Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.7994
Peer-review started: February 8, 2022
First decision: May 11, 2022
Revised: May 18, 2022
Accepted: June 21, 2022
Article in press: June 21, 2022
Published online: August 6, 2022
Processing time: 163 Days and 18.4 Hours
Over the past 20 years, we have gained a deep understanding of the biological heterogeneity of diffuse large B cell lymphoma (DLBCL) and have developed a range of new treatment programs based on the characteristics of the disease, bringing us to the era of immune-chemotherapy. However, the effectiveness and molecular mechanisms of targeted-immunotherapy remain unclear in DLBCL. Targeted-immunotherapy may be beneficial for specific subgroups of patients, thus requiring biomarker assessment.
Here, we report a case of MCD subtype DLBCL with MYD88L265P and CD79B mutations, considered in the initial stage as lymphoplasmic lymphoma (LPL) or Waldenstrom macroglobulinemia (WM). Flow cytometry supported this view; however, the immunohistochemical results of the lymph nodes overturned the above diagnosis, and the patient was eventually diagnosed with MCD subtype DLBCL. The presence of a monoclonal IgM component in the serum and infiltration of small lymphocytes with a phenotype compatible with WM into the bone marrow led us to propose a hypothesis that the case we report may have trans
This highlights the possible transformation from WM to DLBCL, CD79B mutation may be a potential biomarker for predicting this conversion.
Core Tip: This report highlights the possible transformation from Waldenstrom macroglobulinemia (WM) to diffuse large B cell lymphoma (DLBCL). Bone marrow infiltration by small lymphocytes, with an immunophenotype compatible with WM and the presence of MYD88L265P and CD79B mutations, support the hypothesis that the case may have transformed from lymphoplasmic lymphoma/WM. The CD79B mutation may be a potential biomarker for predicting the conversion of WM to DLBCL. Understanding the biology and mechanisms behind this process is important to identify susceptible patients. Frail patients could benefit from personalized low toxicity therapeutic approaches based on their mutational profile.
- Citation: Huang WY, Weng ZY. Occurrence of MYD88L265P and CD79B mutations in diffuse large b cell lymphoma with bone marrow infiltration: A case report. World J Clin Cases 2022; 10(22): 7994-8002
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/7994.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.7994
The cell of origin (COO) is determined by the algorithm of Hans; thus, diffuse large B cell lymphoma (DLBCL) cases can be classified as germinal center B-cell-like (GCB) or non-GCB[1]. With the progression of clinical practice, COO has been found to be insufficient to explain different treatment responses. The biological heterogeneity of DLBCL may be directly due to its various genetic abnor
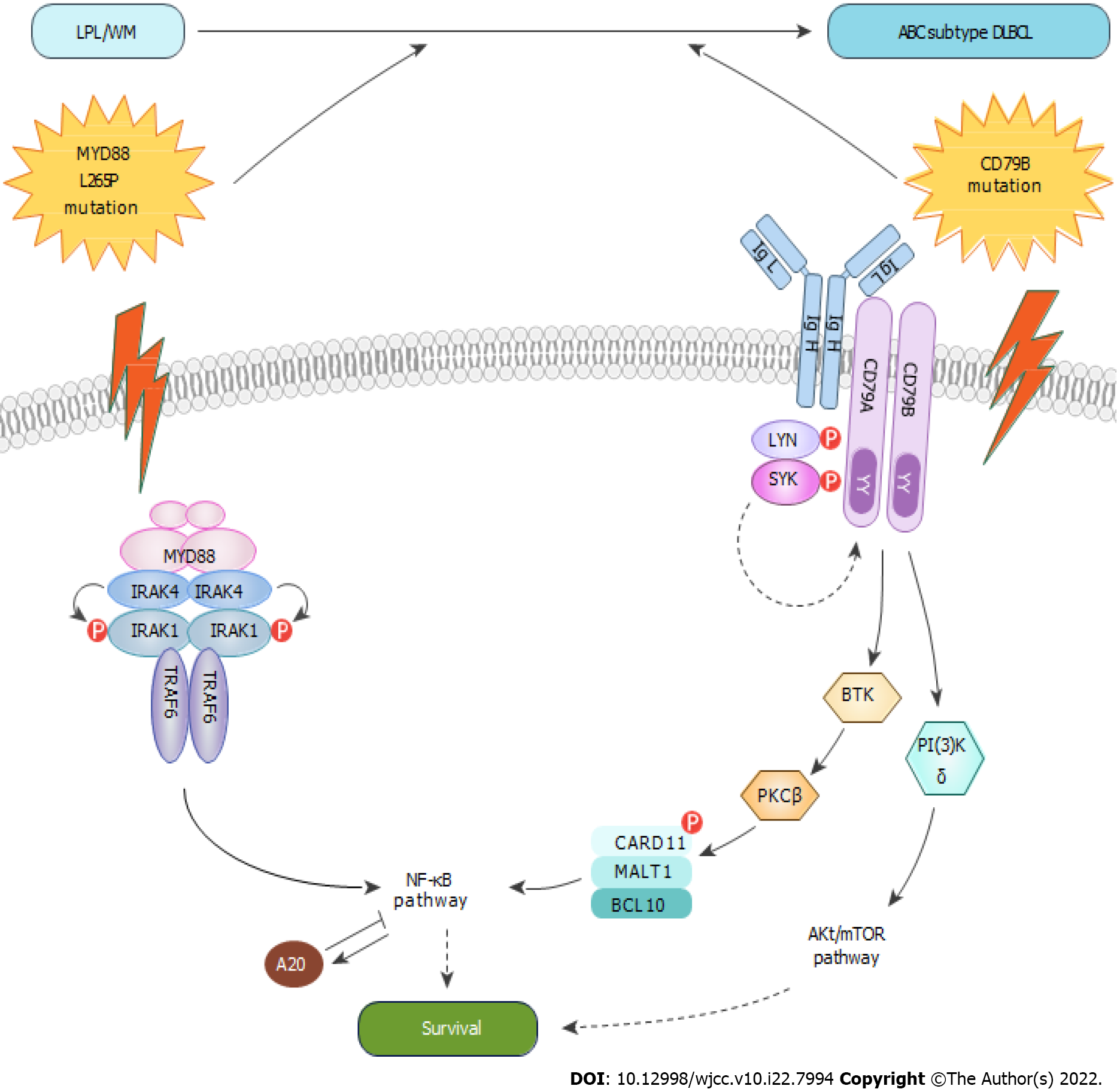
CD79 is a transmembrane protein comprising two distinct peptide chains, CD79A and CD79B, which form a molecular complex with B-cell receptor (BCR). The cytoplasmic ends of the two peptide chains of CD79 contain an immunoreceptor tyrosine-based activation motif, which is involved in activating the nuclear factor kappa light chain enhancer of activated B cell (NF-κB) signaling pathway during antigen-mediated BCR activation[3]. Myeloid differentiation primary response 88 (MYD88) is a soluble adaptor protein in the cytoplasm; it belongs to the Toll/interleukin-1 receptor and death domain family members and mediates NF-κB signaling[4]. A mutation in CD79B was detectable in 30% of patients with ABC DLBCL and 3% of those with GCB DLBCL. In addition, MYD88 mutations were detected in 28% of ABC DLBCL[5]. They play an important role in DLBCL evolution. In recent years, with extensive research on multi-platform genomes, DLBCL was divided into seven genetic subtypes[2]; DLBCL with MYD88L265P and CD79B mutations were defined as the MCD genetic subtype, 42% of which had double concomitant mutations, mostly observed in the ABC subtype[2].
Here, we present a rare DLBCL case with mutations positive for MYD88L265P and CD79B, named MCD genetic subtype, characterized by discordant bone marrow (BM) infiltration, which may have transformed from indolent lymphoma.
Fever and fatigue.
An 84-year-old bedridden man presented to the clinic with complaints of fever and fatigue and was hospitalized on January 2021.
He had been taking medication for hypertension and diabetes for more than 10 years and underwent interventional treatment for coronary atherosclerotic heart disease 4 years prior.
He had no history of viral hepatitis B or family history of cancer.
Rales could be heard in the lungs, and edema was visible in the lower extremities.
A laboratory exam revealed normocytic anemia (hemoglobin 66 g/L), elevated C reactive protein (169 mg/L), and hydrogen hexachloro platinum (III).
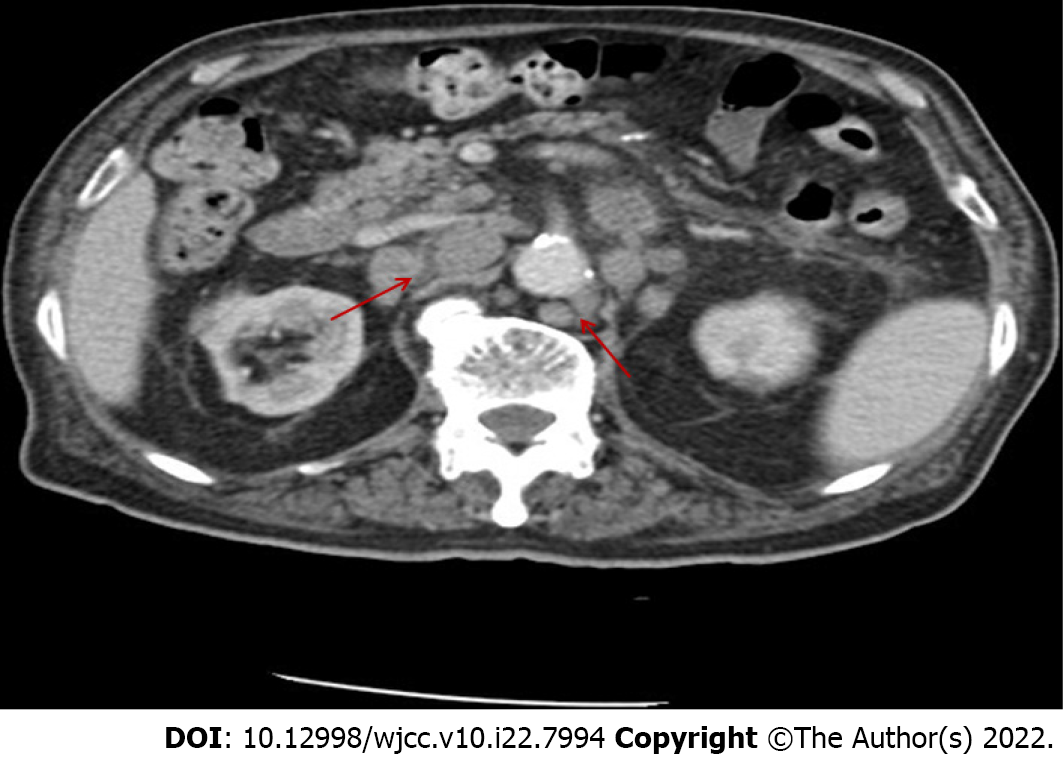
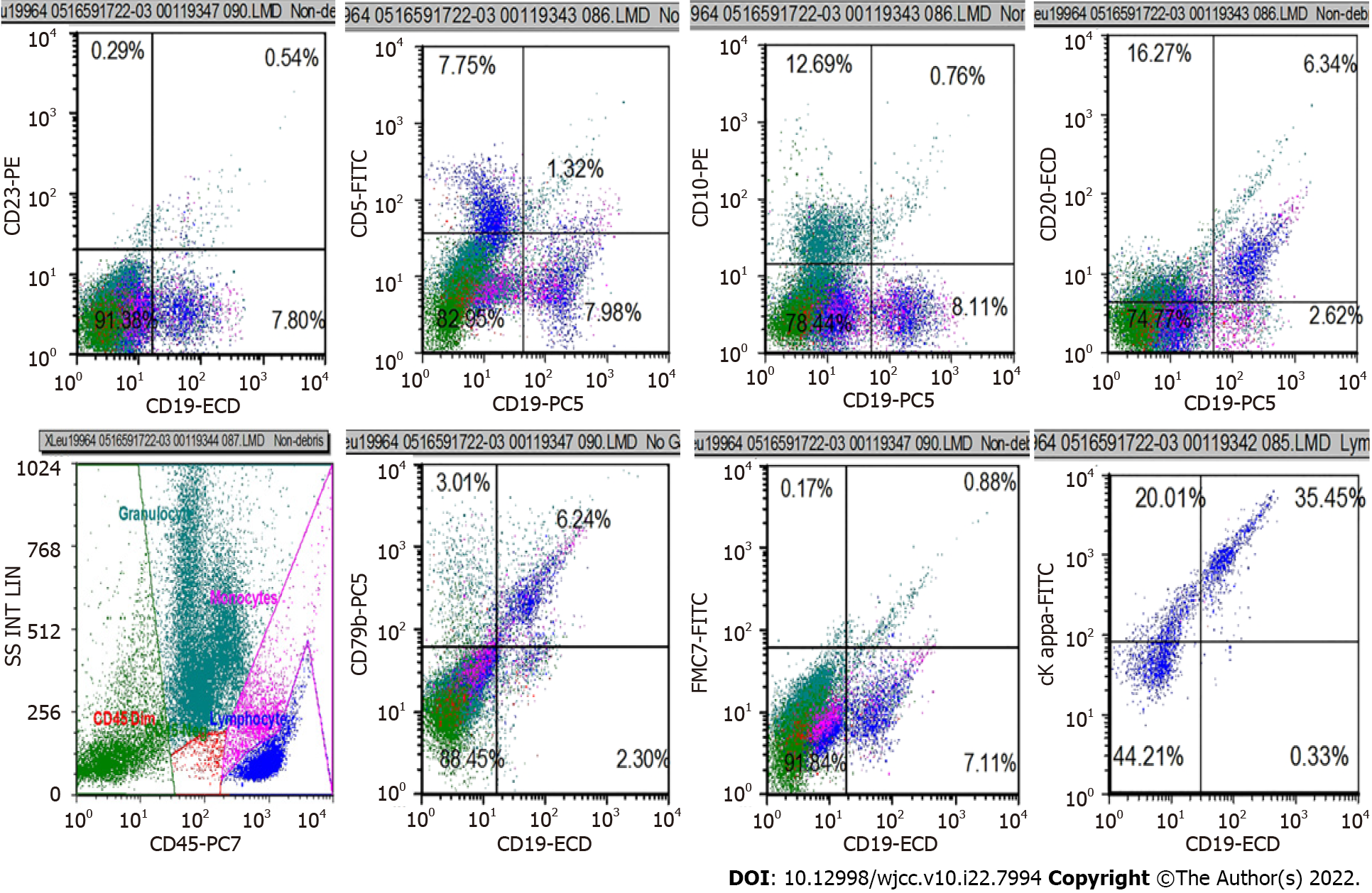
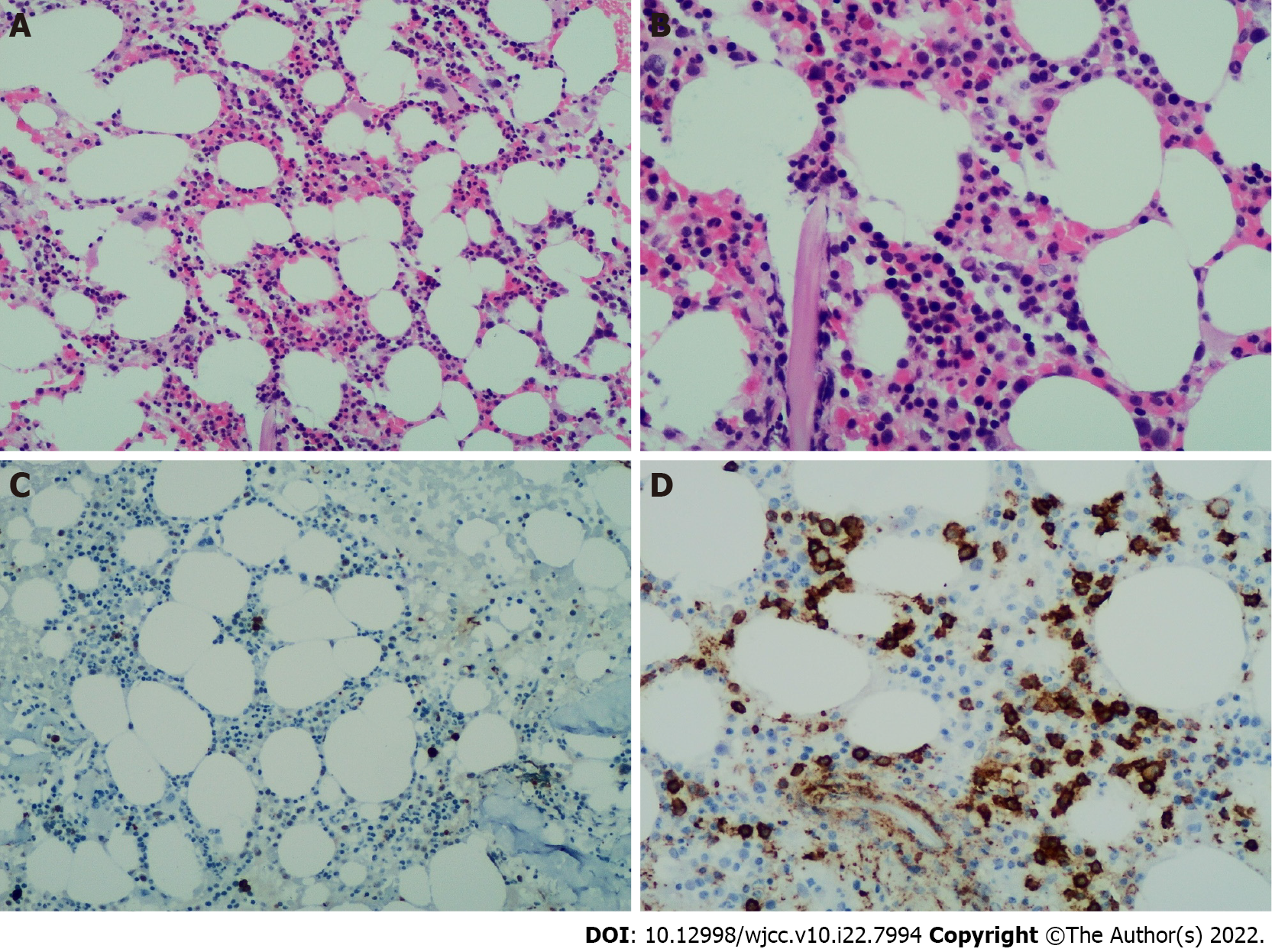
B mode ultrasonography showed several swollen cervical lymph nodes, with the largest being 1.7 cm × 1.1 cm with an abnormal structure. An abdominal computed tomography scan with contrast revealed pulmonary infection, no evidence of splenomegaly, and multiple enlarged lymph nodes in the mediastinum and abdominal region, with a maximum size of 2.5 cm × 2.5 cm (Figure 1). We completed the routine examination of the BM, and no abnormal cells were detected in the smear. However, using BM as a specimen, flow cytometry identified a group of abnormal monoclonal B cells that showed restricted expression of the intracellular kappa light chain. This group of B cells tested positive for CD19, CD20, and CD79b and were negative for CD5, CD10, CD23, and FMC7 (Figure 2). The morphology of the BM biopsy suggested that small B-cell lymphomas were scattered, indicating that the disease involved the BM (Figure 3).
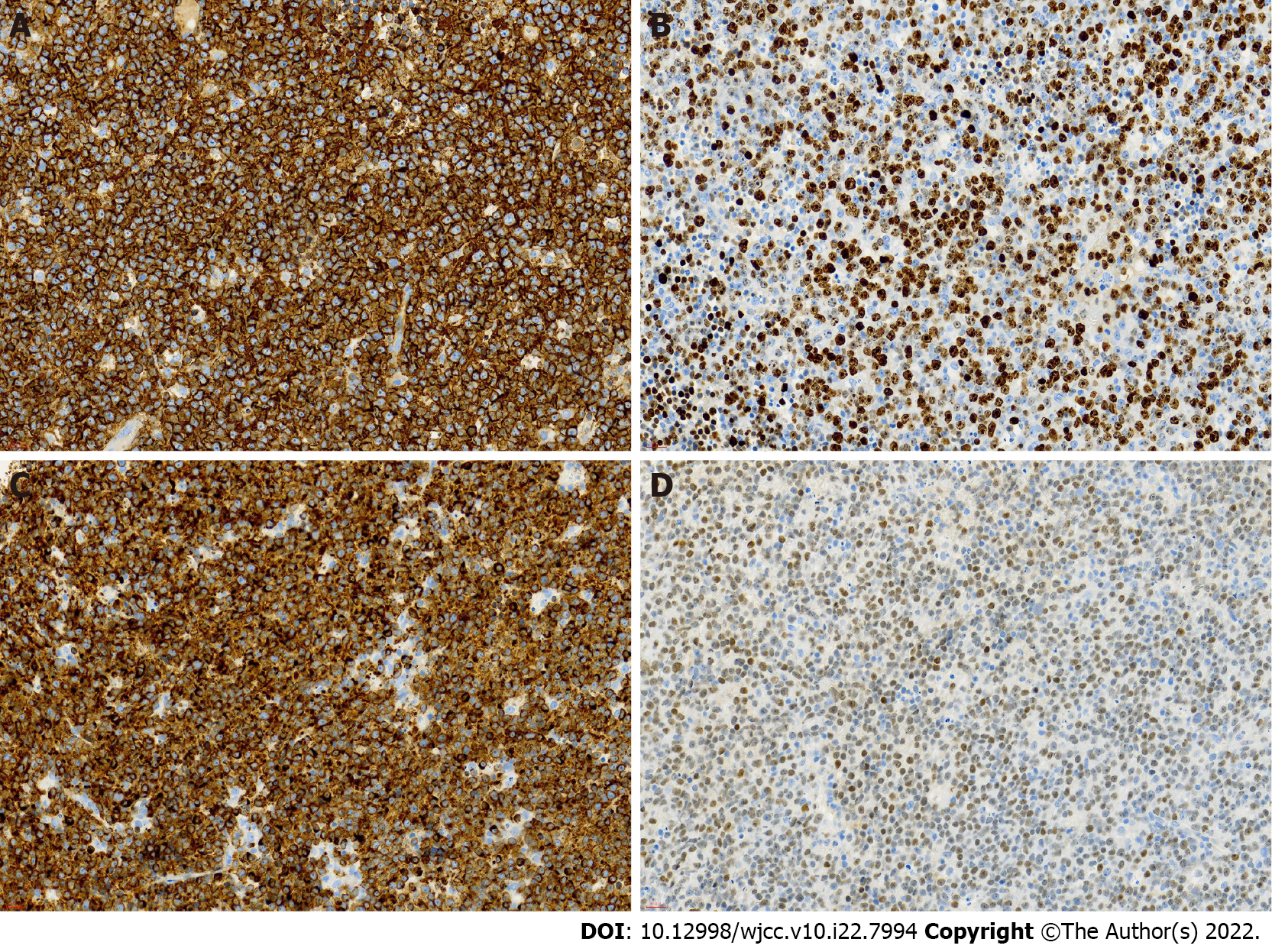
In the case of monoclonal small B-lymphocyte tumors, chronic lymphocytic leukemia (CLL), lymphoplasmacytic lymphoma (LPL), and Waldenstrom macroglobulinemia (WM) should be considered. However, patients with CLL usually also express CD5 and CD23, and the peripheral blood is involved; this patient did not have these characteristics. Our case was characterized by IgM monoclonal gammopathy without plasmacytoid changes. To further confirm the diagnosis, we performed a biopsy of the patient’s cervical lymph nodes. The immunohistochemical results of the lymph nodes are as follows (Figure 4): Bcl-2(+); Bcl-6(+); CD10(-); CD20(+); CD3(-); CD5(-); CMYC(40%); CyclinD1(-); Ki67(70%); MUM-1(+); P53(60%).
The immunohistochemical results support the diagnosis of subtype activated B-cells like that of DLBCL [stage IV B, National Comprehensive Cancer Network International Prognostic Index (IPI) ≥ 6, Eastern Cooperative Oncology Group performance score = 4]. Interestingly, the patient had MYD88L265P and CD79B mutations.
Ibrutinib is an irreversible small-molecule BTK inhibitor and a B lymphocyte signaling protein, which can effectively inhibit the proliferation and survival of malignant B lymphocytes[6]. Studies have found that compared with GCB subtypes, ibrutinib has a better effect on patients with ABC DLBCL[7]. More importantly, the combined application of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone can significantly increase the toxicity of the drug for patients aged ≥60 years, which in turn affects the therapeutic effect[8]. The patient could not tolerate chemotherapy. After a comprehensive assessment, the patient was treated with I-R (ibrutinib 420 mg/d combined with rituxan 375 mg/m2).
Unfortunately, due to financial pressure and being long-term bedridden, this patient eventually refused to receive further treatment and died two months later.
Multiple studies have shown that the MYD88 mutation is present in 90% of patients with LPL/ WM; however, it is not specific to LPL/WM and is also present in DLBCL. Further research confirmed that the MYD88L265P mutation is unique to ABC and rarely occurs in GCB or primary mediastinal diffuse large B-cell lymphoma[5]. Studies have reported that continuous excessive activation of the NF-κB signaling pathway is characteristic of ABC-DLBCL. Dubois et al[9] further emphasized that activation of the NF-κB signaling pathway in the ABC subtype is related to the L265P mutation site, whereas non-L265P mutants harbor a mutational profile more similar to GCB-DLBCL. The impact of MYD88L265P and CD79B mutations on the prognosis of the ABC type is worth discussing, and most reports have shown that these two mutations negatively affect patient outcomes. One study showed that MYD88L265P and CD79B increase the risk of recurrence and progression. In addition, detection of the MYD88L265P mutation can effectively improve the predictive performance of the IPI scores[10]. Using next generation sequencing to detect 361 cases of DLBCL, a study showed that CD79B and MYD88
The mechanism of the emergence of monoclonal IgM is unclear; it may be related to the differentiation of plasma cells in the BM[11]. Cox et al[12] analyzed 151 patients with DLBCL and found that 17 cases (11.2%) had a serum monoclonal IgM component, although none were associated with MYD88L265P mutations, which indicates there is no necessary connection between monoclonal IgM and MYD88L265P. Cho et al[13] studied the relationship between the clonal status of monoclonal immunoglobulin gene rearrangement and histological B cell aggregation in the BM. The results showed that of the 394 patients with DLBCL, 32 patients had BM invasion, and only two patients with large B-cell lymphoma had no gene rearrangement detected[13]. This suggests that patients with BM invasion were more likely to have monoclonal immunoglobulin gene rearrangements. However, there is no correlation with the COO[13]. Although BM infiltration was found in our case, it showed a histological inconsistency compared with the immunohistochemistry of the peripheral lymph nodes. Regarding the biological and clinical impact, concordant BM infiltration was associated with lower progression free survival and a higher incidence of central nervous system relapse independent of the COO and IPI; therefore, the prognostic impact of discordant BM infiltration could be limited to non-CGB cases[14].
The most interesting part of this case is the possible transformation from WM to DLBCL. We support this hypothesis on two pillars: the presence of a monoclonal IgM component in the serum and infiltration of small lymphocytes with a phenotype compatible with WM into the BM. Castillo et al[15] retrospectively analyzed 1466 patients with WM; a total of 20 patients underwent histological transformation. Interestingly, all 20 patients had tissue transformation to DLBCL and had a high IPI score. The median survival time after transformation was not more than 3 years. Among them, 13 patients were tested for Ki67 expression, and it was found that their median value-added index was 90% (range 50%-99%), which suggests that the malignancy in these patients is higher. Two cases in this study were tested for MYD88L265P mutations before and after histological transformation, and the results were positive[15]. This also suggests that ibrutinib may be a potential treatment option for these histological conversion cases. Regarding the biological analysis of patients with diffuse large B lymphoma with BM infiltration, 24% of patients have discordant BM involvement, which manifested as infiltration by small cells forming lymphoid aggregates. This group, classified by flow cytometry (FCM), showed a wide variety of indolent B-cell lymphomas, although only one case showed DLBCL[14]. Genomic analysis technology can further determine whether this group of patients has transformed from indolent lymphoma[16]. As the transformation from WM to DLBCL is very rare, relevant literature on this was collected (Table 1).
| Ref. | No. of case | A/G | BM | Extramedullary site | Histological type | Staging | Therapy | Interval (mo)1 | Ending | Survival (mo) |
| Uchino et al[18] | 1 | 55/M | + | Spleen | Nospecified | IV B | R-CHOP | 408 | CR | > 17 |
| Owen et al[19] | 2 | 80/M | + | Mesenteric mass | ABC | IV | R-CVP | 36 | Dead | No specified |
| Owen et al[19] | 3 | 63/M | + | Lymphadenopath | GCB | IV | R-CHOP | 84 | PR | No specified |
| Shiseki et al[20] | 4 | 63/M | + | Lymph node | No specified | IV | THP-COP | 36 | CR | > 60 |
| Kikukawa et al[21] | 5 | 60/F | + | Brain | ABC | IV | R-MPV, WBRT+Ara-C | 72 | PR | > 6 |
| Okolo et al[22] | 6 | 69/M | + | Retroperitoneal mass | No specified | IV | R-CHOP, DRC | Unknown | CR | No specified |
| Kobayashi et al[23] | 7 | 75/M | + | Liver and ileum | GCB | IV | R-CHOP | 120 | PR | No specified |
| Elimimian et al[24] | 8 | 60/M | + | Inguinal lymph node | ABC | IV A | O+CHOP, DHAP | 168 | Dead | 4 |
The other interesting hypothesis concerns the role of mutations in CD79B in the transformation to DLBCL. A small sample study found that CD79B mutations may be a potential biomarker for predicting the conversion of WM to DLBCL[16]. We propose a transformation hypothesis on the basis of the antiapoptotic NF-κB pathway. The mutation of MYD88 is the most important pathogenic mechanism of LPL/WM. When LPL/WM acquires the mutation of CD79B, the reduced activity of LYN contributes to their increased surface BCR expression and constitutive BCR signaling. Chronically active BCR signaling and MYD88L265P-dependent signaling synergy promotes the conversion of WM to DLBCL (Figure 5). However, the genetic pathogenesis is a complex process that may incorporate other genetic changes to facilitate its transition to DLBCL. Schmitz et al[17] proposed the classification of genetic subtypes that uncovered the interrelationship between this genetic nosology and the oncogenic signaling pathway. MYD88L265P and CD79B played an important role in the evolution of WM to DLBCL, which fits the genetic characteristics of the MCD genetic subtype. It further explains why the MCD genetic subtype responds to ibrutinib[7].
In summary, with the development of genetic testing technology, research has further focused on the classification of DLBCL and its pathogenic mechanism. In the era of immune-chemotherapy, the molecular classification of DLBCL appears to offer greater prognostic interest than IPI. Furthermore, the molecular profile could help us in the choice of the optimal therapy. Ibrutinib appears a good option in patients with MCD molecular subtype DLBCL: MYD88+ CD79B+. Bone marrow infiltration by small lymphocytes, with an immunophenotype compatible with WM and the presence of MYD88L265P and CD79B mutations, supports the hypothesis that the case may have transformed from LPL/WM. Furthermore, frail patients could benefit from personalized low toxicity therapeutic approaches based on their mutational profile.
The CD79B mutation may be a potential biomarker for predicting the conversion of WM to DLBCL. Understanding the biology and mechanisms behind this process is important in identifying susceptible patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Baysal M, Turkey; Watanabe T, Japan S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO, Chan WC. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2826] [Cited by in RCA: 3158] [Article Influence: 143.5] [Reference Citation Analysis (1)] |
| 2. | Wright GW, Huang DW, Phelan JD, Coulibaly ZA, Roulland S, Young RM, Wang JQ, Schmitz R, Morin RD, Tang J, Jiang A, Bagaev A, Plotnikova O, Kotlov N, Johnson CA, Wilson WH, Scott DW, Staudt LM. A Probabilistic Classification Tool for Genetic Subtypes of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer Cell. 2020;37:551-568.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 750] [Article Influence: 150.0] [Reference Citation Analysis (0)] |
| 3. | Phelan JD, Young RM, Webster DE, Roulland S, Wright GW, Kasbekar M, Shaffer AL 3rd, Ceribelli M, Wang JQ, Schmitz R, Nakagawa M, Bachy E, Huang DW, Ji Y, Chen L, Yang Y, Zhao H, Yu X, Xu W, Palisoc MM, Valadez RR, Davies-Hill T, Wilson WH, Chan WC, Jaffe ES, Gascoyne RD, Campo E, Rosenwald A, Ott G, Delabie J, Rimsza LM, Rodriguez FJ, Estephan F, Holdhoff M, Kruhlak MJ, Hewitt SM, Thomas CJ, Pittaluga S, Oellerich T, Staudt LM. A multiprotein supercomplex controlling oncogenic signalling in lymphoma. Nature. 2018;560:387-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 288] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 4. | de Groen RAL, Schrader AMR, Kersten MJ, Pals ST, Vermaat JSP. MYD88 in the driver's seat of B-cell lymphomagenesis: from molecular mechanisms to clinical implications. Haematologica. 2019;104:2337-2348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, Kohlhammer H, Xu W, Yang Y, Zhao H, Shaffer AL, Romesser P, Wright G, Powell J, Rosenwald A, Muller-Hermelink HK, Ott G, Gascoyne RD, Connors JM, Rimsza LM, Campo E, Jaffe ES, Delabie J, Smeland EB, Fisher RI, Braziel RM, Tubbs RR, Cook JR, Weisenburger DD, Chan WC, Staudt LM. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1041] [Cited by in RCA: 1179] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 6. | Shanafelt TD, Wang XV, Kay NE, Hanson CA, O'Brien S, Barrientos J, Jelinek DF, Braggio E, Leis JF, Zhang CC, Coutre SE, Barr PM, Cashen AF, Mato AR, Singh AK, Mullane MP, Little RF, Erba H, Stone RM, Litzow M, Tallman M. Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N Engl J Med. 2019;381:432-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 571] [Article Influence: 95.2] [Reference Citation Analysis (0)] |
| 7. | Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, Lih CJ, Williams PM, Shaffer AL, Gerecitano J, de Vos S, Goy A, Kenkre VP, Barr PM, Blum KA, Shustov A, Advani R, Fowler NH, Vose JM, Elstrom RL, Habermann TM, Barrientos JC, McGreivy J, Fardis M, Chang BY, Clow F, Munneke B, Moussa D, Beaupre DM, Staudt LM. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21:922-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 915] [Article Influence: 91.5] [Reference Citation Analysis (0)] |
| 8. | Younes A, Sehn LH, Johnson P, Zinzani PL, Hong X, Zhu J, Patti C, Belada D, Samoilova O, Suh C, Leppä S, Rai S, Turgut M, Jurczak W, Cheung MC, Gurion R, Yeh SP, Lopez-Hernandez A, Dührsen U, Thieblemont C, Chiattone CS, Balasubramanian S, Carey J, Liu G, Shreeve SM, Sun S, Zhuang SH, Vermeulen J, Staudt LM, Wilson W; PHOENIX investigators. Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non-Germinal Center B-Cell Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2019;37:1285-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 432] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 9. | Dubois S, Viailly PJ, Bohers E, Bertrand P, Ruminy P, Marchand V, Maingonnat C, Mareschal S, Picquenot JM, Penther D, Jais JP, Tesson B, Peyrouze P, Figeac M, Desmots F, Fest T, Haioun C, Lamy T, Copie-Bergman C, Fabiani B, Delarue R, Peyrade F, André M, Ketterer N, Leroy K, Salles G, Molina TJ, Tilly H, Jardin F. Biological and Clinical Relevance of Associated Genomic Alterations in MYD88 L265P and non-L265P-Mutated Diffuse Large B-Cell Lymphoma: Analysis of 361 Cases. Clin Cancer Res. 2017;23:2232-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 10. | Vermaat JS, Somers SF, de Wreede LC, Kraan W, de Groen RAL, Schrader AMR, Kerver ED, Scheepstra CG, Berenschot H, Deenik W, Wegman J, Broers R, de Boer JD, Nijland M, van Wezel T, Veelken H, Spaargaren M, Cleven AH, Kersten MJ, Pals ST. MYD88 mutations identify a molecular subgroup of diffuse large B-cell lymphoma with an unfavorable prognosis. Haematologica. 2020;105:424-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Jardin F, Delfau-Larue MH, Molina TJ, Copie-Bergman C, Brière J, Petrella T, Canioni D, Fabiani B, Jais JP, Figeac M, Leroy K, Mareschal S, Salles GA, Coiffier B, Delarue R, Peyrade F, Bosly A, André M, Ketterer N, Haioun C, Tilly H. Immunoglobulin heavy chain/light chain pair measurement is associated with survival in diffuse large B-cell lymphoma. Leuk Lymphoma. 2013;54:1898-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Cox MC, Di Napoli A, Scarpino S, Salerno G, Tatarelli C, Talerico C, Lombardi M, Monarca B, Amadori S, Ruco L. Clinicopathologic characterization of diffuse-large-B-cell lymphoma with an associated serum monoclonal IgM component. PLoS One. 2014;9:e93903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Cho YA, Yang WI, Song JW, Min YH, Yoon SO. The prognostic significance of monoclonal immunoglobulin gene rearrangement in conjunction with histologic B-cell aggregates in the bone marrow of patients with diffuse large B-cell lymphoma. Cancer Med. 2016;5:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Alonso-Álvarez S, Alcoceba M, García-Álvarez M, Blanco O, Rodríguez M, Baile M, Caballero JC, Dávila J, Vidriales MB, Esteban C, Arias P, Díaz LG, Tamayo P, Caballero MD, Gutiérrez NC, González M, Martín A. Biological Features and Prognostic Impact of Bone Marrow Infiltration in Patients with Diffuse Large B-Cell Lymphoma. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Castillo JJ, Gustine J, Meid K, Dubeau T, Hunter ZR, Treon SP. Histological transformation to diffuse large B-cell lymphoma in patients with Waldenström macroglobulinemia. Am J Hematol. 2016;91:1032-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Jiménez C, Alonso-Álvarez S, Alcoceba M, Ordóñez GR, García-Álvarez M, Prieto-Conde MI, Chillón MC, Balanzategui A, Corral R, Marín LA, Gutiérrez NC, Puig N, Sarasquete ME, González M, García-Sanz R. From Waldenström's macroglobulinemia to aggressive diffuse large B-cell lymphoma: a whole-exome analysis of abnormalities leading to transformation. Blood Cancer J. 2017;7:e591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, Roulland S, Kasbekar M, Young RM, Shaffer AL, Hodson DJ, Xiao W, Yu X, Yang Y, Zhao H, Xu W, Liu X, Zhou B, Du W, Chan WC, Jaffe ES, Gascoyne RD, Connors JM, Campo E, Lopez-Guillermo A, Rosenwald A, Ott G, Delabie J, Rimsza LM, Tay Kuang Wei K, Zelenetz AD, Leonard JP, Bartlett NL, Tran B, Shetty J, Zhao Y, Soppet DR, Pittaluga S, Wilson WH, Staudt LM. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med. 2018;378:1396-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1316] [Cited by in RCA: 1566] [Article Influence: 223.7] [Reference Citation Analysis (0)] |
| 18. | Uchino K, Sameshima H, Miyamoto T, Iino T, Kato K, Henzan H, Aoki K, Nagafuji K, Gondo H, Harada M. Successful treatment of diffuse large B-cell lymphoma following Waldenström's macroglobulinemia with CHOP chemotherapy followed by combination therapy of CHOP with rituximab. Intern Med. 2004;131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Owen RG, Bynoe AG, Varghese A, de Tute RM, Rawstron AC. Heterogeneity of histological transformation events in Waldenström's macroglobulinemia (WM) and related disorders. Clin Lymphoma Myeloma Leuk. 2011;176-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Shiseki M, Masuda A, Watanabe N, Fujii M, Kimura T, Yoshinaga K, Mori N, Teramura M, Motoji T. Development of diffuse large B-cell lymphoma in a patient with Waldenström's macroglobulinemia/lymphoplasmacytic lymphoma: clonal identity between two B-cell neoplasms. Hematol Rep. 2011;e10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Kikukawa Y, Yamamura-Fujimoto A, Endo S, Miyagawa E, Kawano Y, Ueno S, Mitsuya H, Hata H, Okuno Y. Successful Treatment of Bing-Neel Syndrome Accompanying Waldenström's Macroglobulinemia with R-MPV: A Case Report. J Clin Exp Hematop. 2015;113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Okolo ON, Johnson AC, Yun S, Arnold SJ, Anwer F. Rare transformation to double hit lymphoma in Waldenstrom's macroglobulinemia. Immunotherapy. 2017;709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Kobayashi H, Asada N, Egusa Y, Ikeda T, Sakamoto M, Abe M, Ennishi D, Sakata M, Takaki A, Kawahara S, Meguri Y, Nishimori H, Fujii N, Matsuoka KI, Sato Y, Yoshino T, Maeda Y. Transformation to diffuse large B-cell lymphoma with germinal center B-cell like subtype and discordant light chain expression in a patient with Waldenström macroglobulinemia/lymphoplasmacytic lymphoma. Int J Hematol. 2021;401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Elimimian EB, Bilani N, Diacovo MJ, Sirvaitis S, Fu CL. Histologic Transformation in an Untreated Waldenstrom's Macroglobulinemia After 14 Years: Case Report and Review of the Literature. J Hematol. 2021;25-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |