Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.7924
Peer-review started: November 22, 2021
First decision: January 11, 2022
Revised: January 23, 2022
Accepted: June 17, 2022
Article in press: June 17, 2022
Published online: August 6, 2022
Processing time: 241 Days and 15.4 Hours
Intracranial Listeria infections are common in newborns and immunocompromised individuals, but brainstem abscesses are rare.
We report a rare case of brainstem abscesses caused by Listeria monocytogenes in a previously healthy adult patient. The patient’s magnetic resonance imaging examination showed multiple brain abscesses, and his second cerebrospinal fluid culture test indicated the presence of Listeria monocytogenes. Despite early empirical therapy, the patient’s condition progressively deteriorated. Because the patient's abscesses were located in the brainstem and multiple lobes, surgery was not possible. The patient died 40 d after admission.
This case highlights the importance of rational clinical use of drugs to avoid potentially serious infectious complications.
Core Tip: Listeria monocytogenes, an opportunistic pathogen, can be life-threatening when it infects the central nervous system (CNS). Herein, we report the case of a patient presenting with fever, headache, emesis, and perturbed consciousness. His condition rapidly deteriorated after empiric antibiotic therapy. He was finally diagnosed with Listeria monocytogenes infection after re-examination. Despite a timely change in his medication regimen, the patient died. This case highlights the importance of rational clinical antibiotic therapy to avoid potentially serious infectious complications. When empiric antibiotic therapy fails, and Listeria infection of the CNS is suspected, bacterial culture should be repeated for timely adjustment of antibiotics.
- Citation: Wang J, Li YC, Yang KY, Wang J, Dong Z. Brainstem abscesses caused by Listeria monocytogenes: A case report. World J Clin Cases 2022; 10(22): 7924-7930
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/7924.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.7924
Listeria monocytogenes is a Gram positive bacterium that can opportunistically cause listeriosis, including bacteremia and neurolisteriosis. Listeriosis is rarely seen in humans but has a high mortality rate of approximately 30%[1]. Patients diagnosed with neurolisteriosis develop meningitis and cerebral hemisphere inflammation, with a 20%–30% mortality[1]; brainstem encephalitis appears in 17% of patients, with a 51% mortality[1,2].The percentage of cases with brain abscess is low (2%)[1], and those formed in the brainstem are more rare, with only a few case reports described. Despite clinically appropriate antibiotic therapy, the mortality rate remains high, owing to the difficulty in diagnosis, resistance to cephalosporin, and rapid progression of the disease. Early diagnosis and appropriate and adequate anti-microbial therapy are essential to decrease mortality and sequelae.
Here, we report the case of a patient whose cerebrospinal fluid (CSF) culture was positive for Listeria and who accepted positive anti-infective therapy. Multiple abscesses formed at the brainstem and both cerebral hemispheres rapidly during the course of treatment. The patient’s condition subsequently deteriorated, and he ultimately died. This case report aims to optimize the anti-infection treatment protocols for brainstem abscesses caused by Listeria monocytogenes and to provide a reference for rational drug administration by clinicians.
A history of fever and headache for 6 d, emesis for 1 d, and disturbance of consciousness for 4 h.
The patient began to experience headache and fever, and vomiting and disturbance of consciousness appeared later.
The patient was previously healthy.
The patient had no personal and family history.
At the time of admission, the patient’s body temperature was 38 ℃; neurological examination showed confusion and irritability; his bilateral pupils were equally large and round and insensitive to light; the shallow sensation on the right limb was lower than that on the left limb; the Kernig sign was positive; and neck resistance was observed, at the submental three horizontal fingers. Both lungs produced an audible sputum sound, and the right external auditory canal had purulent secretion.
On the 14th day, the patient’s condition suddenly worsened and included drowsiness, left pupil dilation, a slow light response, and limited left and right abduction. On the 24th day, the patient was in shallow-moderate coma and left eyelid insufficiency. On the 39th day, his body temperature rose up to 41.3 ℃, and he develop vomiting, a rapid heart rate, and a decrease in blood oxygen saturation to approximately 70%.
Lumbar puncture was performed that day, and the pressure exceeded 330 mmH2O. CSF cytology revealed a white blood cell (WBC) count of 2520/mm3, and the proportions were 68% neutrophilic granulocytes, 25% monocytes, and 7% lymphocytes. CSF biochemistry showed high protein (2875 mg/L), low glucose (1.34 mmol/L), and low chloride (100.9 mmol/L). No abnormalities were found in the CSF smear. The WBC count was 21.5 × 109/L, the neutrophilic granulocyte percentage was 91.7%, and the original calcitonin was 3.28 ng/mL in the blood tests. Cultures of blood, CSF, and purulent secretions were examined simultaneously.
On the 7th day, a lumbar puncture reexamination showed a decrease in CSF WBC count (32/mm3), with 100% lymphocytes, but an increase in protein (3938 mg/L). Blood tests indicated a decrease in WBC count to the normal range. On the 14th day, CSF analysis revealed a WBC count of 401/mm3 (62% neutrophils, 14% monocytes, and 23% lymphocytes), glucose level of 4.37 mmol/L, chlorine level of 114 mmol/L, and protein level of 1990 mg/L. The results of the second CSF culture test indicated the presence of Listeria monocytogenes (but repeated medical history did not include questions about the history of contaminated food intake), and drug susceptibility testing indicated sensitivity to teicoplanin, linezolid, erythromycin, and amikacin. A blood culture was negative, and sputum culture showed hydrocarbon-resistant Acinetobacter baumannii. On the 24th day, abnormal liver function, electrolyte disorder (low sodium, chlorine, and calcium), and hypoproteinemia gradually appeared during the course of the disease. On the 27th day, the lumbar puncture was reexamined. The CSF WBC count was 30/mm3, with 100% lymphocytes, the glucose level was 3.91 mmol/L, the chlorine level was 113 mmol/L, and the protein level was 1270 mg/L. CSF culture suggested methicillin-resistant staphylococcus, and sensitivity to teicoplanin and linezolid. On the 39th day, blood gas analysis suggested type I respiratory failure.
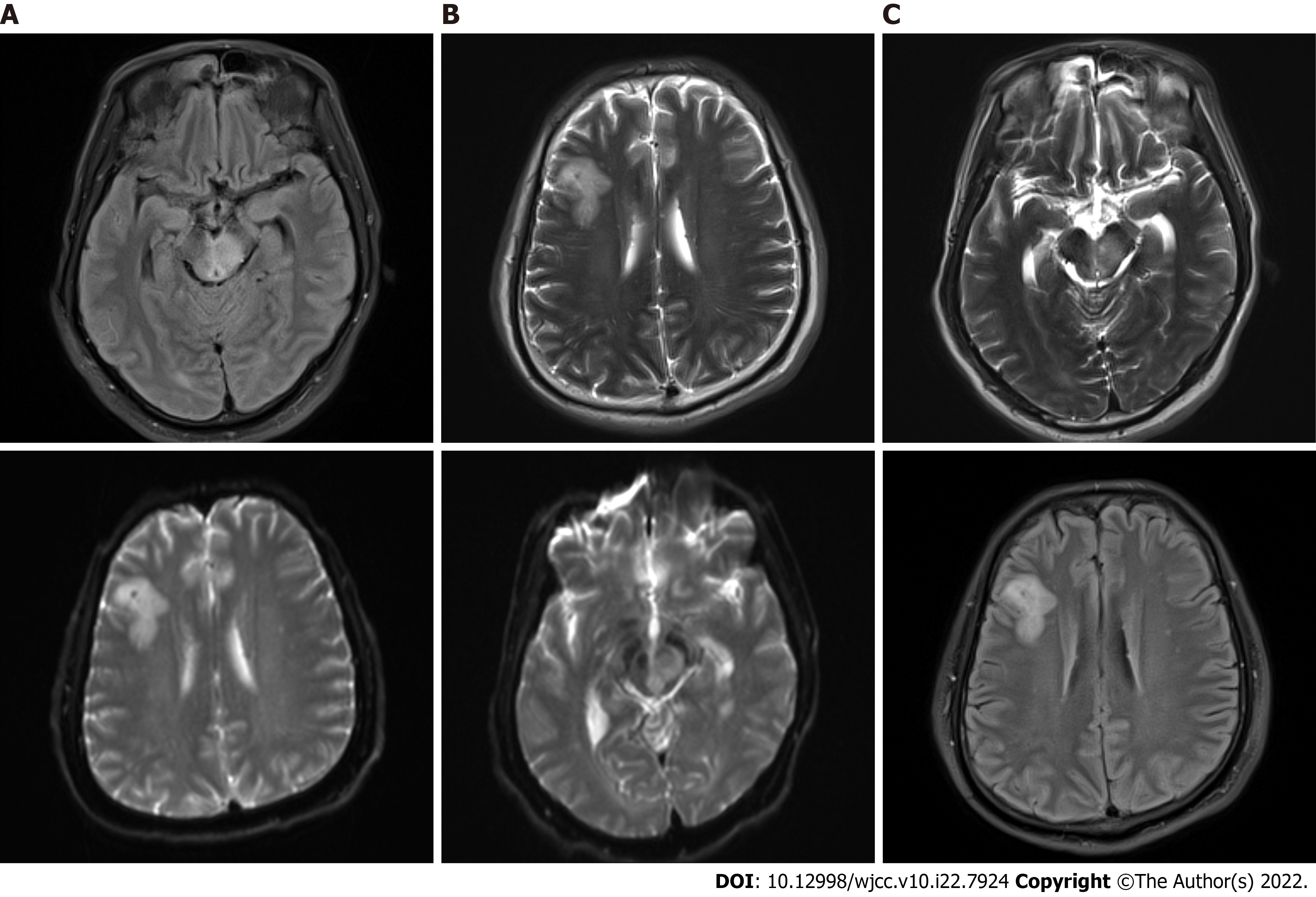
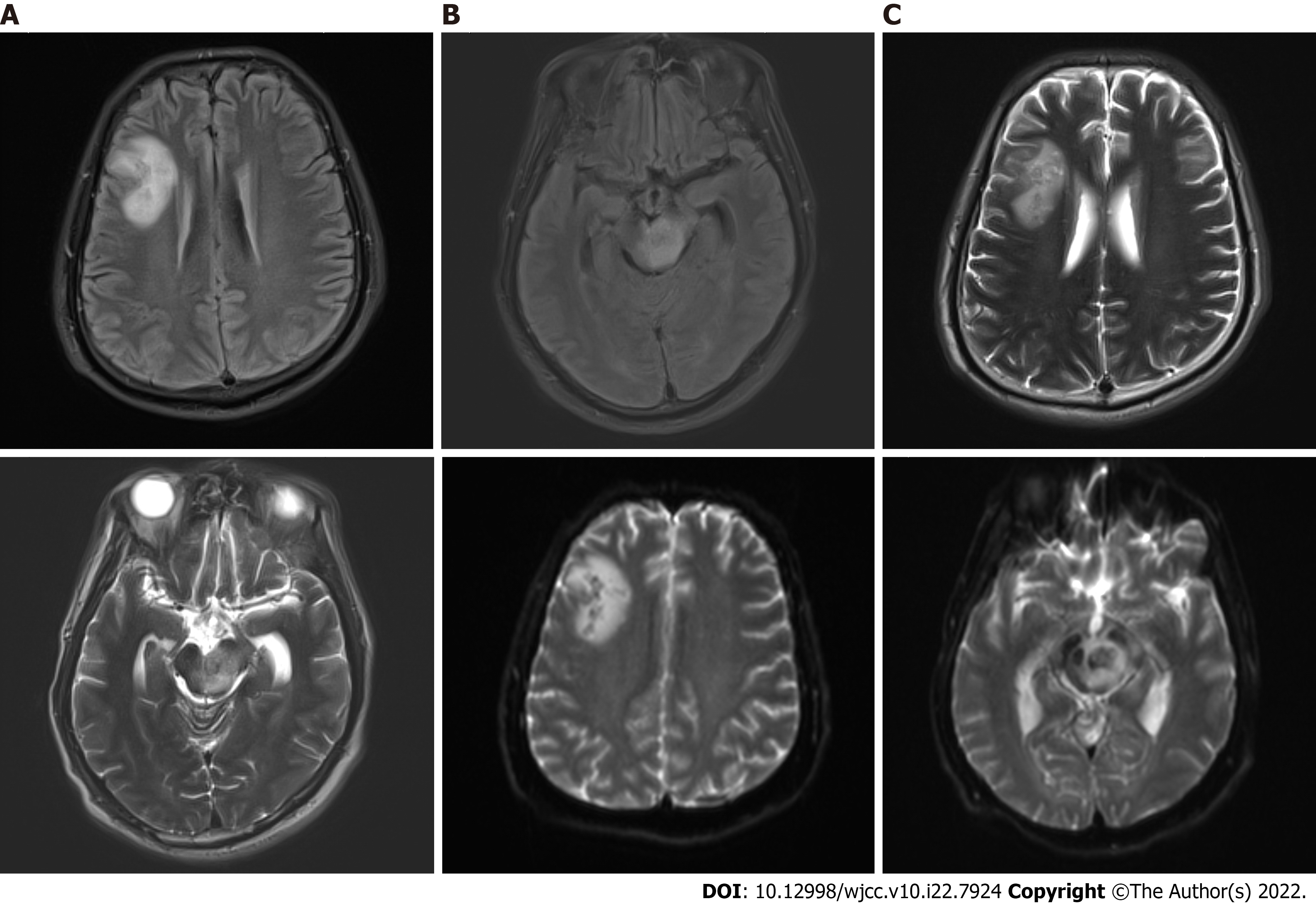
At admission, computed tomography (CT) of the head showed brain swelling, and CT of the chest suggested pulmonary infection. On the fifth day, a head CT reexamination showed no significant change, whereas a chest CT reexamination showed serious infection of the right lower lung, with clear consolidation. On the 14th day, head CT revealed a new oval slightly low-density shadow in the right frontal lobe. On the 15th day, head magnetic resonance imaging (MRI) examination was completed, and multiple abnormal signal shadows were found in the right basal ganglia region, lateral ventricle, bilateral frontal lobes, left craniocerebral foot, pons, right bridge-arm, left ventricle trigonometry, and bilateral parioccipito-temporal sulcus (Figure 1). On the 27th day, head MRI showed multiple abnormal signal shadows in the right basal ganglia region, lateral ventricle, bilateral frontal lobe, left craniocerebral foot, thalamus, pons, medulla oblongata, and left temporal horn, which had become enlarged (Figure 2).
Brainstem abscesses caused by Listeria monocytogenes.
On admission, the patient was treated with vancomycin (1 g, every 12 h) and ceftriaxone (2 g, every 12 h) to resist infection; mannitol and glycerol fructose to decrease the intracranial pressure; and other treatments for his symptoms. On the fifth day, treatment was adjusted to vancomycin (1 g, every 12 h) and cefoperazone and sulbactam sodium (3 g intravenous infusion once every 8 h). On the 15th day, the antibiotics were adjusted to a combination of teicoplanin, penicillin, cefoperazone, and sulbactam sodium.
On the 40th day and, the patient died outside the hospital.
Listeria are food-borne bacteria whose food sources in China are mainly meat and poultry products. In addition, dairy products are possible sources[3]. Listeria can adapt to harsh environments, such as high salinity, low temperature, and acidic or alkaline pH[4]. Because the incubation period varies widely, ranging from 3 to 70 d[5], identifying the source of infection is difficult in most patients. Despite repeated questioning, the history of intake of contaminated food intake by this patient was not obtained. Studies have suggested that Listeria can cross the blood-brain barrier, be transported by migratory immune cells, or be transmitted in a retrograde manner through nerves, such as the trigeminal and olfactory nerves[6-8]. Most patients with central nervous system (CNS) Listeria infection are the elderly, pregnant women, or those who have immunodeficiency, immunosuppression, diabetes, or cirrhosis[9]. However, patients with brainstem encephalitis are often healthy individuals, and this condition is believed to be associated with the typing of Listeria now[10]. Experimental studies have found that some subtypes of Listeria are neurotropic, and can cause brainstem encephalitis through food-borne transmission, and symptoms such as abnormal gait and balance or dyskinesia in mice, despite being negative in the blood. Other strains can enter the brain only if the high levels are present in the blood, and most cause meningoencephalitis not involving the brainstem[10]. According to previous studies, the pathogen affecting our patient may have been the neurotropic Listeria subtype, but this possibility has not been confirmed. Patients with central Listeria infection may have symptoms of systemic infection, such as fever, headache, vomiting, and diarrhea, as well as symptoms of nervous system damage or irritation, such as disturbance of consciousness, epilepsy, aphasia, hemiplegia, cranial nerve palsy, ataxia, or dysarthria[11]. Most patients are in critical condition after admission, and approximately 33% require endotracheal intubation; 19% develop multiple organ failure after a long hospitalization (15-33 d); approximately 44% have neurological sequelae; and 30% die within 3 mo after diagnosis[1]. Our patient showed signs of improvement several times during hospitalization but was unable to survive. This outcome was considered to be associated with an enlarged brainstem abscess. Patients with CNS Listeriosis often present with mild abnormalities in the CSF and are diagnosed on the basis of the detection of Listeria in the CSF, or unexplained neurological symptoms together with the detection of Listeria in the blood (other pathogens are negative)[1]. Previous diagnosis has mainly relied on culture of CSF and blood or PCR techniques. In recent years, large-scale clinical investigations have shown that the positive rate in blood culture is 63%, the positive rate in direct detection of CSF is 32%, the positive rate in culture is 84%, and the positive rate in PCR detection of CSF is 62.5%[1]. In patients with brain abscess, the positive rate in CSF culture is even lower. A retrospective study has reported that although blood cultures from patients with Listeria brain abscesses were higher (28 out of 33 (86%)), only 11 (38%) of 29 patients with CSF reports were positive[12], thus hindering early diagnosis of patients with Listeria brain abscesses. In recent years, second-generation sequencing has been able to detect pathogens in the CSF of culture-negative patients with CNS Listeria infection, thus improving the diagnostic efficiency[13,14]. In our case, the initial CSF assay hinted at a significantly high WBC count, high protein, and low sugar and chlorine. The first cultures of the blood and CSF were negative, and the results of the second CSF culture suggested Listeria positivity after the patient's condition had worsened. Antibiotics were adjusted according to the results of drug susceptibility testing, and our patient’s condition improved and then worsened, and eventually died. Our case suggested that, although Listeria infection of the CNS is rare, the CSF and blood cultures should be tested early and even repeatedly if the first results are negative but the patient's condition fluctuates. If necessary, second generation sequencing can be used to improve the early pathogen detection rate to enable timely treatment and improve the prognosis. Regarding imaging, MRI is better than CT for observing lesions. Previous studies have reported that most patients with Listeria brain abscesses have a single abscess lesion, but 22% have more than one. MRI showed that most multiple abscesses were located in one hemisphere and distributed along the white matter fiber bundles, thus supporting the hypothesis that Listeria is transduced along the nerve axons[15]. In our case, head CT was performed many times in the anterior region over 12 d but did not hint at an abscess lesion. Because of the patient's poor condition (mechanical assistance) in early stages, head MRI was unable to be completed. The CT showed a new large frontal lobe abscess on the 14th day after the patient’s condition worsened, and the head MRI on the 15th day showed not only the frontal lobe abscess detected 1 d before but also several new abscess lesions in both the cerebral hemisphere and brainstem. All abscesses had increased in size according to the follow-up MRI. We suggest that intracranial Listeria infection progresses rapidly, and head MRI examination is important in early stages if conditions permit. Large-scale clinical studies have suggested that amoxicillin combined with gentamicin is the first-line drug for Listeria infection[1]. In addition, studies have shown that penicillin, ampicillin, linezolid, and other antibiotics are effective in intracranial Listeria infection[1,16,17]. Notably, Listeria is sensitive to many common antibiotics but resistant to cephalosporins[17]. Because of the difficulty of diagnosis, up to 90% of patients are treated empirically with cephalosporins because the pathogen is not detected. In addition to drug therapy, surgical procedures such as abscess puncture, drainage, and excision have been reported for the treatment of Listeria brain abscesses in patients who have not responded to antibiotic therapy[12]. In our case, there was no intracranial lesion in the initial stage, and no positive results were found in the culture of CSF and blood. Only vancomycin and third generation cephalosporin were used empirically. Later, according to the results of the culture of CSF and drug sensitivity testing, the antibiotics were replaced with teicoplanin and penicillin, but the patient nonetheless died. Because the patient's abscesses were located in the brainstem and multiple lobes, surgery was extremely risky, and a surgical approach was not possible. In retrospect, the patient’s condition fluctuated after early empirical anti-infective therapy, and the number of CSF cells decreased significantly in the initial stage. However, the abscesses continued to expand, and the patient experienced recurrence and eventually died. Analysis of the reasons for this outcome led us to the following conclusions. First, because of the lack of awareness of CNS Listeria infection, targeted antibiotics such as penicillin and gentamicin were not used early in the treatment course. Second, Listeria infection is a dangerous disease; and the patient's condition was exacerbated by brainstem abscess, and the patient eventually died.
In summary, we describe a rare case of multiple abscesses in the brainstem and cerebral hemispheres after Listeria infection. Our findings suggest that intracranial infection with Listeria may improve during the course of disease, but the disease is nonetheless dangerous. Although the disease is more common in immunocompromised patients, CNS Listeria infections should be considered in previously healthy patients with intracranial infection. In terms of treatment, antibiotics should be given as early and sufficiently as possible, and should be adjusted in a timely manner according to the results of CSF culture and second-generation sequencing. In addition, repeated searching for pathogens and reexamination of head images are necessary to track changes in patients. Because of the non-specific CSF/blood findings and low positivity rate of cultures, early diagnosis of Listeria infections remains a clinical challenge. Large-scale clinical studies to identify prognostic factors and the efficacy of empiric and definitive antibiotic treatments are also important.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Emran TB, Bangladesh; Garg P, India S-Editor: Xing YX L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Charlier C, Perrodeau É, Leclercq A, Cazenave B, Pilmis B, Henry B, Lopes A, Maury MM, Moura A, Goffinet F, Dieye HB, Thouvenot P, Ungeheuer MN, Tourdjman M, Goulet V, de Valk H, Lortholary O, Ravaud P, Lecuit M; MONALISA study group. Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. Lancet Infect Dis. 2017;17:510-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 338] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 2. | Armstrong RW, Fung PC. Brainstem encephalitis (rhombencephalitis) due to Listeria monocytogenes: case report and review. Clin Infect Dis. 1993;16:689-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 167] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Li W, Bai L, Fu P, Han H, Liu J, Guo Y. The Epidemiology of Listeria monocytogenes in China. Foodborne Pathog Dis. 2018;15:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Bajard S, Rosso L, Fardel G, Flandrois JP. The particular behaviour of Listeria monocytogenes under sub-optimal conditions. Int J Food Microbiol. 1996;29:201-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Angelo KM, Jackson KA, Wong KK, Hoekstra RM, Jackson BR. Assessment of the Incubation Period for Invasive Listeriosis. Clin Infect Dis. 2016;63:1487-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Wei P, Bao R, Fan Y. Brainstem Encephalitis Caused by Listeria monocytogenes. Pathogens. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Pägelow D, Chhatbar C, Beineke A, Liu X, Nerlich A, van Vorst K, Rohde M, Kalinke U, Förster R, Halle S, Valentin-Weigand P, Hornef MW, Fulde M. The olfactory epithelium as a port of entry in neonatal neurolisteriosis. Nat Commun. 2018;9:4269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Radoshevich L, Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol. 2018;16:32-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 523] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 9. | Goulet V, Hebert M, Hedberg C, Laurent E, Vaillant V, De Valk H, Desenclos JC. Incidence of listeriosis and related mortality among groups at risk of acquiring listeriosis. Clin Infect Dis. 2012;54:652-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Senay TE, Ferrell JL, Garrett FG, Albrecht TM, Cho J, Alexander KL, Myers-Morales T, Grothaus OF, D'Orazio SEF. Neurotropic Lineage III Strains of Listeria monocytogenes Disseminate to the Brain without Reaching High Titer in the Blood. mSphere. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Maertens De Noordhout C, Devleesschauwer B, Maertens De Noordhout A, Blocher J, Haagsma JA, Havelaar AH, Speybroeck N. Comorbidities and factors associated with central nervous system infections and death in non-perinatal listeriosis: a clinical case series. BMC Infect Dis. 2016;16:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Eckburg PB, Montoya JG, Vosti KL. Brain abscess due to Listeria monocytogenes: five cases and a review of the literature. Medicine (Baltimore). 2001;80:223-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Lan ZW, Xiao MJ, Guan YL, Zhan YJ, Tang XQ. Detection of Listeria monocytogenes in a patient with meningoencephalitis using next-generation sequencing: a case report. BMC Infect Dis. 2020;20:721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Yao M, Zhou J, Zhu Y, Zhang Y, Lv X, Sun R, Shen A, Ren H, Cui L, Guan H, Wu H. Detection of Listeria monocytogenes in CSF from Three Patients with Meningoencephalitis by Next-Generation Sequencing. J Clin Neurol. 2016;12:446-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Bojanowski MW, Seizeur R, Effendi K, Bourgouin P, Magro E, Letourneau-Guillon L. Spreading of multiple Listeria monocytogenes abscesses via central nervous system fiber tracts: case report. J Neurosurg. 2015;123:1593-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Yılmaz PÖ, Mutlu NM, Sertçelik A, Baştuğ A, Doğu C, Kışlak S. Linezolid and dexamethasone experience in a serious case of listeria rhombencephalitis. J Infect Public Health. 2016;9:670-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Thønnings S, Knudsen JD, Schønheyder HC, Søgaard M, Arpi M, Gradel KO, Østergaard C; Danish Collaborative Bacteraemia Network (DACOBAN). Antibiotic treatment and mortality in patients with Listeria monocytogenes meningitis or bacteraemia. Clin Microbiol Infect. 2016;22:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |