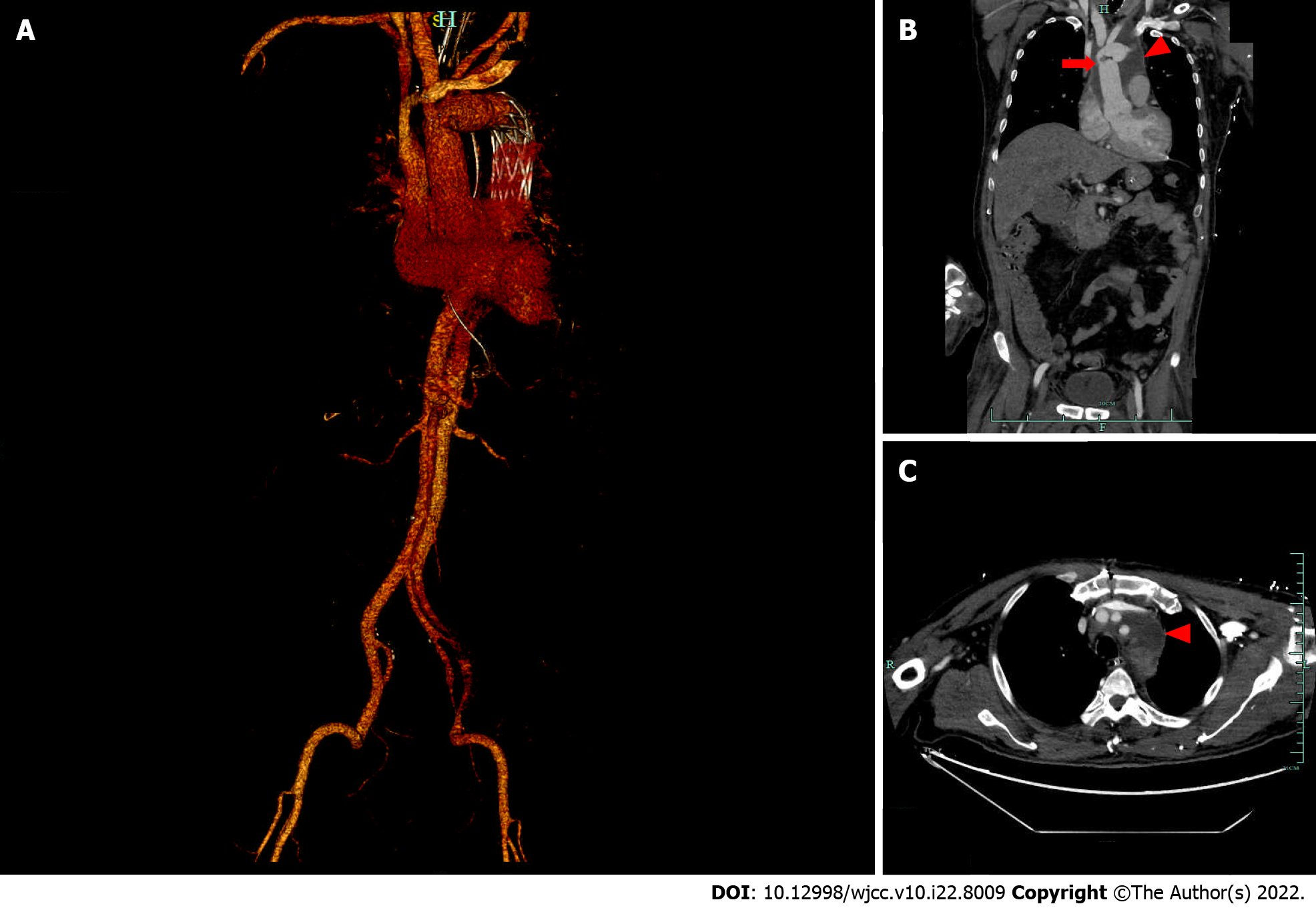
Cranial computed tomography (CT) scan revealed bilateral basal ganglia and lateral ventricular paraventricular infarct lesions (Figure 1). This patient had a National Institute of Health Stroke Scale score of 9. In this patient, recombinant tissue fibrinogen activator was used to perform intravenous thrombolysis (IVT). The patient became less conscious and had grade 0 muscle strength in the left limb. On the next day, he was transferred from the local hospital to our hospital for further treatment. He was examined at our hospital and found to have a D-dimer greater than 100000 mg/L. Cranial CT was performed, which revealed multiple cerebral infarctions in the right basal ganglia (Figure 2A) and right frontotemporoparietal (Figure 2B). CT perfusion showed multiple ischemic cores in the right frontotemporoparietal lobe as well as relatively extensive hypoperfusion areas (Figure 3). Emergency head and neck CT angiography (CTA) revealed aortic dissection in the ascending aorta, innominate artery, and right common carotid artery. Then, CTA of thoracoabdominal aorta was performed, which revealed aortic dissection in the ascending aorta, innominate artery, right common carotid artery, abdominal trunk, common hepatic artery, splenic artery, superior mesenteric artery, left common iliac artery, and left external iliac aorta (Figure 4). CTA revealed a Stanford type A aortic dissection that extended from aortic root to the left external iliac artery.
Stanford type A AAD complicated by AIS was considered.
The patient was immediately scheduled for cardiovascular surgery. On the next day, he was subjected to aortic arch and ascending aortic replacement and aortic valvuloplasty. Laceration of AAD was intraoperatively found in the lesser curvature of the aortic arch. Postoperatively, CTA at 19 d revealed reconstructed and remodeled aortic arch and thoracic aorta with periaortic hematoma (Figure 5).
The patient had grade 1 muscle strength in the left limb and was transferred to a rehabilitation facility for further care and management. Six months after discharge, the patient still has hemiparesis of the left limb. The patient’s modified Rankin Scale score was 4.
We report a patient with a case of cerebral infarction as the first presentation who received intravenous thrombolytic therapy after a missed AAD diagnosis, and who ultimately had a poor prognostic outcome. A limited number of studies have reported cases with cerebral infarction as the first symptom with concomitant AAD[4-10]. Clinical management of these patients is below optimal thresholds. We report this case and review the relevant literature to provide a reference for clinicians.
AAD is a high mortality disease, and its incidence is about 3 cases per 100000 people per year[11]. Risk factors for AAD include long-term arterial hypertension, smoking, dyslipidemia, drug abuse (cocaine, crack cocaine, or amphetamine), connective tissue disorders, vascular inflammation, deceleration trauma and iatrogenic factors (catheter or instrument intervention as well as valvular or aortic surgery)[3]. Hypertension is a common risk factor for aortic coarctation, with up to 75% of AAD patients suffering from hypertension[12]. The patient in this article had no obvious history of trauma, surgery, or drugs use, but had a history of untreated hypertension. Therefore, hypertension may be the main cause of AAD in this patient. Through various mechanisms, including extension of the dissection to common carotid artery, to intracranial carotid artery[13], thromboembolism and cerebral hypoperfusion[14], Stanford A AAD can be complicated by stroke[15]. Studies have reported that AAD often involves the right common carotid artery. Therefore, patients often present with pulse weakness and left-sided hemiparesis[6]. Consistent with previous reports, the patient in this report had a right-sided common carotid artery dissection and first presented with left-sided hemiplegia. We hypothesize that the patient had an unstable embolus in the right common carotid artery dissection, which dislodged and led to an acute stroke.
Chest or back pains comprise the most common AAD symptoms. Most patients with AAD combined with neurological symptoms present with initial pain, but one third of patients have no pain symptoms[16]. Patients with neurological symptoms only, and without pain, may be missed for AAD at the time of diagnosis. This was the case with our reported patient. He initially presented with painless neurological symptoms and eventually, the disease. Aortic dissection was missed upon first admission at the local hospital. In addition, some patients have aphasia or a reduced level of consciousness, and are unable to report chest and back pain, leading to undiagnosed or delayed diagnosis of AAD[8]. About 1% of patients with acute ischemic stroke have AAD[6]. Therefore, for patients with cerebral infarction, in addition to focusing on patient’s chest and back pains, physicians should pay attention to any unexplained hypotension, mild dyspnea, asymmetry in blood pressure between the arms (differences in systolic pressure over 20 mmHg), loss of consciousness, electrocardiogram changes, heart murmurs and cold extremities[17].
In addition to clinical symptoms, some ancillary tests can provide some assistance in the diagnosis of AAD. Yoshimuta et al[18] reported that D-dimer levels are significantly elevated in patients with ischemic stroke and AAD than in those without AAD. They concluded that D-dimer is a potential early diagnostic marker for AAD with isolated neurological symptoms in ischemic stroke patients. Our reported patient had elevated D-dimer levels (7.190 mg/L) in blood at the time of presentation, implying that he was at risk of AAD. It has also been reported that chest x-ray can provide valuable diagnostic information by detecting widened mediastinum and abnormally shaped aorta in 80% of AAD patients[16]. Although abnormal chest radiography may be helpful in evaluation of suspected AAD, it may be normal in a subset of patients[2]. Ordinary CT can detect a certain proportion of AAD patients, but it is also negative in a significant proportion of AAD patients. CTA plays a central role in the diagnosis of AAD, but some patients with cerebral infarction cannot be routinely subjected to CTA to determine whether they have a combined AAD and whether they are eligible for thrombolytic therapy because of the urgent time window. Magnetic resonance imaging (MRI) can be used to comprehensively evaluate aortic dissection, but it is slower than CT imaging. Moreover, some AIS patients do not have sufficient time to perfect MRI. In addition, MRI is difficult for unstable AAD patients during imaging.
Ultrasound is a non-invasive, bedside, real-time diagnostic tool that can be used to detect AAD in patients with cerebral infarction in a timely manner[8]. Carotid ultrasound is an effective method for the diagnosis of AAD-associated carotid artery dissection[4,8,19]. Tsivgoulis et al[4] emphasized that simultaneous ultra-early ultrasound evaluation and clinical assessment of acute stroke patients can help in the early diagnosis of AAD and prevent inadvertent use of intravenous thrombolytic agents in such patients.
Failure of physicians to take an adequate history or/and physical examination, failure to identify atypical symptoms, failure to arrange or interpret diagnostic tests, and failure to arrange appropriate specialized consultation were the main factors contributing to misdiagnosis of AAD in the emergency department[20]. Due to the high mortality rate of AAD, reducing missed diagnoses of AAD can prevent potential medical disputes.
IVT is an effective treatment for AIS within 4.5 h of symptom onset. However, IVT is contraindicated in patients with AIS combined with AAD, as it may lead to aortic dissection[21], or delay life-saving surgery[22]. IVT in patients with acute stroke caused by AAD has been associated with poor prognosis[16,23]. The patient in this article presented with worse muscle strength and consciousness after IVT. This outcome could be attributed to various reasons. When alteplase was used for IVT, more thrombus disintegrated and were dislodged from the intima of the right common carotid artery, leading to excessive embolization of the right cerebral hemisphere and, ultimately, to a more severe cerebral infarction in the patient.
For some patients with AIS due to large-vessel occlusion, mechanical thrombectomy within 24 h after symptom onset may improve functional outcomes[24]. In patients with severe functional impairment possibly caused by large vessel occlusion, a CTA or magnetic resonance angiogram of the head and neck should be performed to determine the occlusion location and the eligibility for mechanical throm
Acute Type A AAD has a mortality rate of 50% within the first 48 h without surgery, and surgery remains the best therapy for reducing the risk of mortality[25]. However, it has not been conclusively determined whether surgery should be performed in patients with Type A AAD presenting with neurological deficits or coma. Coma, shock secondary to pericardial tamponade, malperfusion of coronary or peripheral arteries, and stroke are significant predictors of postoperative mortality[25]. Although AAD patients with coma or cerebral malperfusion have a poor postoperative prognosis, some patients have been reported to recover if rapid cerebral reperfusion is achieved[26,27], especially if the time between symptom onset and arrival at the operating room is less than 5 h[28]. Ueyama et al[29] successfully treated a patient with Stanford type A AAD combined with cerebral malperfusion through urgent surgical therapy. Surgery can also provide IVT opportunities for patients with cerebral infarction after AAD treatment. Intravenous recombinant tissue-type plasminogen activator therapy for ischemic stroke has been shown to be effective and safe several days after surgical treatment of AAD[30].
AAD is a serious and lethal disease. Some AAD patients with acute stroke present atypical symptoms, with only neurological deficits as first symptoms, making it easy to miss or delay AAD diagnosis. When treating AIS patients, clinicians should rule out AAD before IVT, if there is sufficient time. When timing of IVT is urgent, simultaneous ultrasound evaluation and clinical assessment of the patient can be performed to exclude AAD and avoid adverse consequences due to IVT.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jensen-Kondering U, Germany; Mesrati MA, Tunisia S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA. 2015;313:1451-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 504] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 2. | Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;879-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2417] [Cited by in RCA: 2333] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 3. | Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet. 2015;385:800-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 449] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 4. | Tsivgoulis G, Vadikolias K, Heliopoulos I, Patousi A, Iordanidis A, Souftas V, Piperidou C. Aortic arch dissection causing acute cerebral ischemia: an uncommon contraindication for intravenous thrombolysis. Circulation. 2011;124:657-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Veyssier-Belot C, Cohen A, Rougemont D, Levy C, Amarenco P, Bousser MG. Cerebral infarction due to painless thoracic aortic and common carotid artery dissections. Stroke. 1993;24:2111-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Iguchi Y, Kimura K, Sakai K, Matsumoto N, Aoki J, Yamashita S, Shibazaki K. Hyper-acute stroke patients associated with aortic dissection. Intern Med. 2010;49:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Wang J, Wu LR, Xie X. Stanford type a aortic dissection with cerebral infarction: a rare case report. BMC Neurol. 2020;20:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Rodríguez-Luna D, Vilar RM, Peinazo M, del Villar A, Claramonte B, Vilar C, Geffner D. Intravenous thrombolysis in an elderly patient with acute ischemic stroke masking aortic dissection. J Stroke Cerebrovasc Dis. 2011;20:559-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Folgoas E, Toulgoat F, Sévin M, Boutoleau-Bretonnière C, Guillon B. [Ischemic stroke related to pauci-symptomatic acute aortic dissection. Risks of intravenous thrombolysis]. Rev Neurol (Paris). 2012;168:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Hama Y, Koga M, Tokunaga K, Takizawa H, Miyashita K, Iba Y, Toyoda K. Carotid Ultrasonography Can Identify Stroke Patients Ineligible for Intravenous Thrombolysis Therapy due to Acute Aortic Dissection. J Neuroimaging. 2015;25:671-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 606] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 12. | Baguet JP, Chavanon O, Sessa C, Thony F, Lantelme P, Barone-Rochette G, Mallion JM. European Society of Hypertension scientific newsletter: hypertension and aortic diseases. J Hypertens. 2012;30:440-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Stanley I, Sharma VK, Tsivgoulis G, Lao AY, Alexandrov AV. Painless aortic dissection with unusual extension into intracranial internal carotid arteries. Cerebrovasc Dis. 2007;24:314-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Khan IA, Nair CK. Clinical, diagnostic, and management perspectives of aortic dissection. Chest. 2002;122:311-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 368] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 15. | Gaul C, Dietrich W, Erbguth FJ. Neurological symptoms in aortic dissection: a challenge for neurologists. Cerebrovasc Dis. 2008;26:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Gaul C, Dietrich W, Friedrich I, Sirch J, Erbguth FJ. Neurological symptoms in type A aortic dissections. Stroke. 2007;38:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 17. | Koga M, Iguchi Y, Ohara T, Tahara Y, Fukuda T, Noguchi T, Matsuda H, Minatoya K, Nagatsuka K, Toyoda K. Acute ischemic stroke as a complication of Stanford type A acute aortic dissection: a review and proposed clinical recommendations for urgent diagnosis. Gen Thorac Cardiovasc Surg. 2018;66:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Yoshimuta T, Yokoyama H, Okajima T, Tanaka H, Toyoda K, Nagatsuka K, Higashi M, Hayashi K, Kawashiri MA, Yasuda S, Yamagishi M. Impact of Elevated D-Dimer on Diagnosis of Acute Aortic Dissection With Isolated Neurological Symptoms in Ischemic Stroke. Circ J. 2015;79:1841-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Bonnin P, Giannesini C, Amah G, Kevorkian JP, Woimant F, Levy BI. Doppler sonograpy with dynamic testing in a case of aortic dissection extending to the innominate and right common carotid arteries. Neuroradiology. 2003;45:472-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Morello F, Santoro M, Fargion AT, Grifoni S, Nazerian P. Diagnosis and management of acute aortic syndromes in the emergency department. Intern Emerg Med. 2021;16:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Marian AJ, Harris SL, Pickett JD, Campbell E, Fromm RE. Inadvertent administration of rtPA to a patient with type 1 aortic dissection and subsequent cardiac tamponade. Am J Emerg Med. 1993;11:613-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Flemming KD, Brown RD Jr. Acute cerebral infarction caused by aortic dissection: caution in the thrombolytic era. Stroke. 1999;30:477-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Fessler AJ, Alberts MJ. Stroke treatment with tissue plasminogen activator in the setting of aortic dissection. Neurology. 2000;54:1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Mendelson SJ, Prabhakaran S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. JAMA. 2021;325:1088-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 453] [Article Influence: 113.3] [Reference Citation Analysis (0)] |
| 25. | Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwöger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ; ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873-2926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2374] [Cited by in RCA: 3105] [Article Influence: 282.3] [Reference Citation Analysis (0)] |
| 26. | Roselli EE, Rafael A, Soltesz EG, Canale L, Lytle BW. Simplified frozen elephant trunk repair for acute DeBakey type I dissection. J Thorac Cardiovasc Surg. 2013;145:S197-S201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 27. | Pocar M, Passolunghi D, Moneta A, Donatelli F. Recovery of severe neurological dysfunction after restoration of cerebral blood flow in acute aortic dissection. Interact Cardiovasc Thorac Surg. 2010;10:839-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Tsukube T, Hayashi T, Kawahira T, Haraguchi T, Matsukawa R, Kozawa S, Ogawa K, Okita Y. Neurological outcomes after immediate aortic repair for acute type A aortic dissection complicated by coma. Circulation. 2011;124:S163-S167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Ueyama K, Otaki K, Koyama M, Kamiyama H. Urgent simultaneous revascularization of the carotid artery and ascending aortic replacement for type A acute aortic dissection with cerebral malperfusion. Gen Thorac Cardiovasc Surg. 2007;55:284-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Matsuzono K, Suzuki M, Arai N, Kim Y, Ozawa T, Mashiko T, Shimazaki H, Koide R, Fujimoto S. Successful Tissue Plasminogen Activator for a Patient with Stroke After Stanford Type A Aortic Dissection Treatment. J Stroke Cerebrovasc Dis. 2018;27:e132-e134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |