Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.7913
Peer-review started: September 3, 2021
First decision: December 1, 2021
Revised: December 14, 2021
Accepted: June 17, 2022
Article in press: June 17, 2022
Published online: August 6, 2022
Processing time: 321 Days and 23.7 Hours
Multicentric reticulohistiocytosis (MRH) is a rare non-Langerhans histiocytosis of unknown etiology characterized by papulonodular skin lesions and progressive, erosive arthritis. To date, there have been approximately 300 cases of MRH reported worldwide. The majority of patients are Caucasian from western coun
A 38-year-old male was admitted to the hospital with a rash that had persisted for over 2 years and bilateral knee pain for over 1 year. The patient’s symptoms had previously been misdiagnosed as eczema when there were only skin symptoms and was finally diagnosed as MRH after a skin biopsy of the left upper back. The patient was treated with glucocorticoids combined with an immunosuppressive regimen. While the skin lesions on both arms, abdomen, and back subsided, the skin lesions on the rest of the body did not increase. The interphalangeal joints of both thumbs and bilateral knee joints remained swollen and painful.
The case will help clinicians better identify and treat this disease in the absence of epidemiological studies or randomized controlled data.
Core Tip: Multicentric reticulohistiocytosis (MRH) is a rare non-Langerhans histiocytosis of unknown etiology characterized by papulonodular skin lesions and progressive, erosive arthritis. To date, there have been approximately 300 cases of MRH reported worldwide. However, the majority of patients are Caucasian from western countries, and Asian patients are rare. Here, we report a case of MRH in a Chinese patient. We also review the relevant literature and comprehensively analyze the clinical characteristics of MRH. This case report and comprehensive analysis will help clinicians better identify and treat this disease in the absence of epidemiological studies or randomized controlled data.
- Citation: Xu XL, Liang XH, Liu J, Deng X, Zhang L, Wang ZG. Multicentric reticulohistiocytosis with prominent skin lesions and arthritis: A case report. World J Clin Cases 2022; 10(22): 7913-7923
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/7913.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.7913
Multicentric reticulohistiocytosis (MRH) is a rare multisystemic disease of unknown etiology characterized by papulonodular skin lesions and erosive arthritis. MRH is classified as non-Langerhans cell histiocytosis class II b according to the recommendations of the Histiocyte Society. There is currently no published data on the incidence and prevalence of MRH. To date, there have been approximately 300 reported cases of MRH worldwide. Moreover, the majority of patients are Caucasian from western countries and Asian patients are rare. Here, we report a case of MRH in China and provide a comprehensive analysis of the clinical characteristics of MRH.
A 38-year-old male was admitted to our hospital with a rash that had persisted for over two years and bilateral knee pain for over one year.
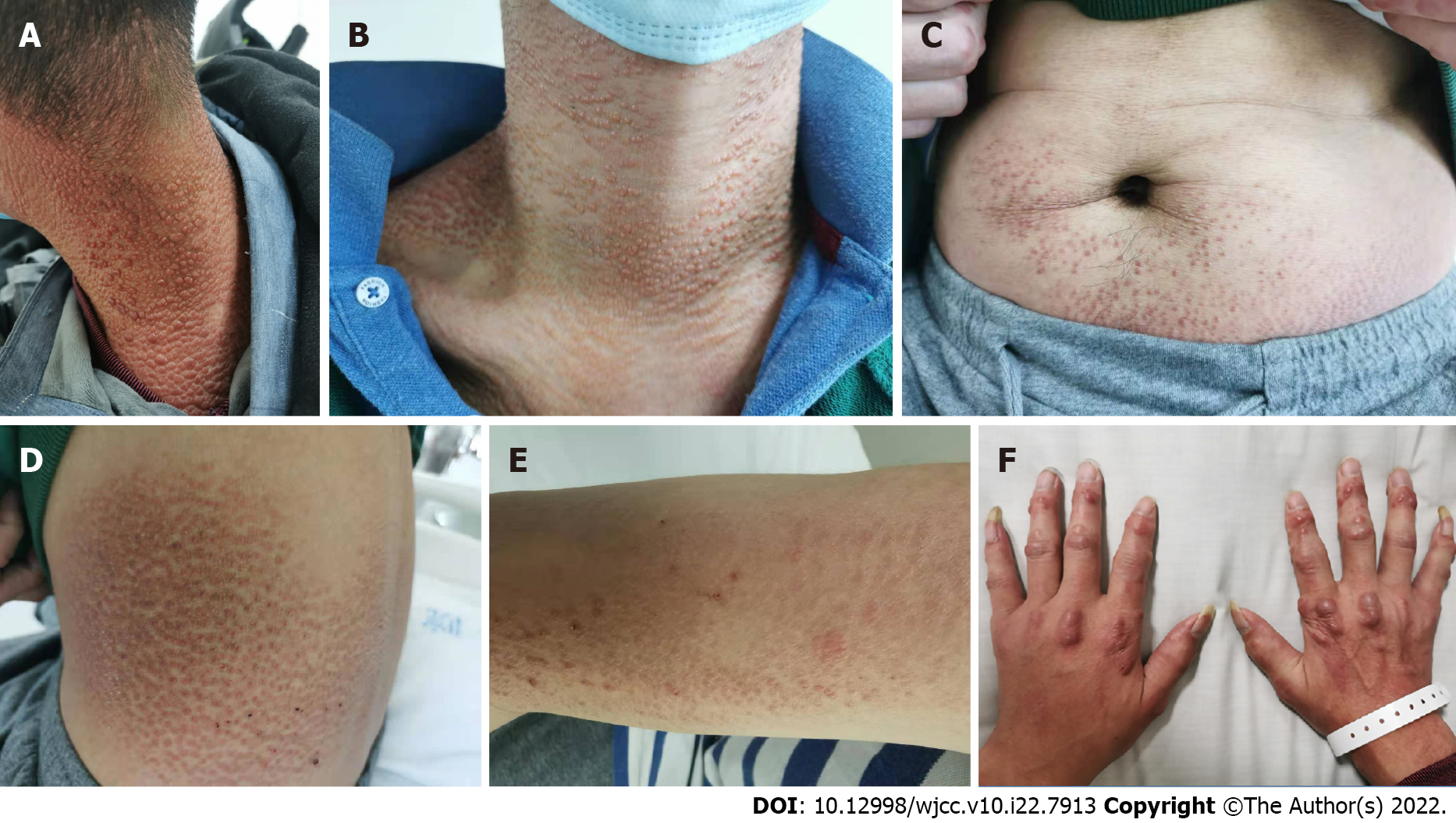
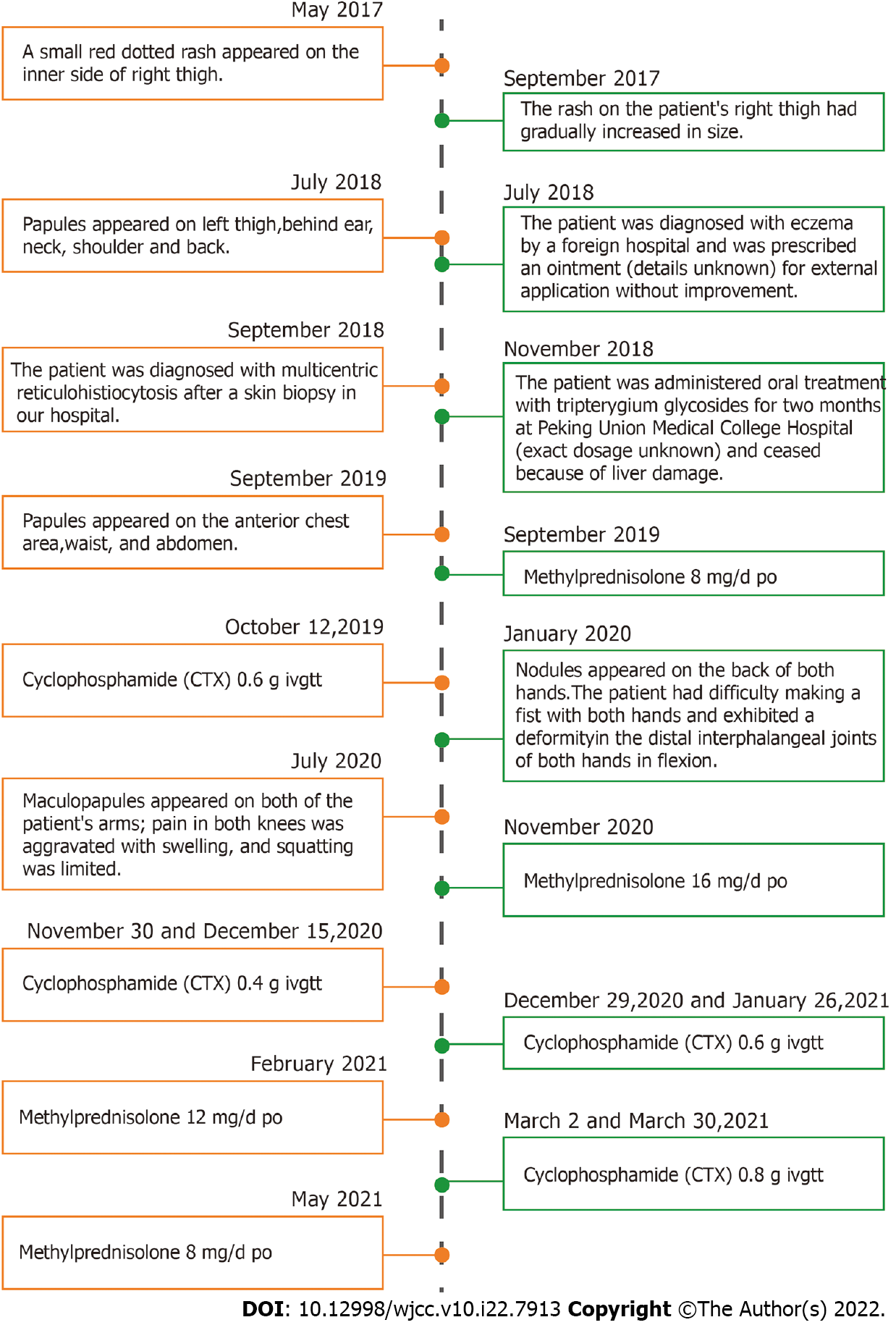
In May 2017, a small red dotted rash appeared on the inner side of the patient’s right thigh slightly above the skin surface, without itching. By September 2017, the rash on the patient’s right thigh had gradually increased in size from a small dot to a chrysanthemum-like pattern. In July 2018, millet-like, brownish-red papules appeared on the left thigh, symmetrically positioned with the right leg. This was followed by the appearance of similar rashes on the back of the ear, neck, shoulder, and back. The patient was diagnosed with eczema by a foreign hospital and was prescribed an ointment (details unknown) for external application without improvement. In September 2018, he was diagnosed with MRH following a skin biopsy obtained from the left upper back in our hospital. In November 2018, the patient was administered oral treatment with tripterygium glycosides for two months at Peking Union Medical College Hospital (exact dosage unknown). He subsequently ceased taking the medicine upon the development of liver damage. From September 2019, red macules and millet-like brown-red papules appeared on the anterior chest area, waist, and abdomen. After re-visiting our hospital, he began taking 8 mg/d oral methylprednisolone, and was administered 0.6 g cyclophosphamide in October 2019 by venous transfusion. Subsequently, the patient took 8 mg/d oral methylprednisolone irregularly without improvement of the skin lesions and joint symptoms. In January 2020, multiple round and oval nodules that were brownish red, hard, with a smooth surface, no rupture, approximately 2-8 mm in size appeared on the back of both hands. The patient had difficulty making a fist with both hands and exhibited a deformity in the distal interphalangeal joints of both hands in flexion. Beginning in July 2020, sesame-sized maculopapules appeared on both of the patient’s arms; pain in both knees was aggravated with swelling; and squatting was limited. The patient had no fever, myalgia, oral ulcers, or Raynaud's phenomenon, was in good spirits, and had no loss of appetite. No significant changes were observed regarding the patient’s weight throughout the course of the illness.
The patient had no previous medical history.
The patient denied a history of smoking and alcohol consumption; denied drug addiction; no history of exposure to industrial poisons, dust, and radioactive substances. There is no similar family history and family genetic disease is denied.
On physical examination, the patient was in a good general condition. A brownish-red maculopapular rash densely covered behind the ears, neck, shoulder, anterior chest, back, waist, abdomen, arms, and inner thighs. Multiple round and oval nodules, 2-8 mm in size, reddish-brown, hard, without rupture, and without pressure pain were observed on the back of both hands (Figure 1) and was associated with poor mobility. The oral mucosa and tongue were not involved. Grade V muscle strength of the extremities was observed. The patient had a "gooseneck-like" deformity involving 2-5 fingers of both hands, swelling of the interphalangeal joints of both thumbs, difficulty making a fist, swelling of the bilateral knee joints with pressure-associated pain, and limited capacity for squatting.
The laboratory tests revealed that liver function, biochemistry, blood lipid level, erythrocyte sedi
Radiographs of both hands revealed that multiple interphalangeal joints and the intercarpal joints of both hands were narrowed. In addition, the bone density of the constituent bones of the joints was reduced, cystic translucent areas were observed, and the left radial carpal joint was narrowed (Figure 2A). Knee MRI revealed a Grade 2 injury to the anterior and posterior angles of the medial and lateral menisci of the left knee; degeneration of the left knee, osteochondral injury to the patellofemoral articular surface; dotted film shadow of the synovial membrane around the left knee; possible synovitis; and effusion in the left knee joint cavity and suprapatellar bursa (Figure 2B). Chest CT revealed interstitial inflammation in both lungs; bilateral interlobular cleft nodules with recommended follow-up; fibrous foci in both lower lobes of the lungs; and cystic foci in the right subscapularis muscle (Figure 2C).
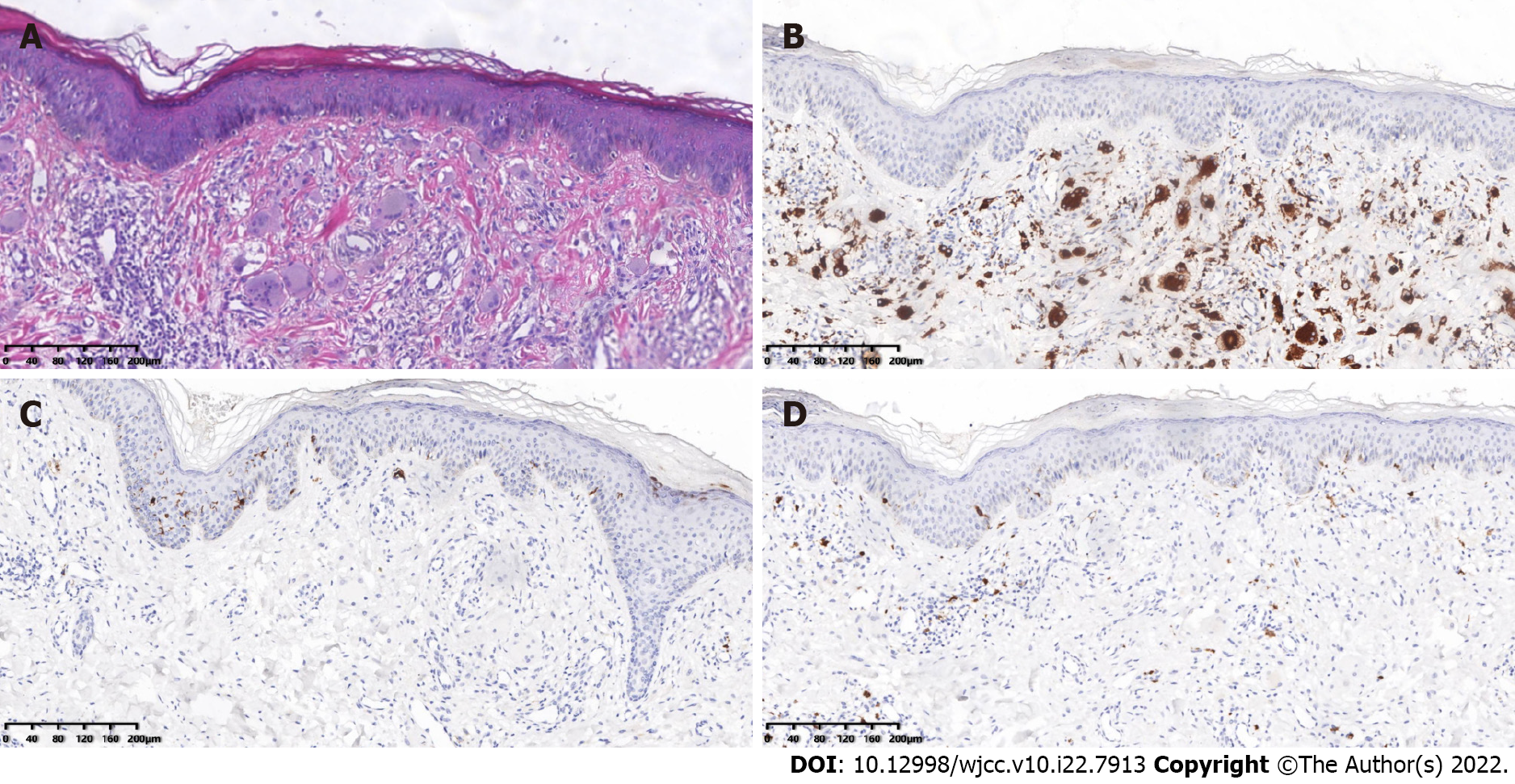
Microscopic, pathological analysis of a skin biopsy taken from the left upper back revealed that the epidermis was generally normal. The dermal papillae showed an increase in the number of histiocytes and multinucleated giant cells with abundant cell cytoplasm and hairy glass-like changes, with a little lymphatic and eosinophilic infiltration around them (Figure 3A). Immunohistochemistry showed CD68(+), CD1a(-), and S100(-) (Figure 3B-D). In an iliac bone aspiration biopsy, the morphological analysis of the bone marrow cells did not exhibit any significant abnormalities in the granulocyte-red-macro triad. The pathological examination revealed hematopoiesis. There was an approximately 40%:60% fat, granulocyte-red ratio < 2.5:1, Alip-, and scattered megakaryocyte lineage, with no obvious tumor component.
The final diagnosis of the presented case is MRH.
As treatment, the patient began taking 16 mg/d oral methylprednisolone tablets regularly on November 2020. The methylprednisolone was reduced to 12 mg/d during February 2021-April 2021, and methylprednisolone was reduced to 8 mg/d from May 2021 onwards. On 30 November and 15 December 2020, 0.4 g cyclophosphamide was intravenously administered once. On 29 December 2020 and 26 January 2021, 0.6 g cyclophosphamide was intravenously administered once. Since then, 0.8 g cyclophosphamide has been administered regularly every month, and blood count, liver, and kidney function have been monitored (Figure 4).
At present, skin lesions on both arms, abdomen, and back had subsided, while the skin lesions on the rest of the body had not increased elsewhere. The interphalangeal joints of both thumbs and bilateral knee joints remained swollen and painful. Thus, we recommend TNF-α antagonists as treatment for patients who are under consideration. The patient treatment and follow up are ongoing.
MRH is a rare multisystemic granulomatous disease and non-Langerhans histiocytosis. Papulonodular skin lesions and destructive arthritis are the two prominent features of MRH. MRH was first identified and reported by Weber and Frudenthal in 1937 and termed Multicentric reticulohistiocytosis by Goltz and Laymon in 1954[1]. While the pathogenesis of MRH remains unclear and may be related to the infiltration of a large number of macrophages in the skin and synovial tissues, it may also be associated with the differentiation of monocytes into osteoclasts, which ultimately leads to joint destruction[2]. To date, there have been approximately 300 cases of MRH reported worldwide. The data demonstrate that most patients are Caucasian and from western countries, whereas Asian patients are rare. The peak age at onset is 40-50 years old, with a male to female ratio of 1:3[3,4].
The skin is the most frequently involved site of MRH. Skin lesions may be the first symptom and are primarily distributed on the face, scalp, behind the ears, neck, anterior chest, back, waist, and abdo{Adamopoulos, 2006 #12}men, arms, hands, and thigh. The manifestations of skin lesions include papular nodules and macules. Papular nodules appear above the skin surface, are hemispherical in shape, hard in texture, millet to soy size, and are usually not accompanied by pruritus. The papular nodules can be of various colors, including skin color, red, and reddish-brown. The small papules are arranged in a linear "coral bead" pattern at the nail folds. In severe cases, facial lesions may have a "lion-like appearance"[5]. Macula is another form of skin lesions that manifest as brown-red and purplish red edematous spots, similar in morphology and distribution to dermatomyositis rash, which is occasionally accompanied by dermatomyositis, and can easily be misdiagnosed. In addition, MRH can also involve mucous membranes (e.g., oral mucosa, gingival mucosa, and laryngeal mucosa).
Arthritis is one of the predominant clinical symptoms of MRH and can appear as the first symptom of MRH, and can occur simultaneously with skin lesions. MRH-associated arthritis can be characterized as diffuse, symmetric, progressive, and destructive. MRH is most commonly involved in the hand joints, especially the distal interphalangeal joint, followed by the knee joint, the shoulder, elbow, hip, ankle, and metatarsophalangeal joint. The affected joints exhibit swelling, pain, an increase in skin temperature, joint effusion, and some patients can also experience morning stiffness. Joint erosion is characterized by the gradual progression from the joint edges to the entire joint surface, eventually leading to an enlargement of the joint cavity, loss of articular cartilage, and resorption of subchondral bone. Unlike other forms of inflammatory arthritis, MRH is mainly free of bone loss and abnormal new bone formation[1,6]. However, joint destruction progresses rapidly and can eventually lead to joint deformities, hypofunction, or loss. Without prompt and effective treatment, this destruction of the proximal interphalangeal and distal interphalangeal joints may result in “opera-glass hand”[1]. It has also been reported that some patients with severe hip destruction require hip arthroplasty[7]. Therefore, X-ray examination can play an important role in the early detection of MRH and in the screening of MRH from other types of arthritis.
MRH can involve additional organs. Patients with muscle involvement present with myalgia or decreased muscle strength. Electromyography may exhibit myogenic lesions in some patients[3]. Patients with lung involvement exhibited dyspnea, pulmonary nodules, pleural effusion, or pulmonary interstitial fibrosis[8]. Patients with heart involvement have been reported as having pericardial effusion and myocarditis[9]. Patients with laryngeal involvement exhibit hoarseness, foreign body sensation in the larynx, or laryngeal abnormalities[4]. In addition, patients with thyroid involvement showed hypothyroidism[10]. Thrombosis has also been reported in patients with MRH[11,12]. A small number of patients have extensive systemic involvement of the larynx, lungs, spleen, and plasma membranes at the same time. In addition to organ involvement, MRH may also present with systemic symptoms, including fatigue, fever, and weight loss.
The laboratory tests for patients with MRH are nonspecific. Some patients may have increased leukocytes, decreased hemoglobin, accelerated sedimentation, and elevated lipids. Positivity for rheumatoid factor, anti-cyclic citrullinated antibodies, and antinuclear antibodies is rare, except for the combination with a systemic autoimmune disease[13].
The diagnosis of MRH is primarily based on a biopsy of the skin or synovial tissue. The histopathological manifestations include a large number of histiocytes and multinucleated giant cells with an eosinophilic cytoplasm and hairy glass-like changes. Immunohistochemistry indicated that the macrophage marker CD68 is positive, which may be considered an essential criterion for MRH characterization. The Langerhans cell tissue markers S-100, CD1a and B cell markers CD19 and CD20 are negative. It has been reported that vimentin, CD45, CD43, Mac387, and lysozyme are positive in varying degrees in some patients[14,15].
Patients with MRH may also have a variety of systemic autoimmune diseases including systemic lupus erythematosus, rheumatoid arthritis, Sjogren's syndrome, dermatomyositis, hypothyroidism, diabetes, and tuberculosis[10,11,16,17]. It is important to note that MRH can be associated with malignancies and the combination rate can be as high as 25%, covering almost all solid tumors and hematologic malignant diseases[3,18]. It has been reported that some patients have also experienced remission of the skin and joint symptoms following tumor treatment; thus, MRH is considered to be a paraneoplastic syndrome[19]. While this view remains controversial, clinicians should be on alert for the presence of mali
MRH is prone to be misdiagnosed as a systemic autoimmune disease due to similar skin and joint symptoms. MRH is also misdiagnosed as it often coexists with systemic autoimmune diseases, including rheumatoid arthritis, dermatomyositis, Sjogren's syndrome, fibroblastic rheumatism, and systemic lupus erythematosus. The diseases most commonly confused with MRH include rheumatoid arthritis and dermatomyositis. Here, we will summarize the key points that distinguish MRH from these two diseases based on relevant literature and clinical experience. Unlike rheumatoid arthritis, joint destruction in MRH is rapid, and the distal interphalangeal joints can be involved during the early stages. MRH is mostly free of bone loss and abnormal new bone formation[1] and synovial biopsy can effectively distinguish MRH from rheumatoid arthritis (Table 1). Unlike dermatomyositis, the main skin lesions in MRH are papular nodules and macules, whereas Gottron papules, heliotrope rash, Gottron signs, erythema along the light site, heterochromia, nail fold changes, scalp involvement, and skin calcification are observed in dermatomyositis. The histopathological manifestations of MRH consist of a large number of histiocytes and multinucleated giant cells with an eosinophilic cytoplasm and hairy glass-like changes. In contrast, the histopathological manifestations of dermatomyositis are dominated by the sporadic or focal infiltration of lymphocytes, plasma cells, and histiocytes. Moreover, the musculoskeletal symptoms of MRH are featured as arthritis, whereas proximal muscle weakness is observed in dermatomyositis[20,21] (Table 2).
| MRH | RA | |
| Joint involvement | Hand joint, knee joint, the shoulder, elbow, hip, ankle, and metatarsophalangeal joint | Double hand joint, wrist joint, foot joint, etc. |
| The distal interphalangeal joints involvement is frequent | The distal interphalangeal joints involvement is rare | |
| Rate of joint destruction | Rapid | Slow |
| Radiologic characteristics | Enlargement of joint cavity | Periarticular osteopenia |
| Loss of articular cartilage and resorption of subchondral bone; no bone loss and abnormal new bone formation | Narrowing of the joint space, and bone erosion |
| DM | MRH | |
| Amyasthenia | More than 90% showed a symmetrical proximal muscle weakness | Rare |
| Increased myoenzyme | Almost all patients with DM (except CADM) have at least one myoenzyme level at some point in the disease course | Rare |
| Skin manifestations | Gottron papules and heliotrope rash are the definitive features of DM; Gottron signs, erythema along the light site, heterochromia, nail fold changes, scalp involvement, and skin calcification are also typical manifestations of DM | The manifestations of skin lesions include popular nodules and macules. Skin lesions are primarily distributed on the face, scalp, behind the ears, neck, anterior chest, back, waist, and abdomen, arms, hands, and thigh |
| Skin biopsy | The sporadic or focal infiltration of lymphocytes, plasma cells, and histiocytes | A large number of histiocytes and multinucleated giant cells with an eosinophilic cytoplasm and hairy glass-like changes |
There is currently no consensus regarding the treatment of MRH, which mainly consists of empirical and individualized therapy. The initial treatment of MRH patients primarily consists of glucocorticoids combined with immunosuppressants regimens. Commonly used immunosuppressants include methotrexate, cyclophosphamide, hydroxychloroquine, and leflunomide. These combination regimens provide varying degrees of relief for the skin lesions and early joint symptoms; however, it is difficult to control joint erosion effectively. It has been reported that bisphosphonates can decrease monocyte/ macrophage-osteoclast differentiation, promote osteoclastic apoptosis, and inhibit osteoclastic resorptive activity. Moreover, bisphosphonates are potent inhibitors of osteoclast activity and can effectively prevent joint erosion[22,23]. Bisphosphonates have also been reported to be effective for both skin and joint symptoms. The mechanism by which bisphosphonates improve skin symptom remains unclear.
As the study progressed, it was found that pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-12) were overexpressed in the MRH skin lesions and levels of TNF-α, IL-1β, IL-6, and IL-8 were also elevated. However, their expression levels in the serum were significantly increased after symptom relief by treatment[24]. Based on these theories, biological agents have been used for the treatment of MRH. The most commonly used biological agents are TNF-α antagonists (e.g., etanercept, adalimumab, and infliximab), IL-6 receptor antagonists (tocilizumab), and IL-1 receptor antagonists (anakinra)[25,26]. The effects of biological agents on the relief of skin and joint symptoms varied greatly among individuals. In addition, it is difficult to assess the superiority of one biological agent over another due to the low number of case reports. It has been reported that biological agents may be taken into consideration when first-line treatments are unable to achieve any effective disease control within 4–6 wk[27]. However, additional prospective investigations are required to standardize their application, including dosage, frequency, duration, and side effects.
MRH is a rare non-Langerhans histiocytosis of unknown etiology with the characteristic clinical features of papulonodular skin lesions and progressive, destructive arthritis. MRH can also co-exist or be confused with a variety of systemic autoimmune diseases. Moreover, MRH may also be associated with various types of malignancies. Laboratory tests are not specific and the diagnosis is primarily based on skin or synovial tissue biopsies. Histopathological analyses revealed a large number of histiocytes and multinucleated giant cells with an eosinophilic cytoplasm and hairy glass-like changes. The immunohistochemical analysis indicates that the samples were positive for the macrophage marker, CD68, but negative for the Langerhans cell tissue markers, S-100, CD1a, as well as B cell markers CD19 and CD20. Most initial treatment for MRH consists of a combined regimen of glucocorticoids and immunosuppressants. Recently, while biological agents have been gradually used to treat MRH, an evaluation of the long-term efficacy and safety requires further evaluation. This case is representative of MRH and we also summarized the characteristics of MRH (Table 3) compared to common differential diagnoses (e.g., rheumatoid arthritis and dermatomyositis). We hope that our work will help clinicians better identify and treat this disease in the absence of epidemiological studies or randomized controlled data. The incidence of MRH is higher in Europe than in Asia according to existing reports; however, no significant differences have been found regarding clinical features, and prognosis between the two regions. Therefore, we will continue to pay attention to this disease in the future.
| Clinical manifestation | |
| Skin lesions | The skin is the most frequently involved site of MRH. Skin lesions may be the first symptom and are primarily distributed on the face, scalp, behind the ears, neck, anterior chest, back, waist, and abdomen, arms, hands, and thigh. The manifestations of skin lesions include papular nodules, and macules. MRH can also involve mucous membranes (e.g., oral mucosa, gingival mucosa, and laryngeal mucosa) |
| MRH-associated arthritis | Arthritis can appear as the first symptom of MRH and also can occur simultaneously with skin lesions. MRH-associated arthritis can be characterized as diffuse, symmetric, progressive, and destructive. MRH is most commonly involved in the hand joints, especially the distal interphalangeal joint, followed by the knee joint, the shoulder, elbow, hip, ankle, and metatarsophalangeal joint. The affected joints exhibit swelling, pain, increase in skin temperature, joint effusion, and some patients can also experience morning stiffness |
| Other clinical manifestations | Muscle involvement: Myalgia or decreased muscle strength; Electromyography (EMG) may exhibit myogenic lesions in some patients; Lung involvement: Dyspnea, pulmonary nodules, pleural effusion, or pulmonary interstitial fibrosis; Heart involvement: Pericardial effusion and myocarditis; Laryngeal involvement: Hoarseness, foreign body sensation in the larynx, or laryngeal abnormalities; Thyroid involvement: Hypothyroidism; Thrombosis has also been reported in patients with MRH. A small number of patients have extensive systemic involvement of the larynx, lungs, spleen, and plasma membranes at the same time. Systemic symptoms: Fatigue, fever, and weight loss |
| Laboratory tests | |
| Non-specific. Some patients may have increased leukocytes, decreased hemoglobin, accelerated sedimentation, and elevated lipids | |
| Histopathology | |
| Histopathological manifestations: a large number of histiocytes and multinucleated giant cells with an eosinophilic cytoplasm and hairy glass-like changes | |
| Immunohistochemistry: macrophage marker CD68 is positive; the Langerhans cell tissue markers S-100, CD1a and B cell markers CD19 and CD20 are negative; CD45, CD43, Mac387, and lysozyme are positive to varying degrees | |
| MRH-associated diseases | |
| Systemic autoimmune diseases: systemic lupus erythematosus, rheumatoid arthritis, Sjogren's syndrome, dermatomyositis, hypothyroidism, diabetes, and tuberculosis | |
| Malignancy: Covering almost all solid tumors and hematologic malignant diseases | |
| Differential diagnosis | |
| MRH is most easily confused with rheumatoid arthritis, dermatomyositis, and psoriatic arthritis | |
| Treatment | |
| Initial treatment: Glucocorticoids combined with immunosuppressants regimens. Commonly used immunosuppressants include methotrexate, cyclophosphamide, hydroxychloroquine, and leflunomide | |
| Biological agents: TNF-α antagonists (e.g., etanercept, adalimumab, and infliximab), IL-6 receptor antagonists (tocilizumab), and IL-1 receptor antagonists (anakinra) | |
| Prognosis | |
| The prognosis of MRH patients varies greatly among individuals; It is related to treatment choice, response to drug therapy, and comorbidities | |
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rheumatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jatuworapruk K, Thailand A-Editor: Lin FY S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Tajirian AL, Malik MK, Robinson-Bostom L, Lally EV. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Adamopoulos IE, Wordsworth PB, Edwards JR, Ferguson DJ, Athanasou NA. Osteoclast differentiation and bone resorption in multicentric reticulohistiocytosis. Hum Pathol. 2006;37:1176-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Selmi C, Greenspan A, Huntley A, Gershwin ME. Multicentric reticulohistiocytosis: a critical review. Curr Rheumatol Rep. 2015;17:511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Barrow MV, Holubar K. Multicentric reticulohistiocytosis. A review of 33 patients. Medicine (Baltimore). 1969;48:287-305. [PubMed] |
| 5. | Lu YY, Lu CC, Wu CH. Leonine facies in the cutaneous form of multicentric reticulohistiocytosis. Intern Med. 2012;51:2069-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Gold RH, Metzger AL, Mirra JM, Weinberger HJ, Killebrew K. Multicentric reticulohistiocytosis (lipoid dermato-arthritis). An erosive polyarthritis with distinctive clinical, roentgenographic and pathologic features. Am J Roentgenol Radium Ther Nucl Med. 1975;124:610-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Saibaba B, Sen RK, Das A, Sharma A. Bilateral Total Hip Arthroplasty in a Rare Case of Multicentric Reticulohistiocytosis. Clin Orthop Surg. 2015;7:509-514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Bogle MA, Tschen JA, Sairam S, McNearney T, Orsak G, Knox JM. Multicentric reticulohistiocytosis with pulmonary involvement. J Am Acad Dermatol. 2003;49:1125-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Yee KC, Bowker CM, Tan CY, Palmer RG. Cardiac and systemic complications in multicentric reticulohistiocytosis. Clin Exp Dermatol. 1993;18:555-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Finelli LG, Tenner LK, Ratz JL, Long BD. A case of multicentric reticulohistiocytosis with thyroid involvement. J Am Acad Dermatol. 1986;15:1097-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Toz B, Büyükbabani N, İnanç M. Multicentric reticulohistiocytosis: Rheumatology perspective. Best Pract Res Clin Rheumatol. 2016;30:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Rentsch JL, Martin EM, Harrison LC, Wicks IP. Prolonged response of multicentric reticulohistiocytosis to low dose methotrexate. J Rheumatol. 1998;25:1012-1015. [PubMed] |
| 13. | Trotta F, Colina M. Multicentric reticulohistiocytosis and fibroblastic rheumatism. Best Pract Res Clin Rheumatol. 2012;26:543-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Luz FB, Gaspar AP, Ramos-e-Silva M, Carvalho da Fonseca E, Villar EG, Cordovil Pires AR, Kalil-Gaspar N. Immunohistochemical profile of multicentric reticulohistiocytosis. Skinmed. 2005;4:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Gorman JD, Danning C, Schumacher HR, Klippel JH, Davis JC Jr. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. 2000;43:930-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Saito K, Fujii K, Awazu Y, Nakayamada S, Fujii Y, Ota T, Tanaka Y. A case of systemic lupus erythematosus complicated with multicentric reticulohistiocytosis (MRH): successful treatment of MRH and lupus nephritis with cyclosporin A. Lupus. 2001;10:129-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Morris-Jones R, Walker M, Hardman C. Multicentric reticulohistiocytosis associated with Sjögren's syndrome. Br J Dermatol. 2000;143:649-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Hinchman KF, Wu JJ, Soden CE Jr, Waldman J, Dyson SW. Multicentric reticulohistiocytosis associated with Burkitt lymphoma and adenocarcinoma. Cutis. 2008;82:113-114. [PubMed] |
| 19. | Islam AD, Naguwa SM, Cheema GS, Hunter JC, Gershwin ME. Multicentric reticulohistiocytosis: a rare yet challenging disease. Clin Rev Allergy Immunol. 2013;45:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Baek IW, Yoo SH, Yang H, Park J, Kim KJ, Cho CS. A case of multicentric reticulohistiocytosis. Mod Rheumatol. 2017;27:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Hsiung SH, Chan EF, Elenitsas R, Kolasinski SL, Schumacher HR, Werth VP. Multicentric reticulohistiocytosis presenting with clinical features of dermatomyositis. J Am Acad Dermatol. 2003;48:S11-S14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Broadwell AW, Calamia KT, Kransdorf MJ, Ginsburg WW. Healing of erosive disease in multicentric reticulohistiocytosis. J Rheumatol. 2010;37:1366-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Goto H, Inaba M, Kobayashi K, Imanishi Y, Kumeda Y, Inui K, Okada F, Nishizawa Y. Successful treatment of multicentric reticulohistiocytosis with alendronate: evidence for a direct effect of bisphosphonate on histiocytes. Arthritis Rheum. 2003;48:3538-3541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Bennàssar A, Mas A, Guilabert A, Julià M, Mascaró-Galy JM, Herrero C. Multicentric reticulohistiocytosis with elevated cytokine serum levels. J Dermatol. 2011;38:905-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Zhao H, Wu C, Wu M, Zhou Y, Zhu H, Li Y, You Y, Luo H, Wang L, Zuo X. Tumor necrosis factor antagonists in the treatment of multicentric reticulohistiocytosis: Current clinical evidence. Mol Med Rep. 2016;14:209-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Pacheco-Tena C, Reyes-Cordero G, Ochoa-Albíztegui R, Ríos-Barrera V, González-Chávez SA. Treatment of multicentric reticulohistiocytosis with tocilizumab. J Clin Rheumatol. 2013;19:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Tariq S, Hugenberg ST, Hirano-Ali SA, Tariq H. Multicentric reticulohistiocytosis (MRH): case report with review of literature between 1991 and 2014 with in depth analysis of various treatment regimens and outcomes. Springerplus. 2016;5:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |