Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.7982
Peer-review started: January 12, 2022
First decision: May 11, 2022
Revised: May 19, 2022
Accepted: July 5, 2022
Article in press: July 5, 2022
Published online: August 6, 2022
Processing time: 191 Days and 2.5 Hours
Nonketotic hyperglycinemia (NKH) is a rare autosomal recessive genetic disorder of abnormal glycine metabolism caused by insufficient activity of the glycine cleavage enzyme system. Glycine is believed to function mainly as an inhibitory neurotransmitter, but it can also act as a co-agonist of the N-methyl-D-aspartate (NMDA) receptor. The accumulation of a large amount of glycine in the brain leads to neuronal and axonal injury via overactivation of NMDA receptors located in the hippocampus, cerebral cortex, olfactory bulb, and cerebellum and to stimulation of the inhibitory function of glycine receptors located in the spinal cord and brain stem, resulting in central apnea, hiccups, and hypotonia in the early stage of the disease.
The child described in this report had typical clinical manifestations of NKH, such as hiccups, disturbance of consciousness, hypotonia, and convulsions, within the first week after birth. Whole-exome genetic testing revealed that the child had a compound heterozygous mutation, namely, c.395C>A (p.S132X) and c.2182G>A (p.G728R), in the GLDC gene, and he was diagnosed with NKH. For treatment, we administered an oral levetiracetam solution and added topiramate and prednisone for epilepsy control, but the epilepsy remained uncontrollable. Ketogenic diet therapy was started at 6 mo of age, his seizures were significantly reduced, and there were no obvious adverse reactions during ketogenic treat
This case shows that plasma glycine levels cannot be used to evaluate the prognosis of NKH, that the development of the corpus callosum can be affected by NKH, and that a ketogenic diet may be effective for seizure control in NKH patients.
Core Tip: Herein, we present the case of a child who had typical clinical manifestations of nonketotic hyperglycinemia (NKH), such as hiccups, disturbance of consciousness, hypotonia, and convulsions within the first week after birth. These symptoms combined with the results of gene testing led to a diagnosis of classical nonketotic hyperglycinemia caused by compound heterozygous variants in the GLDC gene. Plasma glycine levels cannot be used to evaluate the prognosis of NKH, and the corpus callosum can be affected by NKH. A ketogenic diet may be effective for seizure control in NKH patients.
- Citation: Ning JJ, Li F, Li SQ. Clinical and genetic analysis of nonketotic hyperglycinemia: A case report. World J Clin Cases 2022; 10(22): 7982-7988
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/7982.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.7982
Nonketotic hyperglycinemia (NKH), also known as glycine encephalopathy, is an autosomal recessive genetic disease with abnormal glycine metabolism caused by insufficient activity of the glycine cleavage enzyme system (GCS), and NKH is clinically characterized by the abnormal accumulation of glycine in all tissues of the human body, especially in the serum and cerebrospinal fluid[1]. According to an epidemiological survey of 55000 newborns, the incidence of NKH is approximately 1/63000[2]. The GCS is composed of glycine decarboxylase (P protein), aminomethyl transferase (T protein), hydrogen carrier protein (H protein), and dihydroamide dehydrogenase (L protein). The P, T, and H proteins are encoded by the GLDC (OMIM 238300), AMT (OMIM 238310), and GCSH (OMIM 238330) genes, respectively. Variations in these genes can cause a decrease in GCS activity and lead to glycine accumulation, and 70%-75% of NKH patients carry GLDC variations[3]. Here, we present the case of a young boy who presented with clinical features of NKH and was ultimately diagnosed by whole-exome genetic testing.
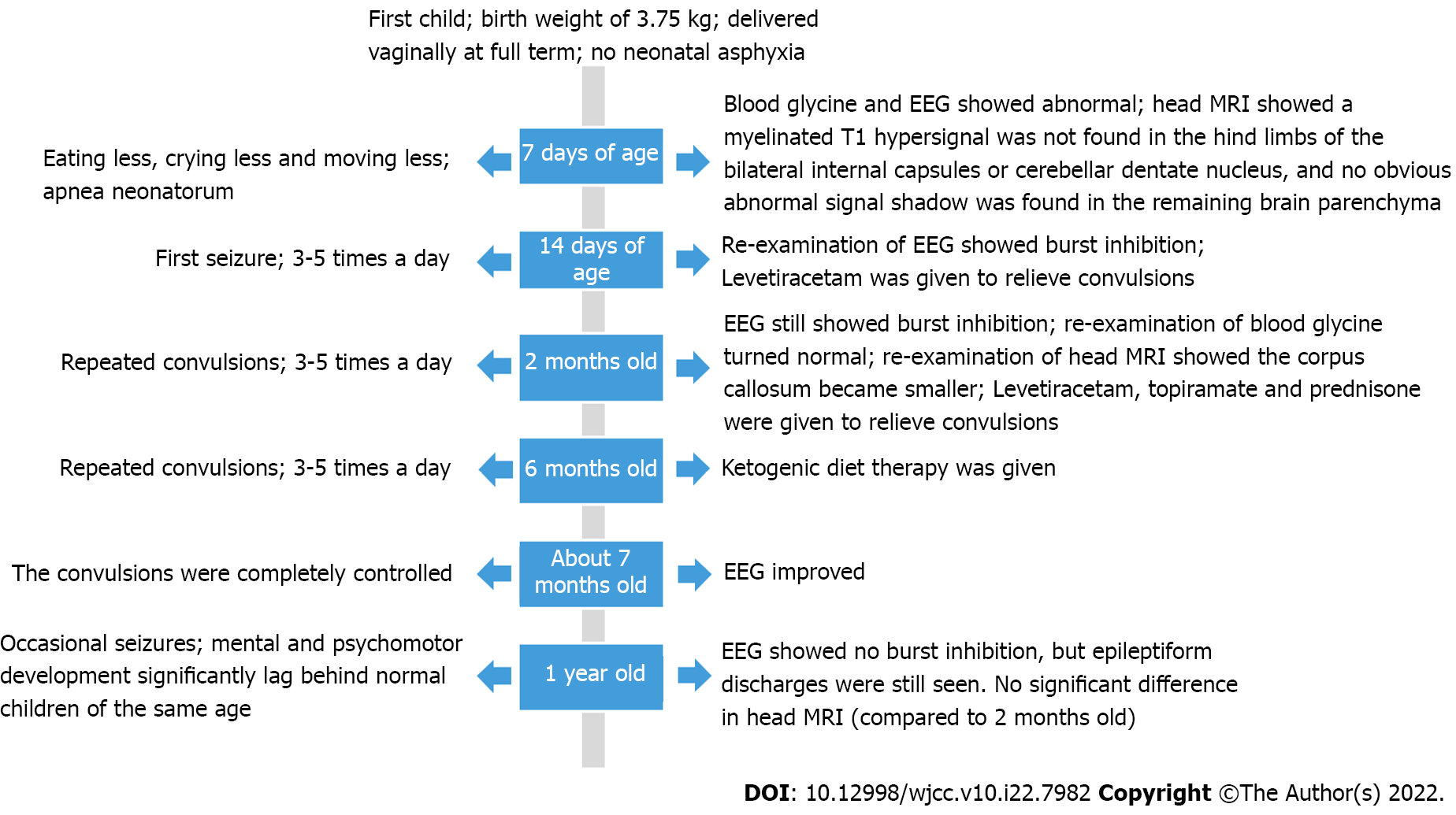
A 7-day-old male child was admitted to the neonatology department of our hospital on December 30, 2020 due to "eating less, crying less, and moving less for 7 d".
The patient was the firstborn child and was delivered vaginally at full term, with a birth weight of 3.75 kg. The Apgar scores at 1, 5, and 10 min after birth were all 10 points. He was provided a reasonable amount of food after birth but had low sucking power, hiccups, and occasional apnea. The mother denied a history of exposure to poisons, chemicals, or radiation and had regular prenatal examinations during pregnancy; no abnormality was found. The parents did not have blood relations.
No history of past illness.
There was no history of family hereditary diseases.
Upon admission examination, the following was observed: Body temperature, 36.8 ℃; heart rate, 128 beats/min; respiratory rate, 34 times/min; arterial blood pressure, 83/46 mmHg; SpO2, 95%; slightly dry skin; poor elasticity; no rash or ecchymosis on the skin; irregular breathing; no obvious dyspnea; trachea in the middle; no abnormal breath sounds heard in both lungs. Examination of the heart and abdomen did not reveal any abnormalities. Neurological examination showed the following: No response after stimulation; the anterior fontanelle measuring 1.0 cm × 1.0 cm that was flat and soft; hypotonia; and an inability to elicit primitive reflexes. A few hours after admission, the child was observed to have frequent apnea neonatorum.
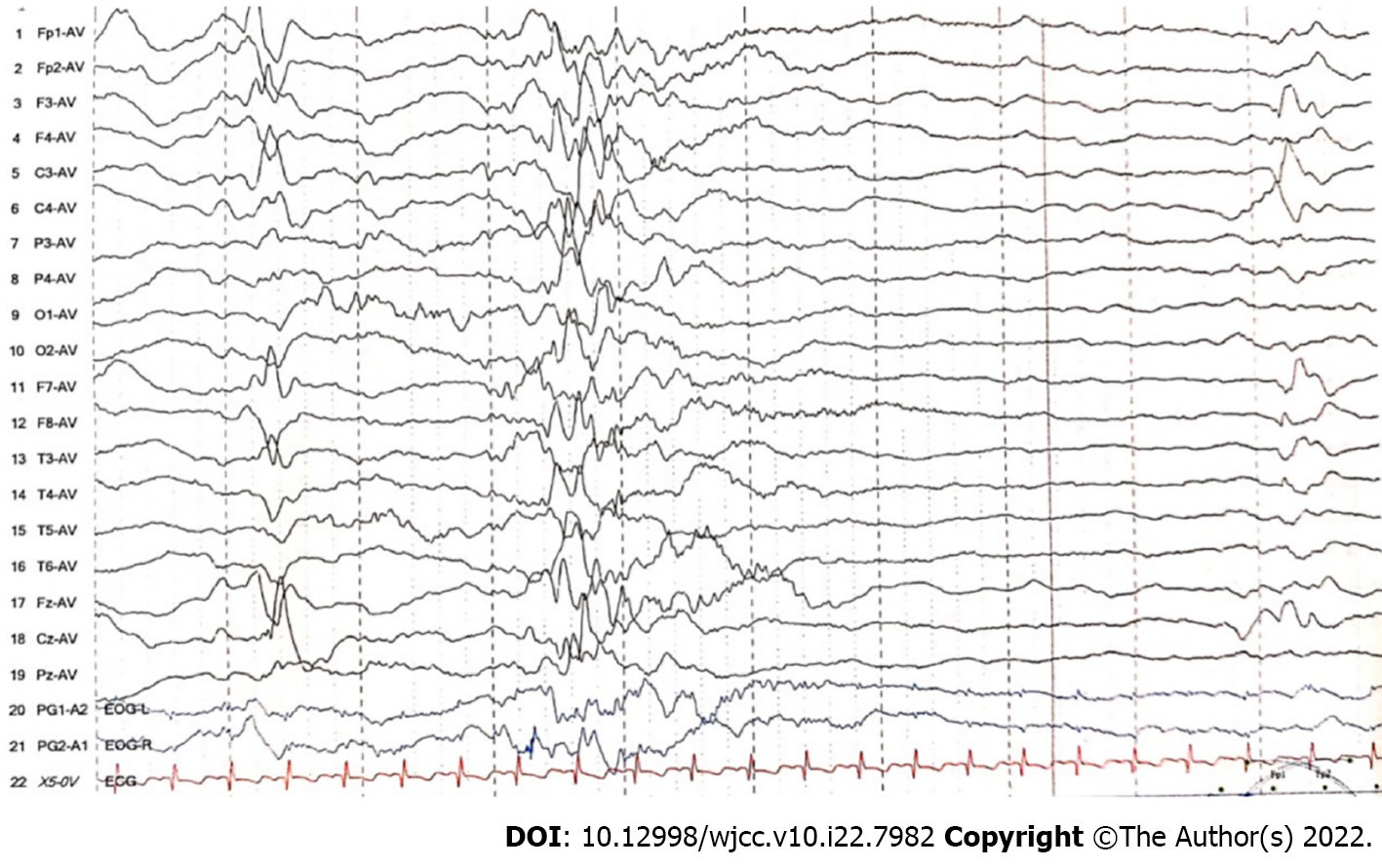
Arterial blood gas analysis showed the following: pH, 7.16 (reference range: 7.35–7.45); PCO2, 96 mmHg (reference range: 35–45 mmHg); PO2, 276 mmHg (reference range: 80–100 mmHg); HCO3-, 34.2 mmol/L (reference range: 21.4–27.3 mmol/L); extracellular fluid base excess, 5.5 mmol/L (reference range: -3–3 mmol/L); lactic acid, 0.9 mmol/L (reference range: 0.5–2.2 mmol/L); and blood ammonia, 100 μmol/L (reference range: 18–72 μmol/L). An electroencephalogram (EEG) showed that diffuse low-amplitude irregular 1–6 Hz δ and θ waves and low-amplitude β waves were mixed in the quiet state, and the external stimulation background did not change. The EEG activity voltage was low, which represented a moderately abnormal neonatal EEG. Serum tandem mass spectrometry showed that the glycine concentration was 850.05 μmol/L (reference range: 130–650 μmol/L), and urine organic acid analysis showed no obvious abnormality. CSF glycine levels were not measured. Routine blood test, routine blood coagulation test, myocardial enzyme, C-reactive protein, procalcitonin, liver and kidney function tests, electrolyte assessment, and cerebrospinal fluid and biochemistry tests did not show obvious abnormalities.
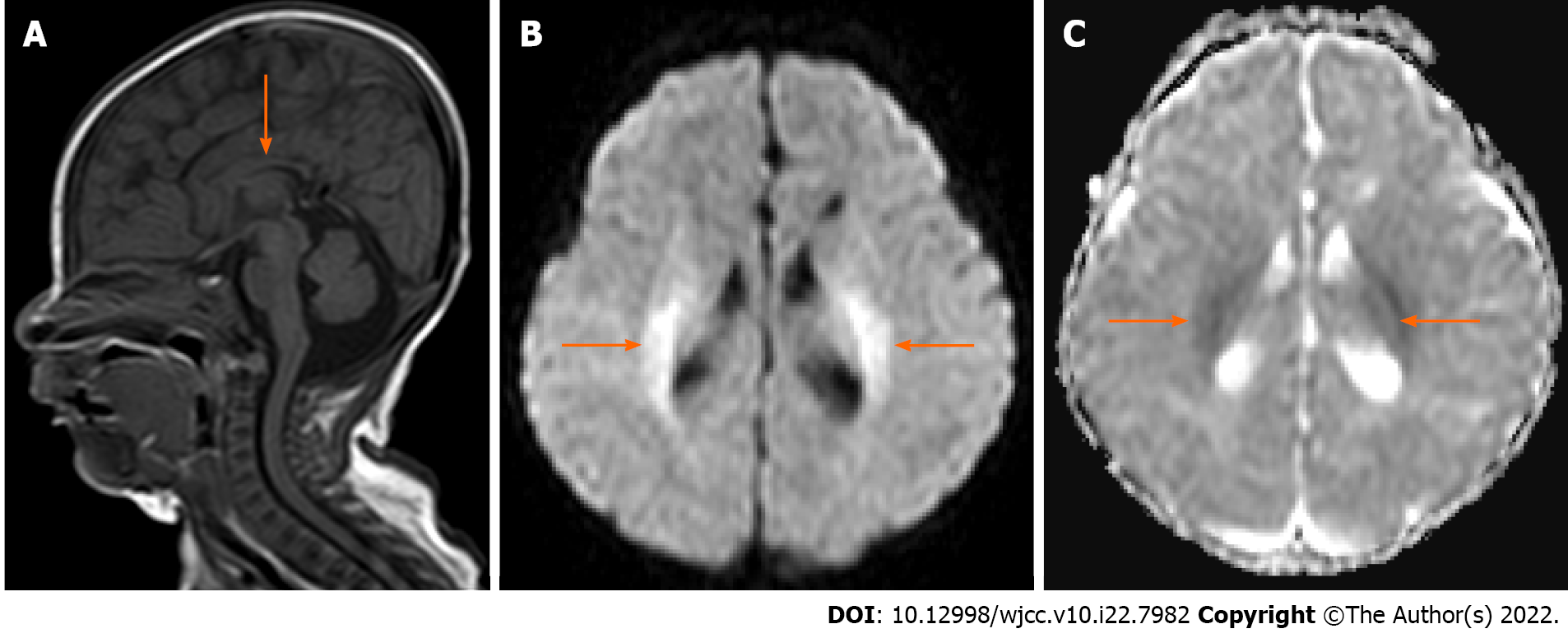
Head magnetic resonance imaging (MRI) in the neonatal period (aged 7 d old) showed that a myelinated T1 hypersignal was not found in the hind limbs of the bilateral internal capsules or cerebellar dentate nucleus, and no abnormal corpus callosum was found. When the child was 2 mo old, re-examination of head MRI showed that the corpus callosum was smaller than it was on earlier imaging; the bilateral ventricles were full and irregular (more pronounced on the left side); the corticospinal tract, the white matter of bilateral ventricles, and the parietal lobe showed symmetrical high signal intensity on diffusion-weighted imaging; and the apparent diffusion coefficient map showed slightly low signal intensity (Figure 1).
The proband has a variant on exon 8, position chr9:6620259G>T, NM_000170.3:c.395C>A, p.(Ser132*) and a variant on exon 18, position chr9:6556173C>T, NM_000170.3:c.2182G>A, p.(Gly728Arg). The p.(Ser132*) variant has been described in one individual in the gnomAD database v3.1.1 (entry: 9-6620259-G-T). Its allele frequency is 0.000006573. It is reported in dbSNP (rs386833576). According to the American College of Medical Genetics and Genomics (ACMG) guidelines, this variation was judged to be a pathogenic variation based on the supporting evidence (PVS1 + PM2 + PM3). In the pedigree analysis, the father of the proband has no mutation at this site, while the mother of the proband has a heterozygous mutation at this site. The variant p.(Gly728Arg) has been reported on ClinVar as likely pathogenic (accession number VCV000580932.2), and it has been described in dbSNP (rs386833542). Its allele frequency in gnomAD database v2.1.1 is 0.000003977. According to the ACMG guidelines, this variation was judged to be a pathogenic variation based on the supporting evidence (PS1 + PM1 + PM2 + PM5 + PP3). By pedigree analysis, the father of the proband has heterozygous variation at this site, while the mother has no variation at this site. The parents of the child are heterozygous, with a normal phenotype, which is consistent with the pathogenesis of autosomal recessive compound heterozygous genetic diseases.
The final diagnosis was classical NKH and epilepsy syndrome.
After admission, invasive ventilation, aggressive anti-infective therapy, and symptomatic treatments were given. After 1 wk, the child could obviously breathe spontaneously, the arterial blood gas analysis was basically normal after re-examination, and the ventilator was successfully withdrawn. However, during hospitalization, the child developed convulsions, characterized by loss of consciousness, staring in both eyes, clenching of fists with both hands, and chewing movements of the lips. After demon
When the child was 2 months old, he had good suckling and swallowing but still had repeated convulsions, with hypotonia in the extremities. Re-examination by EEG showed a burst-inhibition state. The blood glycine level became normal. For treatment, we continued to administer an oral levetiracetam solution (60 mg/kg/d) and added topiramate (5 mg/kg/d) and prednisone (2 mg/kg/d) for epilepsy control. Unfortunately, the epilepsy of the child remained uncontrollable. Ketogenic diet therapy with a 4:1 (lipid:nonlipid) ketogenic milk formula was started at 6 mo of age, e daily calorie and protein requirements were ensured, and the child's urinary ketones were monitored daily. The number of attacks and adverse reactions were recorded during ketogenic diet treatment. On the 25th day after ketogenic treatment, the child's seizures were completely controlled, and the EEG improved after review. No serious adverse reactions occurred during the ketogenic treatment (Figure 3).
NKH is a rare inherited genetic metabolic disease with variable clinical manifestations. Three types of glycine encephalopathy have been identified according to the clinical phenotype and the presence or absence of genetic variation: Classic, atypical, and transient. Most neonatal NKH cases are classified into the classic type, showing a normal phenotype at birth. In most cases, drowsiness, coma, hiccups, hypotension, and myoclonic seizures gradually appear in the first week after birth and develop into central apnea requiring ventilator-assisted breathing, with a mortality rate as high as 50% at this time[4]. These symptoms subside on their own after 1 to 3 wk, but surviving infants experience serious nervous system sequelae within 6 mo, such as epileptic encephalopathy, developmental delay, and growth retardation[5,6]. Atypical NKH is rare and has heterogeneous and nonspecific disease courses, which make the diagnosis more difficult. If hypotonia, developmental delay, and epilepsy occur in infancy and the symptoms are milder than those of classic NKH, it is necessary to pay attention to this type of possibility. Transient NKH is even rarer; although it develops after birth, as the activity of glycine lyase increases, it may heal itself within a few months.
In NKH, serum and cerebrospinal fluid glycine levels are elevated, and the ratio of cerebrospinal fluid to plasma glycine is greater than 0.08. Absence of ketoacidosis and urine organic acid abnor
The child described in this report had typical clinical manifestations of NKH, such as hiccups, disturbance of consciousness, hypotonia, and convulsions, within the first week after birth. Although cerebrospinal fluid glycine was not measured, no organic acid abnormality was found by blood and urine tandem mass spectrometry and gas chromatography. Combined with the gene detection results for the child, a diagnosis of classic NKH caused by compound heterozygous variations in the GLDC gene was made. To date, only four NKH patients with compound heterozygote variations in the GLDC gene have been reported in China[7-9]. In this study, a nonsense mutation (c.395C>A) was found in the GLDC gene, which led to the replacement of serine at position 132 of the coding region by a termination codon (p.S132X), which may lead to the loss of gene function. This is the first time that this mutation has been found in the Chinese population. Compound heterozygosity with another pathogenic mutation may be the basis for the pathogenesis of NKH in this child, which enriches the variation spectrum of the GLDC gene.
A retrospective cross-sectional study showed that a small corpus callosum is the most common structural abnormality of NKH and that this structural abnormality is directly related to the severity of the clinical phenotype[10]. In this case, no abnormal corpus callosum was found on neonatal MRI, but with the development of the disease, the corpus callosum became dysplastic, suggesting that the corpus callosum could be affected by glycine metabolism. Other studies have found that EEG can evaluate the therapeutic effect at each stage and provide a clinical basis for adjusting the administration scheme and its dosage. Plasma glycine levels cannot be used to evaluate the prognosis of NKH, as this study found that a high serum glycine level can decrease or even disappear by itself, but the EEG will still show a burst-inhibition state, which further indicates that NKH is an irreversible brain injury. At present, there is no effective treatment for this rare disease, and the focus of treatment is to rationally use antiepileptic drugs to control epileptic seizures, reduce the plasma concentration of glycine by injecting sodium benzoate, and antagonize N-methyl-D-aspartate receptors by injecting ketamine or oral dextromethorphan. The ketogenic diet is a high-fat, low-carbohydrate, and moderate protein diet that is used mainly for the adjuvant treatment of drug-resistant epilepsy and epileptic encephalopathy[11]. It has been reported that a ketogenic diet has a good effect on infantile spasm, Dravet syndrome, Lennox–Gastaut syndrome, and epileptic encephalopathy caused by gene mutations such as SCN1A, KCNQ2, STXBP1, and SCN2A[12]. This case showed that a ketogenic diet may be a valuable treatment modality for refractory seizure control in classical NKH.
This study found that a high serum glycine level can decrease or even disappear on its own, indicating that plasma glycine levels cannot be used to evaluate the prognosis of NKH. With the development of the disease, the corpus callosum can be affected by glycine metabolism. A ketogenic diet may be effective for seizure control in classical NKH patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: El-Karaksy H, Egypt; Portius D, Germany S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Poothrikovil RP, Al Thihli K, Al Futaisi A, Al Murshidi F. Nonketotic Hyperglycinemia: Two Case Reports and Review. Neurodiagn J. 2019;59:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Applegarth DA, Toone JR, Lowry RB. Incidence of inborn errors of metabolism in British Columbia, 1969-1996. Pediatrics. 2000;105:e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 273] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 3. | Brunel-Guitton C, Casey B, Coulter-Mackie M, Vallance H, Hewes D, Stockler-Ipsiroglu S, Mercimek-Mahmutoglu S. Late-onset nonketotic hyperglycinemia caused by a novel homozygous missense mutation in the GLDC gene. Mol Genet Metab. 2011;103:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Bin Arif T, Ahmed J, Malik F, Nasir S, Khan TM. Neonatal Nonketotic Hyperglycinemia: A Rare Case from Pakistan. Cureus. 2020;12:e7235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Hoover-Fong JE, Shah S, Van Hove JL, Applegarth D, Toone J, Hamosh A. Natural history of nonketotic hyperglycinemia in 65 patients. Neurology. 2004;63:1847-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Swanson MA, Coughlin CR Jr, Scharer GH, Szerlong HJ, Bjoraker KJ, Spector EB, Creadon-Swindell G, Mahieu V, Matthijs G, Hennermann JB, Applegarth DA, Toone JR, Tong S, Williams K, Van Hove JL. Biochemical and molecular predictors for prognosis in nonketotic hyperglycinemia. Ann Neurol. 2015;78:606-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Gao ZJ, Jiang Q, Chen Q, Xu KM. [Clinical and molecular genetic study of nonketotic hyperglycinemia in a Chinese family]. Zhongguo Dang Dai Er Ke Za Zhi. 2017;19:268-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Liu S, Wang Z, Liang J, Chen N, OuYang H, Zeng W, Chen L, Xie X, Jiang J. Two novel mutations in the glycine decarboxylase gene in a boy with classic nonketotic hyperglycinemia: case report. Arch Argent Pediatr. 2017;115:e225-e229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Lin Y, Zheng Z, Sun W, Fu Q. A novel compound heterozygous variant identified in GLDC gene in a Chinese family with non-ketotic hyperglycinemia. BMC Med Genet. 2018;19:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Stence NV, Fenton LZ, Levek C, Tong S, Coughlin CR 2nd, Hennermann JB, Wortmann SB, Van Hove JLK. Brain imaging in classic nonketotic hyperglycinemia: Quantitative analysis and relation to phenotype. J Inherit Metab Dis. 2019;42:438-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Luat AF, Coyle L, Kamat D. The Ketogenic Diet: A Practical Guide for Pediatricians. Pediatr Ann. 2016;45:e446-e450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 12. | Ko A, Jung DE, Kim SH, Kang HC, Lee JS, Lee ST, Choi JR, Kim HD. The Efficacy of Ketogenic Diet for Specific Genetic Mutation in Developmental and Epileptic Encephalopathy. Front Neurol. 2018;9:530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |