Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.7989
Peer-review started: January 21, 2022
First decision: March 23, 2022
Revised: April 3, 2022
Accepted: June 13, 2022
Article in press: June 13, 2022
Published online: August 6, 2022
Processing time: 181 Days and 21.1 Hours
Ectopic Cushing syndrome (ECS) is a rare condition commonly associated with neuroendocrine tumors (NET), mainly bronchial carcinoids. The association of paraneoplastic syndrome with Merkle cell carcinoma (MCC) is limited to individual case reports.
In this article we report an unusual and striking presentation of ECS in a patient with known metastatic MCC. An elderly patient presented with new onset severe hypertension, hyperglycemia and hypokalemia, muscle wasting, and peripheral edema. A diagnosis of adrenocorticotropic hormone dependent, non-pituitary, Cushing syndrome was established. Medical therapy inhibiting adrenal function was promptly started but unfortunately the patient survived only a few days after diagnosis.
The occurrence of an aggressive form of ECS in patients with NET should be recognized as an ominous event. To our knowledge, the association of this complication in a patient with MCC had not been reported.
Core Tip: Merkel cell carcinoma (MCC) is an uncommon but highly aggressive skin cancer with neuroendocrine features. Its incidence and mortality are increasing. We describe an elderly patient with a 2-year history of metastatic MCC, with no apparent cutaneous lesion at diagnosis, who presented with uncontrolled hypertension, diabetes mellitus, and hypokalemia. A diagnosis of ectopic Cushing syndrome was established. The occurrence of ectopic Cushing syndrome in patients with neuroendocrine tumor is a major cause of poor prognosis. To our knowledge, this is the first reported case of ectopic Cushing syndrome linked to the rapid progression of a metastatic MCC.
- Citation: Ishay A, Touma E, Vornicova O, Dodiuk-Gad R, Goldman T, Bisharat N. Ectopic Cushing's syndrome in a patient with metastatic Merkel cell carcinoma: A case report. World J Clin Cases 2022; 10(22): 7989-7993
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/7989.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.7989
Merkel cell carcinoma (MCC) is an uncommon but highly aggressive skin cancer with neuroendocrine features[1]. It was first described by Toker in 1972 as "trabecular carcinoma of the skin"[2]. Evidence suggests that its incidence and mortality are increasing across the world[3]. Ectopic Cushing syndrome (ECS) is a rare condition due to ectopic production of adrenocorticotropic hormone by non-pituitary tumors. The adrenocorticotropic hormone producing neoplasms usually originate from neuroendocrine tumors (NET) and can present as benign indolent tumors or aggressive metastatic tumors with a poor prognosis[4]. Although in the past elevated adrenocorticopic hormone levels in plasma and tumoral tissue were demonstrated in patients with MCC[5,6], there are no reports of adrenocorticopic hormone producing MCC that fulfill the diagnostic criteria for ECS. We describe a patient with metastatic MCC who developed an aggressive form of ECS.
An 82-year-old man with a 2-year history of MCC was referred for evaluation and treatment of uncontrolled high blood pressure and new onset hyperglycemia.
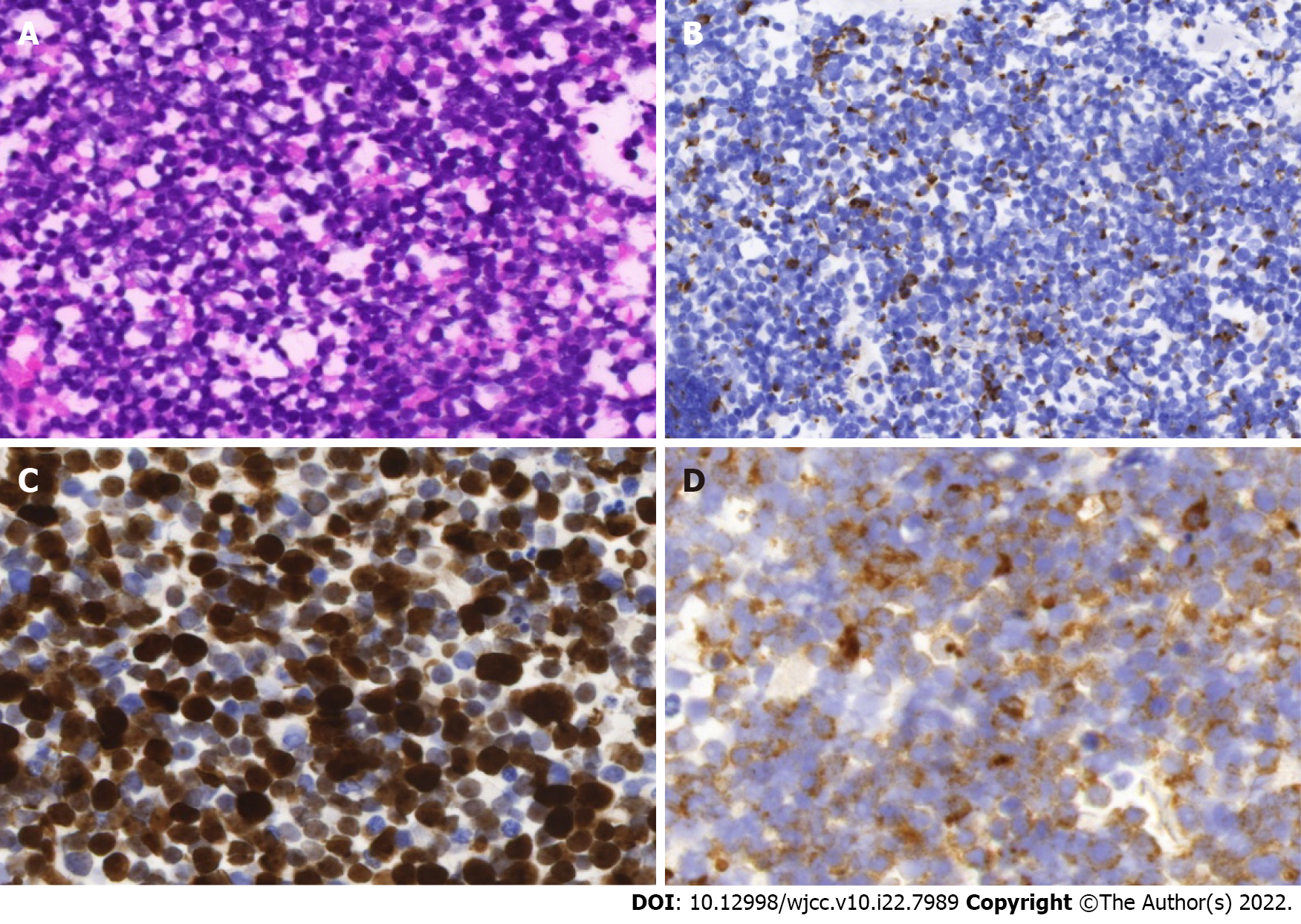
In 2018, a cervical lymphadenopathy biopsy showed metastatic MCC with no apparent primary cutaneous lesion (Figure 1). Multiple bone metastases were demonstrated by an 18F (18-fluorodeoxyglucose) PET/CT scan (18FDG-PET/CT). No pathological uptake was seen in the lungs. The patient achieved good response to avelumab initially as disclosed by a significant reduction of uptake intensity in cervical lymph nodes and in the skeleton on a subsequent 18FDG-PET/CT scan. But thereafter, the disease progressed despite adjuvant radiotherapy and systemic therapy including etoposide, carboplatin, and topotecan. Indeed, a further 18FDG-PET/CT scan showed intensification of the uptake in bones, and new metastases in mediastinal lymph nodes and multiple cutaneous lesions. Noticeably, no disease was present in the lungs.
The patient’s history was significant for multiple surgical treatments for squamous cell carcinoma and basal cell carcinoma of the skin.
Multiple surgical treatments for squamous cell carcinoma and basal cell carcinoma of the skin.
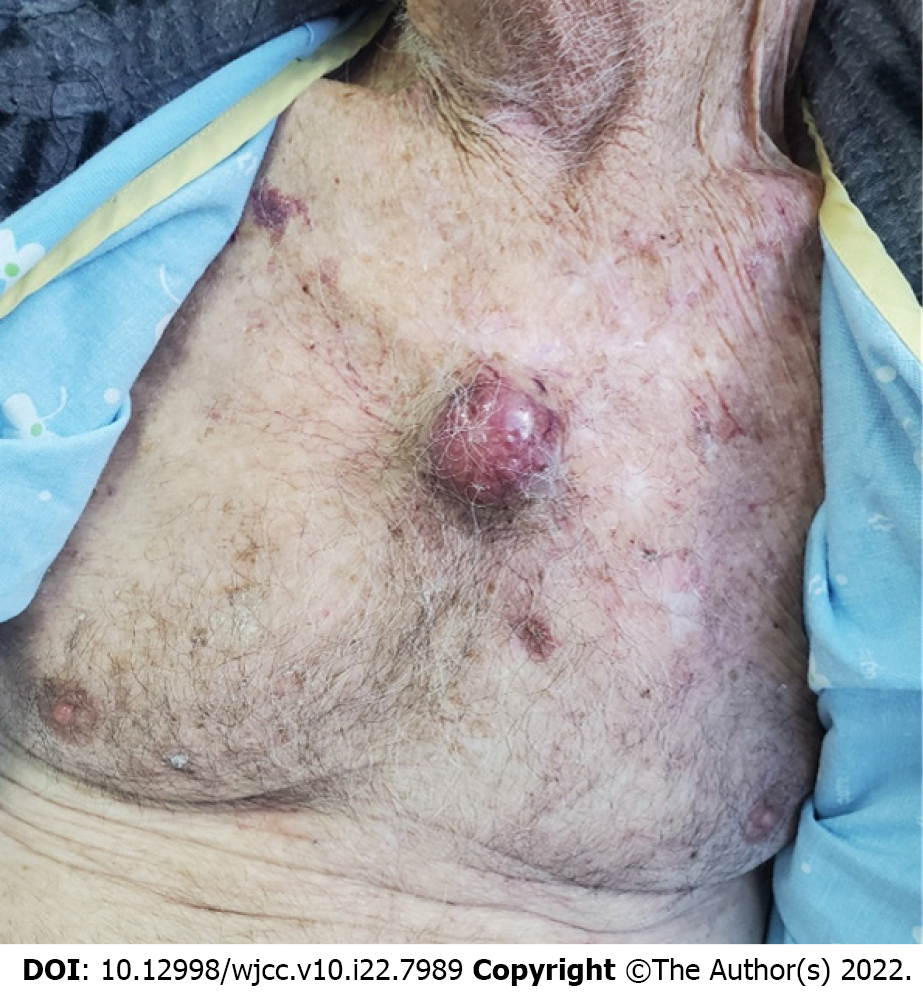
Physical examination revealed high blood pressure (198/100), and a 4 cm-sized purplish-blue tumor in his central chest (Figure 2), bilateral axillary lymphadenopathy, and bilateral lower extremities pitting edema. The clinical phenotype was dominated by weight loss and muscle wasting.
Initial blood work revealed a glucose level of 241 mg/dL with hypokalemia (2.7 nmol/L). The 24-h free urine Cortisol level was 9986 nmol/24 hr (normal values: 57.7-806.8). After high dose (8 mg) overnight dexamethasone suppression test (HDST), the 8-am serum cortisol was 44.9 µg/dL (normal values: 4.3-22.4). Serum adrenocorticopic hormone level was elevated: 106 pg/mL (0.0-46). Testing with intravenous corticotropin releasing hormone (CRH) administration did not affect adrenocorticopic hormone or cortisol levels, which is typical of ECS (Table 1)
| Time (mn) | -15 | 0 | 15 | 30 | 45 | 60 | 90 | 120 |
| Cortisol (µg/d) | 52.22 | 48.92 | 45.48 | 56.11 | 51.44 | 52.1 | 53.08 | 56.65 |
| ACTH (pmol/mL) | 92.9 | 110 | 129 | 128 | 122 | 96.1 | 119 | 120 |
Magnetic resonance imaging scan of the pituitary gland was normal.
Ectopic adrenocorticopic hormone dependent Cushing's syndrome.
The patient was treated with ketoconazole.
The patient’s blood pressure and glucose levels were normalized; however, his general state did not allow additional antineoplastic therapy and he died within few days.
We present an 82-year patient with a metastatic MCC presenting with an overwhelming form of ECS. ECS is a rare condition which accounts for about 10%-20% cases of adrenocorticopic hormone dependent Cushing syndrome. Neuroendocrine tumors (NETs), principally bronchial carcinoids, are the most frequent causes of ECS. Less frequent causes are thymic carcinoids and pancreatic NETs[7]. Small cell lung carcinoma is a known cause of ECS, but in our patient imaging studies did not reveal any lung lesions. Recently, a case of metastatic NET of unknown origin presenting with ECS was reported[8]. Several manifestations of MCC-associated paraneoplastic syndromes have been reported[9], but ECS associated with MCC has not be described, even though a case of metastatic MCC within a cortisol-producing adrenal adenoma has been recently reported[10]. The time elapsed between the first symptoms of hypercortisolism and the diagnosis of ECS may predict the prognosis of the underlying malignancy. The shorter it is, the poorer is the prognosis. In addition to the grade of NET, the severity of cortisol excess is an independent negative prognostic factor[7]. The molecular mechanisms underlying ECS-associated malignant tumors include aberrant processing of the proopiomelanocortin (POMC) gene leading to release in the circulation of high molecular weight adrenocorticopic hormone precursors like POMC and pro- adrenocorticopic hormone. It is speculated that the ability of the tumor to express aberrant molecules is related to the progression of the disease[11]. If ectopic adrenocorticopic hormone producing malignancy is diagnosed early as a localized disease, surgical removal of the primary tumor is the treatment of choice, but it is rarely achievable in patients with aggressive neoplasms. In this ECS group, a prompt control of hypercortisolism should be attempted by medical treatment or alternatively by adrenalectomy[4].
MCC and neuroendocrine ECS are both rare conditions. The occurrence of ECS in patients with metastatic NETs is a major cause of poor prognosis. The suspicion of Cushing syndrome should receive adequate attention and prompt evaluation to confirm the diagnosis and initiate rapidly the treatment to attain a more favorable prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Israel
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Saglam S, Turkey; Tan HY, China A-Editor: Zhu JQ, China S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Becker JC, Stang A, DeCaprio JA, Cerroni L, Lebbé C, Veness M, Nghiem P. Merkel cell carcinoma. Nat Rev Dis Primers. 2017;3:17077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 393] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 2. | Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105:107-110. [PubMed] |
| 3. | Emge DA, Cardones AR. Updates on Merkel Cell Carcinoma. Dermatol Clin. 2019;37:489-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Araujo Castro M, Marazuela Azpiroz M. Two types of ectopic Cushing syndrome or a continuum? Pituitary. 2018;21:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Iwasaki H, Mitsui T, Kikuchi M, Imai T, Fukushima K. Neuroendocrine carcinoma (trabecular carcinoma) of the skin with ectopic ACTH production. Cancer. 1981;48:753-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Anzai S, Sato T, Takayasu S, Asada Y, Terashi H, Takasaki S. Postoperative hyponatremia in a patient with ACTH-producing Merkel cell carcinoma. J Dermatol. 2000;27:397-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Davi' MV, Cosaro E, Piacentini S, Reimondo G, Albiger N, Arnaldi G, Faggiano A, Mantovani G, Fazio N, Piovesan A, Arvat E, Grimaldi F, Canu L, Mannelli M, Ambrogio AG, Pecori Giraldi F, Martini C, Lania A, Albertelli M, Ferone D, Zatelli MC, Campana D, Colao A, Scaroni C, Terzolo M, De Marinis L, Cingarlini S, Micciolo R, Francia G. Prognostic factors in ectopic Cushing's syndrome due to neuroendocrine tumors: a multicenter study. Eur J Endocrinol. 2017;176:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Bostan H, Duger H, Akhanli P, Calapkulu M, Turkmenoglu TT, Erdol AK, Duru SA, Sencar ME, Kizilgul M, Ucan B, Ozbek M, Cakal E. Cushing's syndrome due to adrenocorticotropic hormone-secreting metastatic neuroendocrine tumor of unknown primary origin: a case report and literature review. Hormones (Athens). 2022;21:147-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Iyer JG, Parvathaneni K, Bhatia S, Tarabadkar ES, Blom A, Doumani R, McKenzie J, Asgari MM, Nghiem P. Paraneoplastic syndromes (PNS) associated with Merkel cell carcinoma (MCC): A case series of 8 patients highlighting different clinical manifestations. J Am Acad Dermatol. 2016;75:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Maier A, Kerut SE, Subauste JS, Curry S. “SUN-181 A Case of Metastatic Merkel Cell Carcinoma Within a Cortisol-Producing Adrenal Adenoma. J Endoc Soc. 2020;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Terzolo M, Reimondo G, Alì A, Bovio S, Daffara F, Paccotti P, Angeli A. Ectopic ACTH syndrome: molecular bases and clinical heterogeneity. Ann Oncol. 2001;12 Suppl 2:S83-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |