Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.7936
Peer-review started: November 18, 2021
First decision: February 7, 2022
Revised: February 21, 2022
Accepted: June 26, 2022
Article in press: June 26, 2022
Published online: August 6, 2022
Processing time: 245 Days and 23.2 Hours
Gastric mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN), which consists of neuroendocrine and non-neuroendocrine components, is quite rare. Until now, most data on gastric MiNEN come from clinical cases, without large-scale retrospective studies or controlled clinical trials. Consequently, no consensus regarding the origin, molecular characteristics, or appropriate treatment of MiNEN has been reached so far. We conducted chemotherapy of irinotecan plus cisplatin (IP regimen) and surgery in two patients with gastric MiNEN, which had never been used in treating this kind of tumor, leading to their long-term survival for more than 3 and 7 years, respectively.
We present two patients (one male and one female) with gastric MiNEN, with the primary manifestation of recurrent upper abdominal pain. After they were referred to our hospital, a diagnosis of gastric MiNEN was defined with the help of CT scan, and histopathological and immunohistochemical examinations on the samples of gastrointestinal endoscopy or radical surgery. The male patient (case 1) were found to have metastases in the reginal lymph nodes and the left liver. He received four cycles of IP regimens first, then the gastrectomy and partial left liver resection, followed by additional two cycles of IP chemotherapy. The female patient (case 2) underwent a laparoscopic gastrectomy, and received six cycles of IP regimen. She was found to have metastatic lesions in the right lung 2 years after that, and underwent video-assisted thoracoscopic surgery (VATS) of the lower lobe of the right lung. The two patients have now survived for more than 3 years and 7 years, respectively, without any evidence of recurrence or metastases.
IP regimen, combined with curative-intent surgery if feasible, could be considered as the priority in the choice of front-line chemotherapy for gastric MiNEN.
Core Tip: Mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN) is a rare, highly aggressive tumor with a poor prognosis (median overall survival less than 12 mo), and no consensus regarding the appropriate treatment has been reached so far. We conducted chemotherapy of irinotecan plus cisplatin regimen and surgery in two patients with gastric MiNEN, which had not been used to treat this kind of tumor before, leading to their long-term survival for more than 3 and 7 years, respectively. Our reports may provide a reference for other clinicians.
- Citation: Woo LT, Ding YF, Mao CY, Qian J, Zhang XM, Xu N. Long-term survival of gastric mixed neuroendocrine-non-neuroendocrine neoplasm: Two case reports. World J Clin Cases 2022; 10(22): 7936-7943
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/7936.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.7936
Gastric mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN), which consists of neuroendocrine and non-neuroendocrine components, is quite rare, accounting for about 7% of all gastric neuroendocrine neoplasms and 25% of all gastric poor differentiated neuroendocrine carcinomas, but their prevalence has not been explored specifically so far[1]. Gastrointestinal tumor with an exocrine and a neuroendocrine component was first described by Cordier in 1924[2]. Many different names had been used since then, causing confusion among clinicians, surgeons, and pathologists, such as composite carcinoid, mucin-producing carcinoid argentaffin cell adenocarcinoma, mixed exocrine-endocrine tumors, mixed adenoneuroendocrine carcinomas, and so on[3]. In the 2019 WHO Classification of Tumors of the Digestive System, the term MiNEN has been used when referring to this kind of tumor[4]. Until now, most data on gastric MiNEN come from clinical cases[5-8], without large-scale retrospective studies or controlled clinical trials. Consequently, no consensus regarding the origin, molecular characteristics, or appropriate treatment of MiNEN has been reached so far.
Due to the lack of knowledge of gastric MiNEN, this tumor has a quite poor prognosis, presenting with a short median survival of less than 12 mo[5,9]. The preferred treatment for high-grade MiNENs is currently suggested to be combining etoposide and a platinum salt (EP regimen) or the combinations of 5-fluorouracil and irinotecan or temozolomide or amrubicin[1]. However, we conducted chemotherapy of irinotecan plus cisplatin (IP regimen) and surgery in two patients with gastric MiNEN, leading to their long-term survival for more than 3 and 7 years, respectively. Here, we present the process of diagnosis and treatment and a brief review of the literature to improve our understanding of the tumor.
Case 1: A 63-year-old man was admitted to the hospital because of frequent upper abdominal pain for over 1 mo.
Case 2: A 54-year-old female patient was admitted to the hospital with recurrent epigastric pain for more than 7 years.
Case 1: The patient felt frequent upper abdominal pain for over 1 mo, so he underwent upper gastrointestinal endoscopy and magnetic resonance imaging at a local hospital. Then he was diagnosed as having gastric MiNEN with metastases in the regional lymph nodes and the left liver. He came to our hospital soon after, and was admitted because of “gastric cancer”.
Case 2: The patient had recurrent epigastric pain for 7 years, and the pain got worse on an empty stomach. She took omeprazole herself without obvious relief. Then she underwent upper gastro
Case 1: This patient had a history of hypertension for more than 10 years and herniorrhaphy surgery 5 years ago.
Case 2: The patient was diagnosed with chronic nasosinusitis, thyroiditis, cholecystolithiasis, hepatic cyst, and hepatic haemangioma.
Case 1: The patient's father was dead, and his mother was healthy.
Case 2: The patient's father was dead; her mother and little brother were alive.
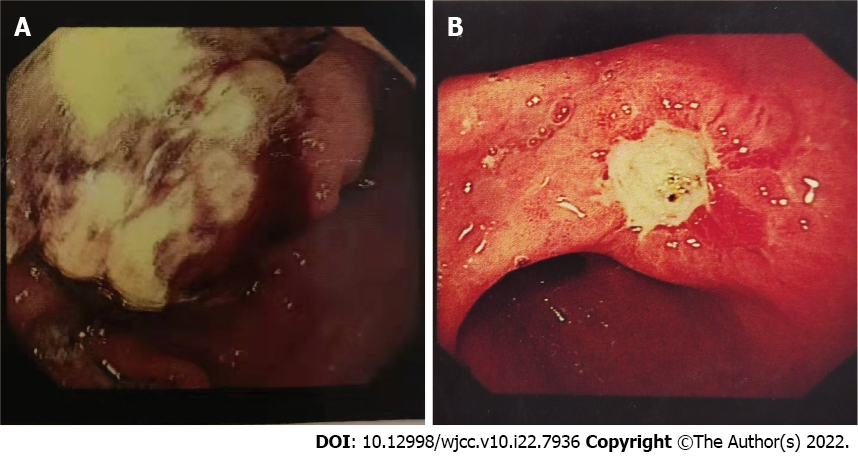
Case 1: The physical examination revealed the following: Temperature: 36.5 °C; pulse: 86/min; respiration rate: 14/min; blood pressure: 122/82mmHg. In the upper gastrointestinal endoscopy, no enlarged superficial lymph nodes, no abdominal wall varicosis, and no gastrointestinal peristalsis (Figure 1A).
Case 2: The physical examination revealed the following: Temperature: 37.1 °C; pulse: 80/min; respiration rate: 16/min; blood pressure: 118/76mmHg. Upper gastrointestinal endoscopy confirmed the gastric cancer (Figure 1B).
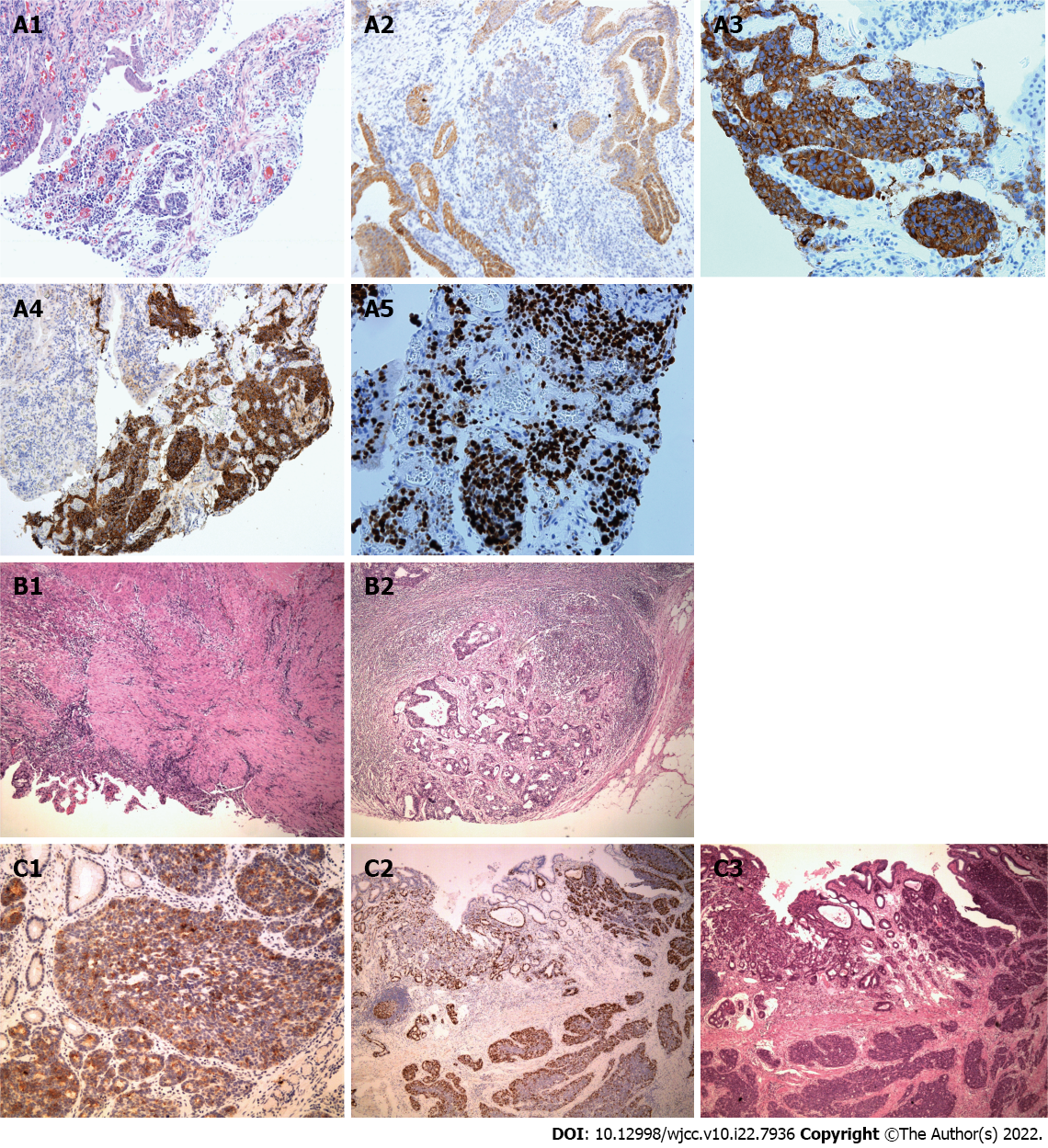
Case 1: Laboratory examinations revealed the following: Red blood cell count (RBC) 4.2 × 1012/L; hemoglobin (Hb) 110 g/L; white blood cell count (WBC) 6.8 × 109/L; platelet count (PLT) 126 × 109/L. The pathological examination and immunohistochemistry (IHC) confirmed the gastric MiNEN and the tumor was composed of two different components. The adenocarcinoma component was positive for cytokeratin 18 (CK18), and the neuroendocrine carcinoma component was positive for chromogranin A (CgA) and synaptophysin (Syn) (Ki67 index 80%) (Figure 2A1-A5). A high mitotic activity was seen (> 20 mitoses/10 high power fields [HPFs]).
Case 2: Laboratory examinations revealed the following: RBC 3.8 × 1012/L; Hb 102g/L; WBC 8.4 × 109/L; PLT 208 × 109/L. The histopathological examination revealed tumor infiltration into the subserosal layer, with 11 regional lymph node metastases (pT4aN3aM0 stage). The tumor was composed of two different components, of which the adenocarcinoma component (positive for CKpan and CK18) accounted for 20% and neuroendocrine carcinoma component (positive for CKpan, CK18, CgA, and Syn; Ki67 index 60%) accounted for 80% (Figure 2C1-3). The mitotic activity was high (about 40 mitoses/10 HPFs).
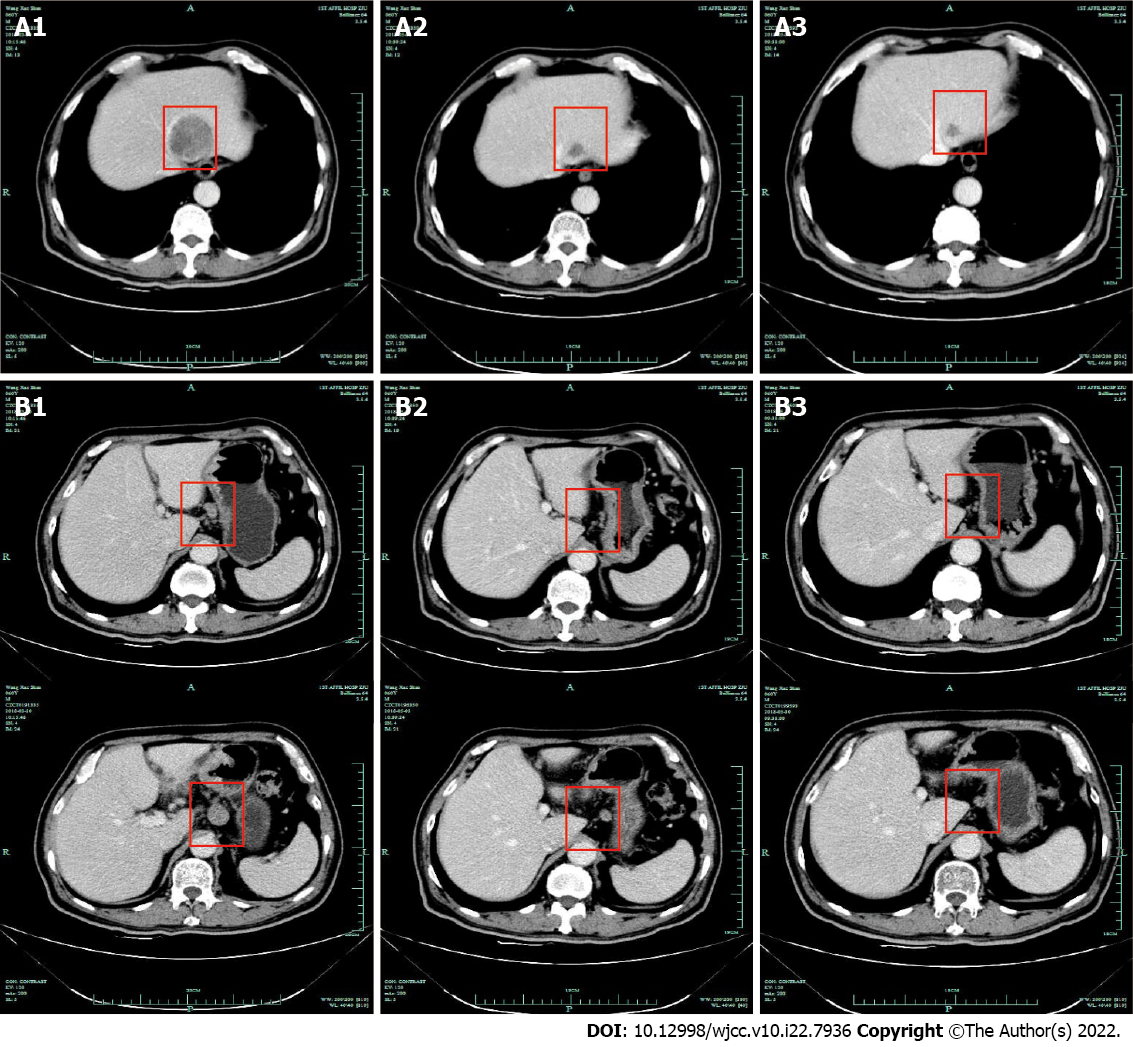
Case 1: CT revealed the tumor infiltration into the omentum majus, with metastases to regional lymph nodes and the left liver (stage IV). Subsequently, the patient received four cycles of IP regimen as first-line chemotherapy. CT after the second and third cycles of chemotherapy revealed that the lesion in the left liver and regional nodes decreased markedly (Figure 3). Then, gastrectomy and partial left liver resection were performed and the histopathological examination confirmed that the neuroendocrine component of those lesions basically disappeared, only with adenocarcinoma component remaining in one regional lymph node (Figure 2B1 and B2). Metastases in the left liver totally disappeared (pT1aN1M0 stage). Two cycles of IP chemotherapy ensued after the operation.
Case 2: CT showed that the tumor infiltrated into the stomach wall and metastasized to regional lymph nodes.
Case 1: Gastric MiNEN (metastases to the left liver).
Case 2: Gastric MiNEN.
Case 1: The patient received four cycles of IP regimen as first-line chemotherapy, then gastrectomy and partial left liver resection were performed.
Case 2: This patient underwent a total of six cycles of IP regimens without serious adverse effects.
Case 1: The patient has survived for more than 3 years without any evidence of recurrence or metastases.
Case 2: Two years after treatment, CT re-examination revealed metastatic lesions in the lower lobe of the right lung and video-assisted thoracoscopic surgery (VATS) was performed. Histopathological examination confirmed the neuroendocrine carcinoma (positive for CK7, CgA, and Syn; Ki67 index 30%) infiltration, with no metastases in regional lymph nodes. After the surgery, the patient did not undergo any further chemotherapy or radiotherapy and has survived for more than 7 years without any evidence of recurrence or metastases.
MiNEN is rare, especially in the stomach. To date, there is no consensus on the definition of MiNEN, especially the minimum proportion of each component. According to the WHO classification of digestive system tumors, MiNEN should contain both adenocarcinoma and neuroendocrine carcinoma components and each component is not less than 30%. However, this cutoff value has not been universally accepted, as it is defined arbitrarily rather than on proven clinical evidence and a minor (i.e., < 30%) poorly differentiated neuroendocrine carcinoma (PDNEC) component can impair prognosis[1,9,10]. Pham et al[5] once reported a case in which the adenocarcinoma component accounted for 10%-20% of the tumor, just as the case in our two patients. Park et al[11] found that a minor proportion (10%-30%) of PDNEC component would negatively influence the prognosis of patients with gastric MiNENs in a study including 88 patients. Consequently, the current 30% threshold, without sufficient prognostic value, may be not mandatory for defining MiNEN.
Most gastrointestinal MiNENs are highly aggressive, with a poor prognosis and median survival of less than 12 mo[5,9]. At present, the diagnosis mainly relies on pathological examination and IHC of surgical specimen[5,10]. CK, carcinoembryonic antigen, and caudal type homeobox 2 are used as markers for adenocarcinoma components, and Syn, CgA, and CD56 for neuroendocrine components[12]. In our two cases, the adenocarcinoma components were positive for CK18 or CKpan, and neuroendocrine component positive for CgA and Syn.
Until now, most studies suggest that surgical resection should be the main treatment for gastrointestinal MiNENs. Pham et al[5] argued that palliative surgery remains essential even if the patients have developed distant metastases. Our two patients underwent resection of the primary lesion and metastatic lesion, respectively, and both of them achieved long-term survival, being in good condition, without any evidence of recurrence to date. Therefore, we believe that curative-intent surgery if feasible, is crucial for the treatment of MiNEN, as recommended by other authors[12-14].
There is still no consensus regarding the standard front-line chemotherapy against MiNENs[5]. Platinum combined with etoposide (EP) regimen is found to be the most recommended first-line therapy for gastroenteropancreatic neuroendocrine carcinomas (GEPNECs)[5,15,16]. The preferred treatment for high-grade MiNENs is also suggested to be EP regimen or the combinations of 5-fluorouracil and irinotecan or temozolomide or amrubicin[1]. Yamaguchi et al[17] compared IP regimen and EP regimen in treating GEPNECs, discovering that the IP group had a higher response rate (50% vs 28%, respectively; P = 0.001). When it comes to irinotecan and etoposide, there were some studies demonstrating a lower incidence of grade 4 adverse events and treatment-related deaths in the irinotecan group than in the etoposide group when treating digestive neuroendocrine carcinoma[15,17]. IP regimen is also better than irinotecan monotherapy when comparing progression-free survival and disease control rate[18]. Therefore, we thought that IP regimen could be used for our two patients. Surprisingly, both of them achieved long-time survival for more than 3 years and 7 years, respectively, which are much longer than those in other studies[5]. It may suggest that IP regimen could be considered as the priority in the choice of front-line chemotherapy for gastric MiNEN. To the best of our knowledge, we were the first to use IP regimen along with surgical resection for patients with gastric MiNENs.
To date, the effect of Ki67 proliferation index variation on prognosis remains unclear. Shi et al[19] discovered that the Ki67 index would rise in 40% (n = 30) patients and decline in 13.3% patients with gastroenteropancreatic NEC during the treatment. In addition, Panzuto, Botling, and their colleagues[20,21] found that the Ki67 index of patients tends to rise at time of disease progression, and median OS was significantly shorter in patients with rising Ki67 index (50.2 vs 115.1 m, hazard ratio = 3.89, 95% confidence interval [CI]: 1.91-7.94, P < 0.001). The Ki67 index of the patient in case 2 declined from 60% to 30% after IP regimen treatment, which was associated with a long-term survival. This, to some extent, may indicate that the decrease of Ki67 index is related to a better prognosis, which still needs further study.
At present, the most common genetic changes found in MiNENs include TP53, KRAS, BRAF, APC, PIK3CA, MYC, etc[22-25]. We wonder if our two patients share some common genetic changes, which could be part of the reason for their long-term survival. Next-generation sequencing tests were performed on the surgical specimens of them, revealing that they were all proved to be microsatellite stable (MSS), and the tumor mutation burden (TMB) was 4.06 mut/Mb and 2.03 mut/Mb, respectively. TP53 mutation was found in patient 1, and BRCA2 mutation, along with copy number increase in nine genes (MET, FGFR1, FGFR4, CDK4, CDK6, CDKN2A, ERBB3, RIT1, and VEGFA) in patient 2. We may assume that MSS and TMB fewer than 10 mut/Mb could be associated with improved response to IP regimen from the tests result. It still needs further studies to explore which genetic changes may indicate a better prognosis in patients with MiNEN receiving IP regimen treatment.
Gastric MiNEN is a rare malignant tumor without specific clinical symptoms. Histopathological and immunohistochemical examinations are requisite for pathologists and physicians to make diagnosis. Palliative surgery remains essential even when patients have undergone distant metastases. In the choice of front-line chemotherapy, we believe that IP regimen could be considered as the priority. More prospective studies are urgently needed to explore better treatment options for patients with gastric MiNEN.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Alzerwi NAN, Saudi Arabia; Moshref L, Saudi Arabia; Tanabe H, Japan S-Editor: Wu YXJ L-Editor: Wang TQ P-Editor: Wu YXJ
| 1. | de Mestier L, Cros J, Neuzillet C, Hentic O, Egal A, Muller N, Bouché O, Cadiot G, Ruszniewski P, Couvelard A, Hammel P. Digestive System Mixed Neuroendocrine-Non-Neuroendocrine Neoplasms. Neuroendocrinology. 2017;105:412-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 2. | Cordier R. Les cellules argentaffines dans les tumeurs intestinales. Arch Int Med Exp. 1924;1:5. [DOI] [Full Text] |
| 3. | La Rosa S, Marando A, Sessa F, Capella C. Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update. Cancers (Basel). 2012;4:11-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 4. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2441] [Article Influence: 488.2] [Reference Citation Analysis (3)] |
| 5. | Pham QD, Mori I, Osamura RY. A Case Report: Gastric Mixed Neuroendocrine-Nonneuroendocrine Neoplasm with Aggressive Neuroendocrine Component. Case Rep Pathol. 2017;2017:9871687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Fujita Y, Uesugi N, Sugimoto R, Eizuka M, Matsumoto T, Sugai T. Gastric mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN) with pancreatic acinar differentiation: a case report. Diagn Pathol. 2019;14:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Robinson L, Schouwstra CM, van Heerden WFP. Oropharyngeal Mixed Neuroendocrine-Nonneuroendocrine Neoplasm (MiNEN): A Case Report and Literature Review. Head Neck Pathol. 2021;15:1415-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Kaneko R, Kimura Y, Sakata H, Ikehara T, Mitomi H, Uekusa T, Ohbu M, Kubo S. A case of primary hepatic mixed neuroendocrine-non-neuroendocrine tumor (MiNEN) associated with gallbladder carcinosarcoma. Clin J Gastroenterol. 2020;13:1280-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | La Rosa S, Sessa F, Uccella S. Mixed Neuroendocrine-Nonneuroendocrine Neoplasms (MiNENs): Unifying the Concept of a Heterogeneous Group of Neoplasms. Endocr Pathol. 2016;27:284-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (2)] |
| 10. | La Rosa S. Challenges in High-grade Neuroendocrine Neoplasms and Mixed Neuroendocrine/Non-neuroendocrine Neoplasms. Endocr Pathol. 2021;32:245-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Park JY, Ryu MH, Park YS, Park HJ, Ryoo BY, Kim MG, Yook JH, Kim BS, Kang YK. Prognostic significance of neuroendocrine components in gastric carcinomas. Eur J Cancer. 2014;50:2802-2809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | de Mestier L, Cros J. Digestive system mixed neuroendocrine-non-neuroendocrine neoplasms (MiNEN). Ann Endocrinol (Paris). 2019;80:172-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Wu QQ, Qiang WG, Wang F, Dai KJ, Xu EC, Luo JD, Li Q, Tang H, Zhou XF, Lu XJ. Management of primary gastric small cell carcinoma in China. Int J Clin Exp Med. 2015;8:1589-1597. [PubMed] |
| 14. | Shen C, Chen H, Yin Y, Han L, Chen J, Tang S, Yin X, Zhou Z, Zhang B, Chen Z. Surgical treatment and prognosis of gastric neuroendocrine neoplasms: a single-center experience. BMC Gastroenterol. 2016;16:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Thomas KEH, Voros BA, Boudreaux JP, Thiagarajan R, Woltering EA, Ramirez RA. Current Treatment Options in Gastroenteropancreatic Neuroendocrine Carcinoma. Oncologist. 2019;24:1076-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Tanaka T, Kaneko M, Nozawa H, Emoto S, Murono K, Otani K, Sasaki K, Nishikawa T, Kiyomatsu T, Hata K, Morikawa T, Kawai K, Watanabe T. Diagnosis, Assessment, and Therapeutic Strategy for Colorectal Mixed Adenoneuroendocrine Carcinoma. Neuroendocrinology. 2017;105:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Yamaguchi T, Machida N, Morizane C, Kasuga A, Takahashi H, Sudo K, Nishina T, Tobimatsu K, Ishido K, Furuse J, Boku N, Okusaka T. Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci. 2014;105:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 18. | Nishikawa K, Koizumi W, Tsuburaya A, Yamanaka T, Morita S, Fujitani K, Akamaru Y, Shimada K, Hosaka H, Nakayama N, Tsujinaka T, Sakamoto J. Meta-analysis of two randomized phase III trials (TCOG GI-0801 and ECRIN TRICS) of biweekly irinotecan plus cisplatin vs irinotecan alone as second-line treatment for advanced gastric cancer. Gastric Cancer. 2020;23:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Shi H, Zhang Q, Han C, Zhen D, Lin R. Variability of the Ki-67 proliferation index in gastroenteropancreatic neuroendocrine neoplasms - a single-center retrospective study. BMC Endocr Disord. 2018;18:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Panzuto F, Cicchese N, Partelli S, Rinzivillo M, Capurso G, Merola E, Manzoni M, Pucci E, Iannicelli E, Pilozzi E, Rossi M, Doglioni C, Falconi M, Delle Fave G. Impact of Ki67 re-assessment at time of disease progression in patients with pancreatic neuroendocrine neoplasms. PLoS One. 2017;12:e0179445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Botling J, Lamarca A, Bajic D, Norlén O, Lönngren V, Kjaer J, Eriksson B, Welin S, Hellman P, Rindi G, Skogseid B, Crona J. High-Grade Progression Confers Poor Survival in Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2020;110:891-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Scardoni M, Vittoria E, Volante M, Rusev B, Bersani S, Mafficini A, Gottardi M, Giandomenico V, Malleo G, Butturini G, Cingarlini S, Fassan M, Scarpa A. Mixed adenoneuroendocrine carcinomas of the gastrointestinal tract: targeted next-generation sequencing suggests a monoclonal origin of the two components. Neuroendocrinology. 2014;100:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Sinha N, Gaston D, Manders D, Goudie M, Matsuoka M, Xie T, Huang WY. Characterization of genome-wide copy number aberrations in colonic mixed adenoneuroendocrine carcinoma and neuroendocrine carcinoma reveals recurrent amplification of PTGER4 and MYC genes. Hum Pathol. 2018;73:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Vortmeyer AO, Lubensky IA, Merino MJ, Wang CY, Pham T, Furth EE, Zhuang Z. Concordance of genetic alterations in poorly differentiated colorectal neuroendocrine carcinomas and associated adenocarcinomas. J Natl Cancer Inst. 1997;89:1448-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 112] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 25. | Woischke C, Schaaf CW, Yang HM, Vieth M, Veits L, Geddert H, Märkl B, Stömmer P, Schaeffer DF, Frölich M, Blum H, Vosberg S, Greif PA, Jung A, Kirchner T, Horst D. In-depth mutational analyses of colorectal neuroendocrine carcinomas with adenoma or adenocarcinoma components. Mod Pathol. 2017;30:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |