Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.7859
Peer-review started: November 10, 2021
First decision: December 3, 2021
Revised: December 4, 2021
Accepted: July 5, 2021
Article in press: July 5, 2022
Published online: August 6, 2022
Processing time: 253 Days and 19.2 Hours
Acute pancreatitis is the most common and severe complication of endoscopic retrograde cholangiopancreatography (ERCP). Recent evidence suggests that combinations based on rectal nonsteroidal anti-inflammatory drugs (NSAIDs) are more beneficial in preventing post-ERCP pancreatitis (PEP). Randomized controlled trials (RCTs) have also demonstrated the efficacy of glyceryl trinitrate (GTN). We conducted a network meta-analysis to compare NSAIDs and GTN for prevention of PEP and to determine whether they are better in combination.
To compare NSAIDs and GTN for prevention of PEP and to determine whether they are better in combination.
A systematic search was done for full-text RCTs of PEP in PubMed, Embase, Science Citation Index, and the Cochrane Controlled Trials database. Inclusion and exclusion criteria were used to screen for eligible RCTs. The major data were extracted by two independent reviewers. The frequentist model was used to conduct this network meta-analysis and obtain the pairwise OR and 95%CI. The data were then extracted and assessed on the basis of the
Twenty-four eligible RCTs were selected, evaluating seven preventive strategies in 9416 patients. Rectal indomethacin 100 mg plus sublingual GTN (OR: 0.21, 95%CI: 0.09–0.50), rectal diclofenac 100 mg (0.34, 0.18–0.65), sublingual GTN (0.34, 0.12–0.97), and rectal indomethacin 100 mg (0.49, 0.33–0.73) were all more efficacious than placebo in preventing PEP. The combination of rectal indomethacin and sublingual GTN had the highest surface under the cumulative ranking curves (SUCRA) probability of (92.2%) and was the best preventive strategy for moderate-to-severe PEP with a SUCRA probability of (89.2%).
Combination of rectal indomethacin 100 mg with sublingual GTN offered better prevention of PEP than when used alone and could alleviate the severity of PEP.
Core tip: Post-endoscopic retrograde cholangiopancreatography pancreatitis (PEP) is a common and serious complication. Several prophylactic measures have been tried. Some guidelines recommend rectal administration of 100 mg diclofenac or indomethacin as routine PEP prophylaxis. glyceryl trinitrate (GTN) has been reported as an effective drug for preventing PEP. In view of some high-quality randomized controlled trials, we conducted this network meta-analysis to compare nonsteroidal anti-inflammatory drugs and GTN for prevention of PEP and to determine whether they are better in combination. Our analysis showed that combination of rectal indomethacin 100 mg with sublingual GTN was the most effective strategy for preventing PEP and reducing its severity.
- Citation: Shi QQ, Huang GX, Li W, Yang JR, Ning XY. Rectal nonsteroidal anti-inflammatory drugs, glyceryl trinitrate, or combinations for prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis: A network meta-analysis. World J Clin Cases 2022; 10(22): 7859-7871
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/7859.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.7859
Endoscopic retrograde cholangiopancreatography (ERCP) is a widely used tool for diagnosing and treating biliary and pancreatic diseases. Despite technological advances and improved operator experience, ERCP has a high potential for complications, such as acute pancreatitis, bleeding, per
Over the past few decades, several prophylactic measures have been explored to solve this thorny problem. These include the placement of pancreatic stents, intravenous fluids, and several pharmacological options[4,5]. Some guidelines recommend rectal administration of 100 mg diclofenac or indomethacin as routine PEP prophylaxis in unselected patients. Its efficacy and safety have been confirmed repeatedly[6]. Nevertheless, increasingly, studies have focused on combination therapy involving nonsteroidal anti-inflammatory drugs (NSAIDs) to investigate whether this might be more effective than NSAIDs alone[5,7].
A meta-analysis has confirmed that glyceryl trinitrate (GTN), an inexpensive and easily administered agent, effectively prevents PEP[8]. It has been suggested that a combination of GTN and NSAIDs may be more effective[9]. Therefore, we conducted a network meta-analysis of RCTs to compare the direct and indirect evidence and identify their effectiveness in preventing PEP.
A comprehensive search was conducted independently by two review authors (Shi QQ and Ning XY). The following databases were searched: PubMed, Embase, Science Citation Index, and the Cochrane Controlled Trials, from initiation to September 10, 2021. The search terms included “pancreatitis” and “cholangiopancreatography, Endoscopic retrograde” or “Endoscopic retrograde cholangiopancreatography” or “ERCP” and “random or randomized controlled trial” or “RCT”. The terms were limited to “title and abstract” and filtered with “human”. Only articles published in English were selected. The reference lists of related systematic reviews or meta-analyses were manually searched to avoid omitting eligible studies.
The inclusion criteria were as follows: (1) RCTs published in full text and English, irrespective of whether double-blind; (2) Patients were subjected to ERCP and administration of rectal NSAIDs, sublingual GTN, or transdermal GTN to prevent PEP; and (3) Incidence of PEP was the primary outcome, and the definition of PEP was explicit. We excluded conference proceedings or abstracts, except where the complete information was available from the authors. We also excluded studies without a record of PEP.
The following data were extracted by two independent investigators (Shi QQ and Ning XY) from eligible RCTs using a common data form: first author, year of publication, country of origin, patient characteristics (ratio of men to women, age distribution), details of intervention and control, PEP definition, PEP severity criteria, sample size, and the incidence of PEP and its severity. The type, dose, route, and timing of medication were also extracted. Any conflicts were resolved through discussion or consultation with a third reviewer (Yang JR). The data were then extracted and assessed on the basis of the
The Cochrane Risk of Bias Assessment Tool was used by two authors to independently evaluate the risk of bias of individual studies (Li W and Huang GX)[10]. The assessment included the following items: Random sequence generation, allocation concealment, blinding of the participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and additional potential practices. Any disagreements were resolved through discussion or consultation with a third reviewer (Yang JR).
This network meta-analysis was undertaken with a Frequentist model using the mumeta and network commands in STATA version 16.0. The pairwise meta-analysis and network meta-analysis were undertaken simultaneously with the random effect model. The OR and 95%CI were used to describe dichotomous outcomes, and the global and local inconsistencies were checked. I2 was used to describe the heterogeneity, where < 50% indicated low heterogeneity and > 50% high heterogeneity. P < 0.05 represented statistical significance. The loop-specific inconsistency was used to assess the discordance between direct and indirect evidence in the loop. If the 95%CI of inconsistency factors included zero or RoR included 1, inconsistency results were considered nonsignificant. The network graph was used to present the treatment comparisons. Interventions were ranked by their posterior probability by the surface under the cumulative ranking curve values.
There was no funding source for this study.
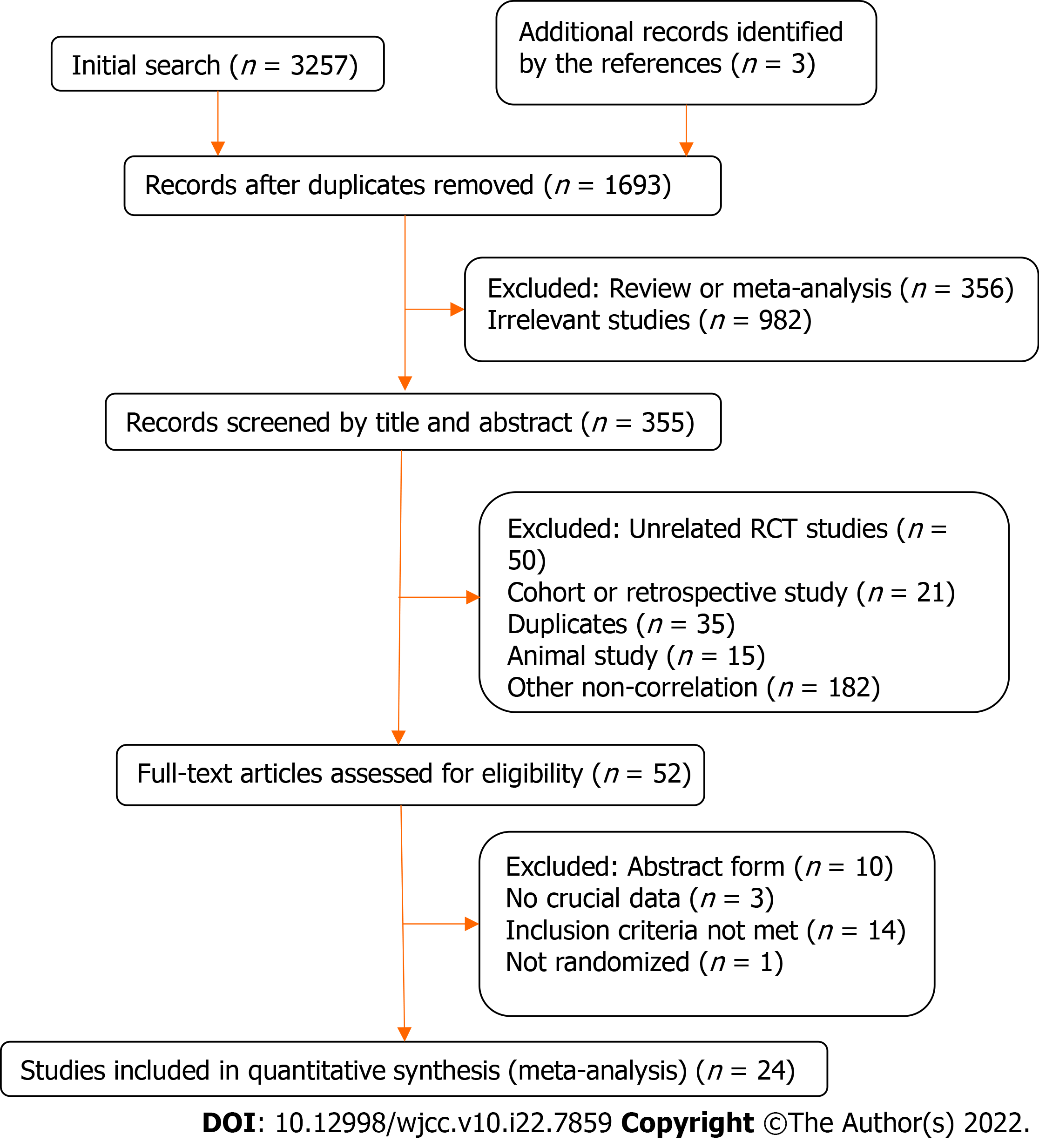
The literature search yielded 3260 titles, of which 2905 articles were excluded because they were duplicates, systematic reviews or meta-analyses, or not relevant. Of the remaining 355 articles, 52 were screened out by scanning the titles and abstracts (Figure 1). Eventually, 24 RCTs (including 9416 patients) were included in this network meta-analysis. Sixteen RCTs involved NSAIDs[11-26] and eight were of GTN[9,27-33]. Two different studies had the same first author[12,33], and both of them were included. One study stratified the patients based on pancreatitis risk after ERCP[21]. In the treatment group, the average-risk patients only received 100 mg of rectal indomethacin before ERCP, but the high-risk patients received a further 100 mg of rectal indomethacin after ERCP. Therefore, we only extracted the data of the average-risk patients. One study only included female patients[9], but the baselines between the experimental and control groups were similar, so we included it.
The main characteristics and the incidence and severity of PEP are presented in Table 1 and Table 2. Among the RCTs that met the inclusion criteria, the first study was published in 2001, and the most recent was in 2020. The sample size ranged from 74 to 2014 subjects. The proportion of women in the RCTs ranged from 37.74% to 100%. A total of 9416 patients were randomly assigned to one of seven different interventions or placebo. The interventions included NSAIDs (100 mg diclofenac, indomethacin, 50 mg diclofenac, naproxen), GTN (sublingual or transdermal), or a combination (indomethacin plus sublingual GTN). The definition and the degree of severity of PEP varied among the included studies, but most of them (66.67%) used the consensus definition[34], with the others using similar definitions. The incidence of PEP was reported in all studies, but four RCTs have no report about the degree of PEP[13,25,27,30].
| Ref. | Country | Intervention | Sample size |
| Murray et al[11], 2003 | Scotland | 100 mg diclofenac after endoscopy | 220 |
| Sotoudehmanesh et al[12], 2007 | Iran | 100 mg indomethacin before ERCP | 490 |
| Khoshbaten et al[13], 2007 | Iran | 100 mg diclofenac after endoscopy | 100 |
| Elmunzer et al[14], 2012 | United States | 100 mg indomethacin after ERCP | 602 |
| Otsuka et al[15], 2012 | Japan | 50 mg diclofenac before ERCP | 104 |
| Döbrönte et al[16], 2014 | Hungary | 100 mg indomethacin 10-15 min before ERCP | 665 |
| Andrade-Dávila et al[17], 2015 | México | 100 mg indomethacin after ERCP | 166 |
| Lua et al[18], 2015 | Malaysia | 100 mg diclofenac after ERCP | 144 |
| Patai et al[19], 2015 | Hungary | 100 mg indomethacin 1 h before ERCP | 539 |
| Levenick et al[20], 2016 | United States | 100 mg indomethacin following attempted cannulation | 449 |
| Luo et al[21], 2016 | China | 100 mg indomethacin within 30 min before ERCP | 2014 |
| Mansour-Ghanaei et al[22], 2016 | Iran | 500 mg naproxen immediately before ERCP | 324 |
| Patil et al[23], 2016 | India | 100 mg diclofenac immediately before or during the ERCP | 400 |
| Mohammad et al[24], 2017 | Iran | 100 mg diclofenac, 100 mg indomethacin or 500 mg naproxen, 30 min before ERCP | 246 |
| Li et al[25], 2019 | China | 100 mg indomethacin before ERCP | 100 |
| Katoh et al[26], 2019 | Japan | 50 mg diclofenac before ERCP | 297 |
| Sudhindran et al[27], 2001 | United Kingdom | Sublingual 2 mg GTN before ERCP | 186 |
| Moretó et al[28], 2003 | Spain | Transdermal 15 mg GTN 30 to 40 minutes before ERCP | 144 |
| Kaffes et al[29], 2006 | Australia | Transdermal 5 mg GTN before ERCP | 318 |
| Hao et al[30], 2009 | China | Sublingual 5 mg GTN 5 min before ERCP | 74 |
| Nøjgaard et al[31], 2009 | France | Transdermal 15 mg GTN before ERCP | 806 |
| Bhatia et al[32], 2011 | India | Transdermal GTN 30 min before ERCP | 250 |
| Sotoudehmanesh et al[33], 2014 | Iran | 100 mg indomethacin, plus 5 mg of sublingual GTN before ERCP | 300 |
| Wang et al[9], 2020 | China | Indomethacin plus 0.5 mg of sublingual GTN 5 min before ERCP | 352 |
| Ref. | Group | Case (n) | PEP | Sex (M:F) | Age (yr) | |
| Mild PEP | Moderate to serve PEP | |||||
| Murray et al[11], 2003 | Diclofenac 100 mg | 110 | 7 | 0 | NA | NA |
| Placebo | 110 | 15 | 2 | NA | NA | |
| Sotoudehmanesh et al[12], 2007 | Indomethacin | 245 | 7 | 0 | 111:134 | 58.4 ± 17.1 |
| Placebo | 245 | 10 | 5 | 115:130 | 58.4 ± 16.8 | |
| Elmunzer et al[14], 2012 | Indomethacin | 295 | 14 | 13 | 66:29 | 44.4 ± 13.5 |
| Placebo | 307 | 25 | 27 | 60:47 | 46.0 ± 13.1 | |
| Otsuka et al[15], 2012 | Diclofenac 50 mg | 51 | 2 | 0 | 20:31 | 75 |
| Placebo | 53 | 7 | 3 | 33:20 | 72 | |
| Döbrönte et al[16], 2014 | Indomethacin | 347 | 16 | 4 | 133:214 | 65.66 ± 16.21 |
| Placebo | 318 | 18 | 4 | 106:212 | 67.68 ± 15.56 | |
| Andrade-Dávila et al[17], 2015 | Indomethacin | 82 | 3 | 1 | 31:51 | 51.59 ± 18.55 |
| Placebo | 84 | 14 | 4 | 25:59 | 54.0 ± 17.85 | |
| Lua et al[18], 2015 | Diclofenac 100 mg | 69 | 4 | 3 | 34:35 | 50.3 ± 17.6 |
| Placebo | 75 | 4 | 0 | 25:50 | 49.6 ± 16.8 | |
| Patai et al[19], 2015 | Indomethacin | 270 | 15 | 3 | 89:181 | 66.25 (23-100) |
| Placebo | 269 | 33 | 4 | 88:181 | 64.51 (20-95) | |
| Levenick et al[20], 2016 | Indomethacin | 223 | 16 | 0 | 105:118 | 64.9 |
| Placebo | 226 | 9 | 2 | 108:118 | 64.3 | |
| Luo et al[21], 2016 | Indomethacin | 992 | 22 | 7 | NA | NA |
| Placebo | 1022 | 48 | 17 | NA | NA | |
| Mansour-Ghanaei et al[22], 2016 | Naproxen | 162 | 8 | 4 | 84:78 | 46.3 ± 8.3 |
| Placebo | 162 | 18 | 10 | 89:73 | 44.7 ± 9.7 | |
| Patil et al[23], 2016 | Diclofenac 100 mg | 200 | 6 | 0 | 72:128 | 45.44 |
| Placebo | 200 | 14 | 9 | 77:23 | 47.86 | |
| Mohammad et al[24], 2017 | Diclofenac 100 mg | 124 | 2 | 3 | 58:66 | 56.5 ± 18.7 |
| Indomethacin | 122 | 3 | 4 | 57:65 | 58.0 ± 16.8 | |
| Naproxen | 126 | 7 | 12 | 60:66 | 54.8 ± 13.7 | |
| Katoh et al[26], 2019 | Diclofenac 50 mg | 147 | 7 | 1 | 82:65 | 74.3 ± 11.8 |
| Placebo | 150 | 4 | 1 | 95:55 | 74.0 ± 12.7 | |
| Moretó et al[28], 2003 | tra-GTN | 71 | 2 | 1 | 44:27 | 66.7 ± 2 |
| Placebo | 73 | 10 | 1 | 43:30 | 65.2 ± 2 | |
| Kaffes et al[29], 2006 | tra-GTN | 155 | 9 | 2 | 59:96 | 60 (47-72) |
| Placebo | 163 | 6 | 4 | 57:106 | 65 (54-75) | |
| Nøjgaard et al[31], 2009 | tra-GTN | 401 | 4 | 14 | 164:237 | 67(18-95) |
| Placebo | 405 | 9 | 20 | 168:237 | 65(19-96) | |
| Bhatia et al[32], 2011 | tra-GTN | 124 | 12 | 0 | 36:88 | 42 (18-76) |
| Placebo | 126 | 13 | 0 | 47:79 | 42.5 (19-90) | |
| Sotoudehmanesh et al[33], 2014 | Indomethacin+sub-GTN | 150 | 8 | 2 | 76:74 | 58.4 ± 17.8 |
| Placebo | 150 | 19 | 4 | 70:80 | 58.6 ± 17.5 | |
| Wang et al[9], 2020 | Indomethacin+sub-GTN | 176 | 5 | 4 | Female | 63.5 ± 14.4 |
| Placebo | 176 | 14 | 20 | Female | 66.87 ± 13 | |
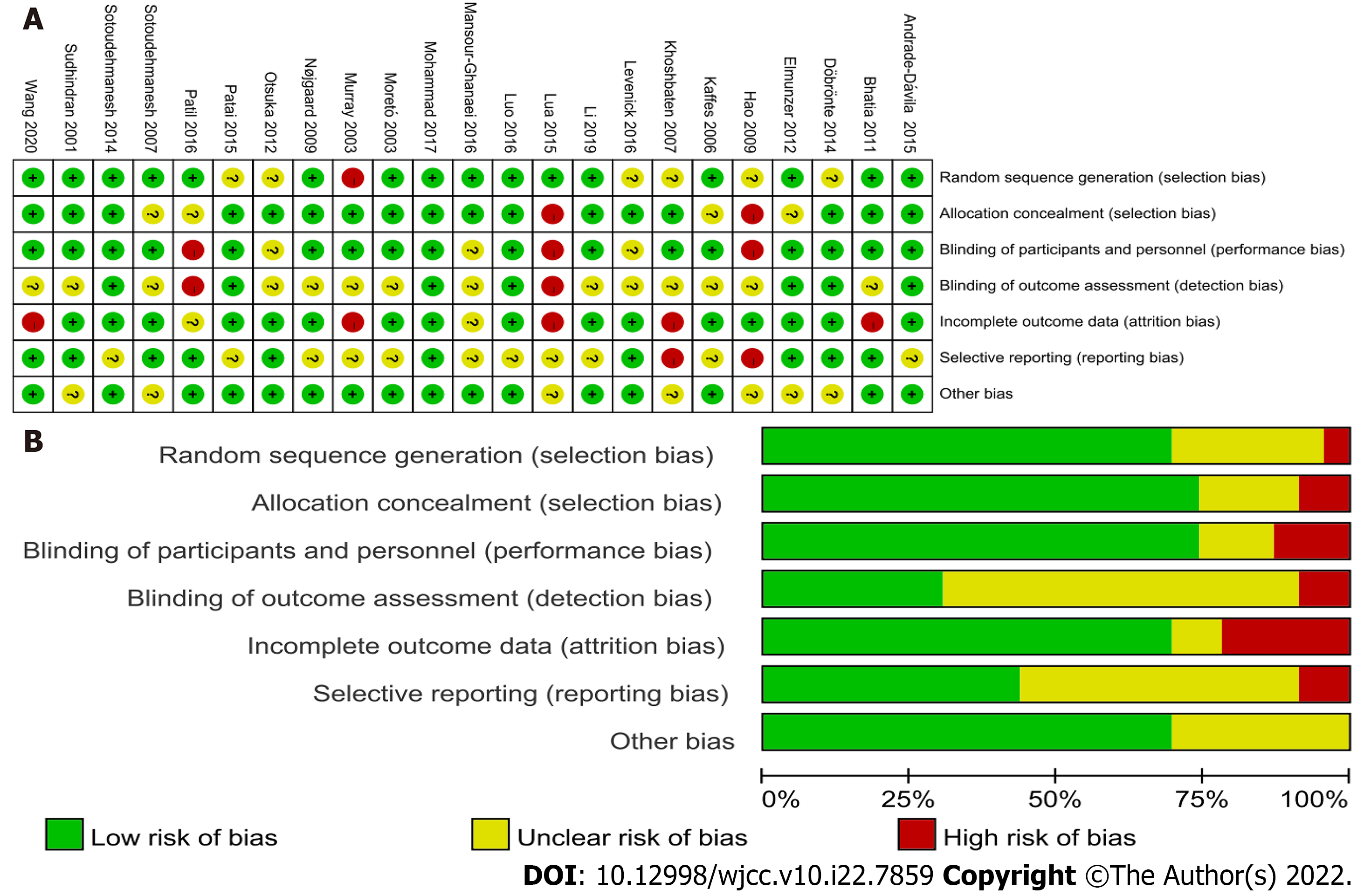
Two authors evaluated the methodological quality of the included RCTs using the Cochrane Collaboration’s Risk of Bias tool. A summary assessment of low, unclear, or high risk of bias was given to each study. The results are presented in Figure 2.
The inconsistency was not significant (I2 = 3.13%, P = 0.37) among the included RCTs, and no evidence of local or loop inconsistency was seen. A sensitivity analysis was conducted by excluding the studies with the largest (n = 2014) and smallest (n = 74) sample sizes. This slightly changed the OR and the SUCRA, indicating low heterogeneity (I2 = 2.47%, P = 0.48). The exclusion of two open-label studies[18,23] also did not change the final results.
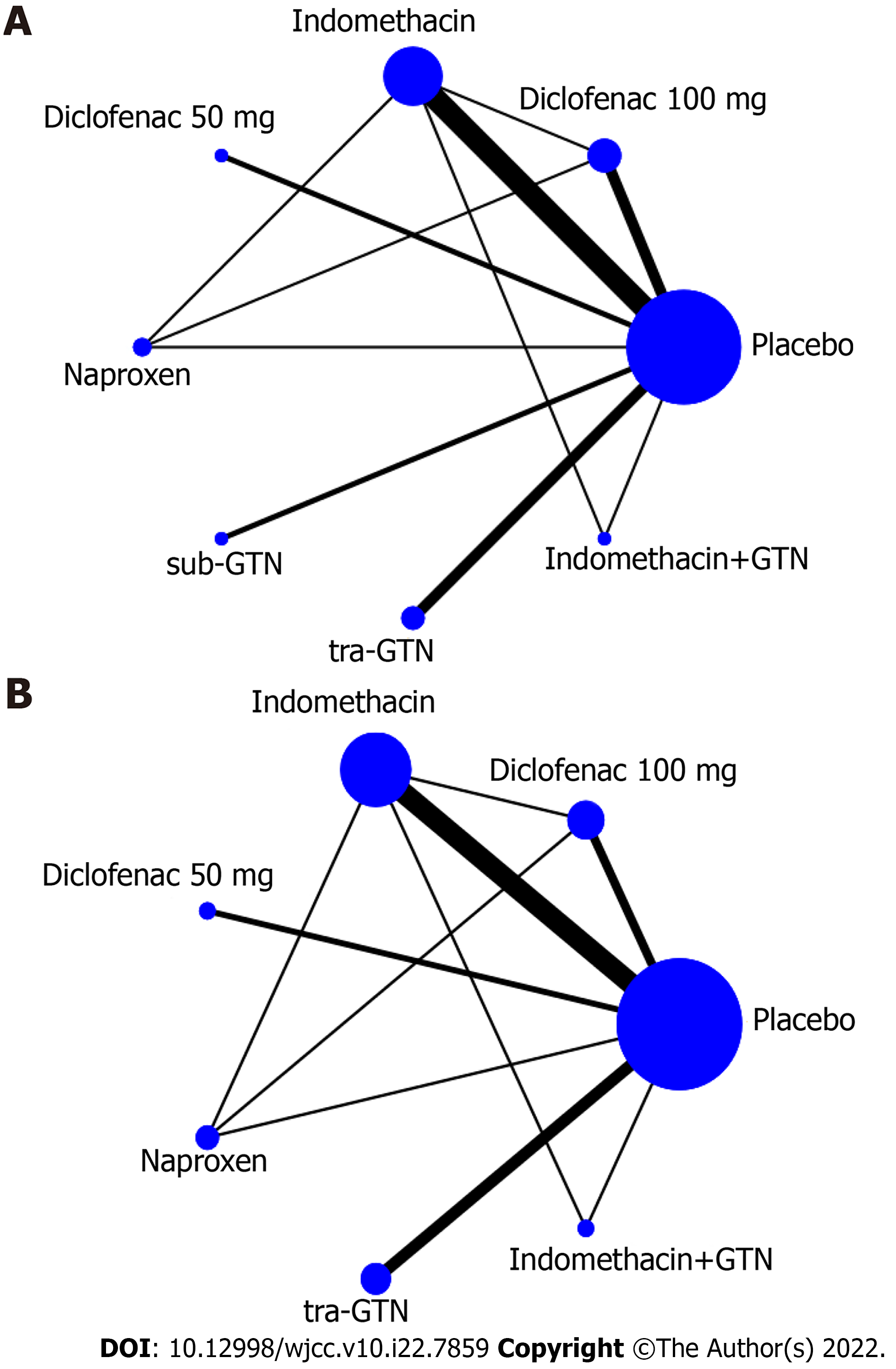
Figure 3A displays the network of all the interventions included in this network meta-analysis, and Figure 3B displays the network of interventions with details of the incidence of mild or moderate-severe PEP recorded. The network meta-analysis included one head-to-head three-arm RCT comparing different NSAIDs, one head-to-head two-arm RCT comparing combined indomethacin and sublingual GTN with indomethacin. All the others were placebo-controlled RCTs.
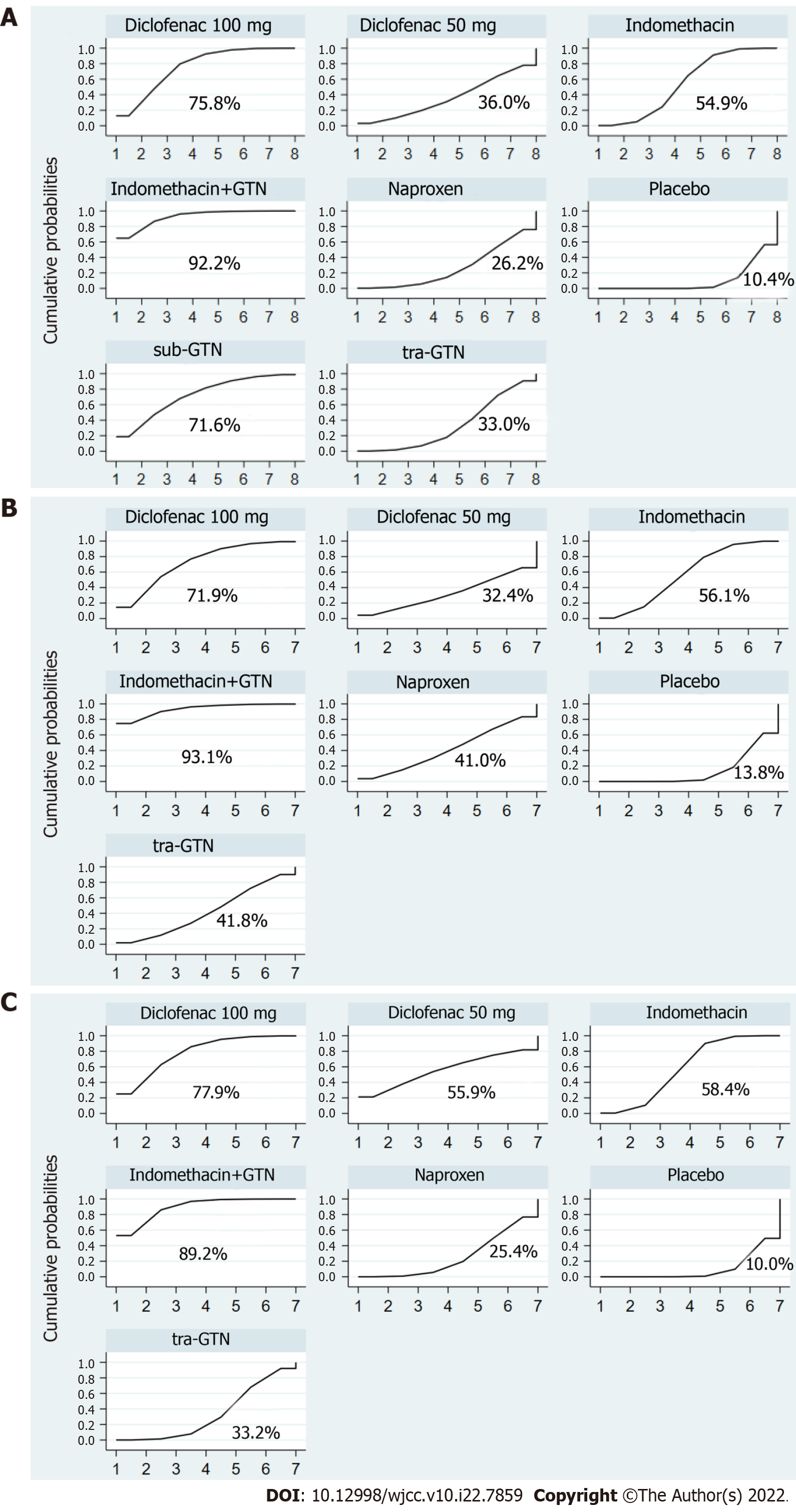
On pairwise comparison with placebo, rectal indomethacin 100 mg plus sublingual GTN (OR: 0.21, 95%CI: 0.09–0.50), rectal diclofenac 100 mg (0.34, 0.18–0.65), sublingual GTN (0.34, 0.12–0.97), and rectal indomethacin 100 mg (0.49, 0.33–0.73) were all more efficacious than placebo in preventing PEP. Rectal indomethacin 50 mg (0.69, 0.22–2.18), transdermal GTN (0.70, 0.37–1.32), rectal naproxen 500 mg (0.80, 0.35–1.83) were found to have no significant effect in preventing PEP (Table 3). Furthermore, the combination of rectal indomethacin 100 mg and sublingual GTN was more effective than rectal naproxen 500 mg (0.26, 0.08–0.86), and transdermal GTN (0.30, 0.10–0.89) in preventing PEP. As shown in Figure 4A, rectal diclofenac 100 mg performed best in the pairwise comparisons of prophylaxis between NSAIDs. Rectal indomethacin 100 mg ranked second. Regarding GTN, sublingual administration was more effective than transdermal in preventing PEP, but the combination achieved the best results.
| Indomethacin+GTN | Diclofenac 100 mg | sub-GTN | Indomethacin | Diclofenac 50 mg | tra-GTN | Naproxen | Pla |
| 0.62 (0.21, 1.82) | |||||||
| 0.61 (0.16, 2.38) | 0.99 (0.29, 3.37) | ||||||
| 0.42 (0.18, 1.02) | 0.69 (0.33, 1.43) | 0.69 (0.23, 2.10) | |||||
| 0.30 (0.07, 1.30) | 0.49 (0.13, 1.86) | 0.50 (0.11, 2.34) | 0.72 (0.21, 2.42) | ||||
| 0.30 (0.10, 0.89) | 0.49 (0.20, 1.22) | 0.49 (0.15, 1.66) | 0.71 (0.33, 1.50) | 0.99 (0.26, 3.68) | |||
| 0.26 (0.08, 0.86) | 0.43 (0.17, 1.10) | 0.43 (0.11, 1.62) | 0.62 (0.26, 1.47) | 0.86 (0.21, 3.58) | 0.87 (0.30, 2.50) | ||
| 0.21 (0.09, 0.50) | 0.34 (0.18, 0.65) | 0.34 (0.12, 0.97) | 0.49 (0.33, 0.73) | 0.69 (0.22, 2.18) | 0.70 (0.37, 1.32) | 0.80 (0.35, 1.83) |
On pairwise comparison with placebo, rectal indomethacin 100 mg plus GTN (0.27, 0.11–0.67), rectal diclofenac 100 mg (0.46, 0.23–0.94), rectal indomethacin 100 mg (0.59, 0.40–0.88) were all more efficacious than placebo in preventing mild PEP (Table 4). The combination of indomethacin with sublingual GTN was also the most effective measure for preventing mild PEP (Figure 4B).
| Indomethacin+GTN | Diclofenac 100 mg | Indomethacin | tra-GTN | Naproxen | Diclofenac 50 mg | Placebo |
| 0.59 (0.19, 1.86) | ||||||
| 0.46 (0.19, 1.12) | 0.77 (0.35, 1.72) | |||||
| 0.38 (0.12, 1.20) | 0.65 (0.24, 1.75) | 0.84 (0.38, 1.85) | ||||
| 0.38 (0.11, 1.35) | 0.64 (0.22, 1.87) | 0.83 (0.32, 2.14) | 0.99 (0.31, 3.14) | |||
| 0.32 (0.08, 1.39) | 0.54 (0.14, 2.11) | 0.70 (0.21, 2.37) | 0.84 (0.22, 3.19) | 0.85 (0.19, 3.71) | ||
| 0.27 (0.11, 0.67) | 0.46 (0.23, 0.94) | 0.59 (0.40, 0.88) | 0.71 (0.36, 1.41) | 0.72 (0.29, 1.78) | 0.84 (0.27, 2.66) |
On pairwise comparison with placebo, rectal indomethacin 100 mg plus GTN (0.19, 0.08–0.48), rectal diclofenac 100 mg (0.27, 0.09–0.79), and rectal indomethacin 100 mg (0.43, 0.28–0.66) were all more efficacious than placebo in preventing moderate-severe PEP (Table 5). The combination of indomethacin with sublingual GTN was more efficacious than transdermal GTN (0.28, 0.09–0.85) and naproxen (0.24, 0.07–0.82) (Table 3) and was the best prevention method for moderate-severe PEP with the highest SUCRA probability (89.2%) (Figure 4C).
| Indomethacin+GTN | Diclofenac 100 mg | Indomethacin | Diclofenac 50 mg | tra-GTN | Naproxen | Placebo |
| 0.71 (0.17, 2.96) | ||||||
| 0.44 (0.17, 1.16) | 0.61 (0.20, 1.87) | |||||
| 0.47 (0.05, 4.40) | 0.66 (0.07, 6.62) | 1.07 (0.13, 8.55) | ||||
| 0.28 (0.09, 0.85) | 0.39 (0.11, 1.36) | 0.63 (0.30, 1.34) | 0.59 (0.07, 4.95) | |||
| 0.24 (0.07, 0.82) | 0.34 (0.11, 1.02) | 0.55 (0.24, 1.27) | 0.52 (0.06, 4.65) | 0.88 (0.32, 2.46) | ||
| 0.19 (0.08, 0.48) | 0.27 (0.09, 0.79) | 0.43 (0.28, 0.66) | 0.41 (0.05, 3.11) | 0.69 (0.37, 1.28) | 0.78 (0.35, 1.77) |
PEP remains the most common and serious complication of ERCP. Various preventive strategies have been used to try to solve this tough problem. Common measures include pancreatic stents, pharmacotherapy, and hydration[7,35]. The prophylactic effect of pancreatic stents and rectal NSAIDs has been recognized by European clinical guidelines[6]. Nevertheless, pancreatic stents have obvious disadvantages, including injury to the pancreatic orifice and failure of placement, which significantly increases the risk of PEP. Recently, more attention has been paid to pharmacotherapy, especially NSAIDs, due to their effectiveness, cheapness and convenience. Both RCTs and meta-analyses found that rectal administration of NSAIDs was better at preventing PEP compared to oral or intramuscular administration[7,36,37].
We did a network meta-analysis of 24 RCTs with a total of 9416 patients to identify the prophylactic efficacy of seven different interventions on PEP and to identify the best-performing dose and best route of administration. We found that rectal diclofenac 100 mg was the most effective rectal NSAID, consistent with the previous meta-analysis[7]. Sublingual GTN administration was more useful than transdermal in preventing PEP. Furthermore, the combination of indomethacin and sublingual GTN might be the best preventive strategy for PEP.
Severe PEP is a well-known complication with significant consequences for patients undergoing ERCP. Therefore, we also concentrated on this challenging complication. A network meta-analysis was also performed on 20 RCTs with a total of 8956 patients, to identify the prophylactic effect of six different interventions on mild or moderate-to-severe PEP. Since the two sublingual GTN studies did not record the severity of the PEP episodes[27,30], the preventive strategy using sublingual GTN was not included in this analysis. We found that rectal diclofenac 100 mg was also the most effective among rectal NSAIDs for preventing mild or moderate-to-severe PEP. The combination of indomethacin with sublingual GTN had the best preventive effect for mild PEP and moderate-to-severe PEP. Based on our results, rectal diclofenac 50 mg, transdermal GTN, and rectal naproxen 500 mg did not prevent or alleviate PEP better than placebo.
The exact mechanism, by which the NSAIDs prevent PEP is still a subject of debate, and there are several hypotheses. It is widely accepted that inflammatory mediators play a vital role in the pathogenesis of pancreatitis and the subsequent inflammatory response[38]. The severity of pancreatitis is also determined by the intensity of the inflammatory cascade and the systemic response. NSAIDs are potent inhibitors of phospholipase A2, which is thought to play a critical role early in the inflammatory cascade[39]. This might explain the ability of NSAIDs to prevent PEP or reduce its severity.
The mechanism of GTN in preventing PEP has not been completely elucidated. The main hypothesis is that the GTN relaxes smooth muscle, which increases pancreatic parenchymal blood flow and lowers the basal pressure and contraction amplitude in the sphincter of Oddi[40]. More studies are needed to confirm the mechanism.
Despite that we believe the combination of NSAIDs with sublingual GTN might be the best preventive strategies in PEP. This analysis had some limitations. First, rectal diclofenac 100 mg is the most efficacious among rectal NSAIDs for PEP prevention, but there was no research on the combination of rectal diclofenac and sublingual GTN. There were only two studies on the combination of indomethacin and sublingual GTN[33,34], and more RCTs are needed to explore this issue in the future. Second, we only searched for RCTs published in English, which may have resulted in sample and geographical biases. Finally, few included studies had results about hyperamylasemia, post-ERCP pain, or perforation. Therefore, we could not compare these complications.
In conclusion, this network meta-analysis confirmed that, of the NSAIDs, rectal diclofenac 100 mg was the best for PEP prophylaxis and sublingual was more effective than transdermal GTN in preventing PEP. Combination of rectal indomethacin 100 mg with sublingual GTN was the most effective strategy for preventing PEP and alleviating its severity. These findings help establish PEP prophylaxis for future study and practice; however, more high-quality, double-blind RCTs are needed for further network meta-analysis.
Clinical application of drugs.
The combination of rectal indomethacin 100 mg with sublingual glyceryl trinitrate (GTN) offered better prevention of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP) than when used alone and could alleviate the severity of PEP. This conclusion needs to be explored in more randomized controlled trials (RCTs) with large samples.
Twenty-four eligible RCTs were selected, evaluating seven preventive strategies in 9416 patients. Rectal indomethacin 100 mg plus sublingual GTN, rectal diclofenac 100 mg, sublingual GTN, and rectal indomethacin 100 mg were all more efficacious than placebo in preventing PEP. The combination of rectal indomethacin and sublingual GTN had the highest surface under the cumulative ranking curves (SUCRA) probability of 92.2% and was the best preventive strategy for moderate-to-severe PEP with a SUCRA probability of 89.2%.
A systematic search was done for full-text RCTs of PEP in PubMed, Embase, Science Citation Index, and the Cochrane Controlled Trials database. Inclusion and exclusion criteria were used to screen for eligible RCTs. The major data were extracted by two independent reviewers. The Frequentist model was used to conduct this network meta-analysis and obtain the pairwise odds ratios and 95%CI.
To compare NSAIDs and GTN in the prevention of PEP and to determine whether they are better in combination.
To explore the role of NSAIDs and GTN for prevention of PEP.
Post-endoscopic retrograde cholangiopancreatography pancreatitis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kitamura K, Japan; Trna J, Czech Republic S-Editor: Xing YX L-Editor: Kerr C P-Editor: Xing YX
| 1. | Talukdar R. Complications of ERCP. Best Pract Res Clin Gastroenterol. 2016;30:793-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Dumonceau JM, Kapral C, Aabakken L, Papanikolaou IS, Tringali A, Vanbiervliet G, Beyna T, Dinis-Ribeiro M, Hritz I, Mariani A, Paspatis G, Radaelli F, Lakhtakia S, Veitch AM, van Hooft JE. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2020;52:127-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 501] [Article Influence: 100.2] [Reference Citation Analysis (1)] |
| 3. | Leerhøy B, Shabanzadeh DM, Nordholm-Carstensen A, Jørgensen LN. Quality of life, performance status, and work capacity after post-endoscopic retrograde cholangiopancreatography pancreatitis. Scand J Gastroenterol. 2018;53:994-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Dubravcsik Z, Hritz I, Keczer B, Novák P, Lovász BD, Madácsy L. Network meta-analysis of prophylactic pancreatic stents and non-steroidal anti-inflammatory drugs in the prevention of moderate-to-severe post-ERCP pancreatitis. Pancreatology. 2021;21:704-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Del Olmo Martínez ML, Velayos Jiménez B, Almaraz-Gómez A. Hydration with Lactated Ringer’s solution combined with rectal diclofenac in the prevention of pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterol Hepatol. 2021;44:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Testoni PA, Mariani A, Aabakken L, Arvanitakis M, Bories E, Costamagna G, Devière J, Dinis-Ribeiro M, Dumonceau JM, Giovannini M, Gyokeres T, Hafner M, Halttunen J, Hassan C, Lopes L, Papanikolaou IS, Tham TC, Tringali A, van Hooft J, Williams EJ. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016;48:657-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 385] [Article Influence: 42.8] [Reference Citation Analysis (1)] |
| 7. | Akshintala VS, Sperna Weiland CJ, Bhullar FA, Kamal A, Kanthasamy K, Kuo A, Tomasetti C, Gurakar M, Drenth JPH, Yadav D, Elmunzer BJ, Reddy DN, Goenka MK, Kochhar R, Kalloo AN, Khashab MA, van Geenen EJM, Singh VK. Non-steroidal anti-inflammatory drugs, intravenous fluids, pancreatic stents, or their combinations for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:733-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Ding J, Jin X, Pan Y, Liu S, Li Y. Glyceryl trinitrate for prevention of post-ERCP pancreatitis and improve the rate of cannulation: a meta-analysis of prospective, randomized, controlled trials. PloS One. 2013;8:e75645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Wang Y, Xu B, Zhang W, Lin J, Li G, Qiu W, Wang Y, Sun D. Prophylactic effect of rectal indomethacin plus nitroglycerin administration for preventing pancreatitis after endoscopic retrograde cholangiopancreatography in female patients. Ann Palliat Med. 2020;9: 4029-4037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Cumpston M, Li T, Page MJ. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2930] [Article Influence: 488.3] [Reference Citation Analysis (0)] |
| 11. | Murray B, Carter R, Imrie C, Evans S, O’Suilleabhain C. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology. 2003;124:1786-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Sotoudehmanesh R, Khatibian M, Kolahdoozan S, Ainechi S, Malboosbaf R, Nouraie M. Indomethacin may reduce the incidence and severity of acute pancreatitis after ERCP. Am J Gastroenterol. 2007;102:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Khoshbaten M, Khorram H, Madad L, Ehsani Ardakani MJ, Farzin H, Zali MR. Role of diclofenac in reducing post-endoscopic retrograde cholangiopancreatography pancreatitis. J Gastroenterol Hepatol. 2008;23:e11-e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Elmunzer BJ, Scheiman JM, Lehman GA, Chak A, Mosler P, Higgins PD, Hayward RA, Romagnuolo J, Elta GH, Sherman S, Waljee AK, Repaka A, Atkinson MR, Cote GA, Kwon RS, McHenry L, Piraka CR, Wamsteker EJ, Watkins JL, Korsnes SJ, Schmidt SE, Turner SM, Nicholson S, Fogel EL; U. S. Cooperative for Outcomes Research in Endoscopy (USCORE). A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366:1414-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 504] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 15. | Otsuka T, Kawazoe S, Nakashita S, Kamachi S, Oeda S, Sumida C, Akiyama T, Ario K, Fujimoto M, Tabuchi M, Noda T. Low-dose rectal diclofenac for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a randomized controlled trial. J Gastroenterol. 2012;47:912-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Döbrönte Z, Szepes Z, Izbéki F, Gervain J, Lakatos L, Pécsi G, Ihász M, Lakner L, Toldy E, Czakó L. Is rectal indomethacin effective in preventing of post-endoscopic retrograde cholangiopancreatography pancreatitis? World J Gastroenterol. 2014;20:10151-10157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Andrade-Dávila VF, Chávez-Tostado M, Dávalos-Cobián C, García-Correa J, Montaño-Loza A, Fuentes-Orozco C, Macías-Amezcua MD, García-Rentería J, Rendón-Félix J, Cortés-Lares JA, Ambriz-González G, Cortés-Flores AO, Alvarez-Villaseñor Adel S, González-Ojeda A. Rectal indomethacin vs placebo to reduce the incidence of pancreatitis after endoscopic retrograde cholangiopancreatography: results of a controlled clinical trial. BMC Gastroenterol. 2015;15:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Lua GW, Muthukaruppan R, Menon J. Can Rectal Diclofenac Prevent Post Endoscopic Retrograde Cholangiopancreatography Pancreatitis? Dig Dis Sci. 2015;60:3118-3123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Patai Á, Solymosi N, Patai ÁV. Effect of rectal indomethacin for preventing post-ERCP pancreatitis depends on difficulties of cannulation: results from a randomized study with sequential biliary intubation. J Clin Gastroenterol. 2015;49:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Levenick JM, Gordon SR, Fadden LL, Levy LC, Rockacy MJ, Hyder SM, Lacy BE, Bensen SP, Parr DD, Gardner TB. Rectal Indomethacin Does Not Prevent Post-ERCP Pancreatitis in Consecutive Patients. Gastroenterology. 2016;150:911-7; quiz e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 21. | Luo H, Zhao L, Leung J, Zhang R, Liu Z, Wang X, Wang B, Nie Z, Lei T, Li X, Zhou W, Zhang L, Wang Q, Li M, Zhou Y, Liu Q, Sun H, Wang Z, Liang S, Guo X, Tao Q, Wu K, Pan Y, Fan D. Routine pre-procedural rectal indometacin versus selective post-procedural rectal indometacin to prevent pancreatitis in patients undergoing endoscopic retrograde cholangiopancreatography: a multicentre, single-blinded, randomised controlled trial. Lancet. 2016;387:2293-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 22. | Mansour-Ghanaei F, Joukar F, Taherzadeh Z, Sokhanvar H, Hasandokht T. Suppository naproxen reduces incidence and severity of post-endoscopic retrograde cholangiopancreatography pancreatitis: Randomized controlled trial. World J Gastroenterol. 2016;22:5114-5121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Patil S, Pandey V, Pandav N, Ingle M, Phadke A, Sawant P. Role of Rectal Diclofenac Suppository for Prevention and Its Impact on Severity of Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis in High-Risk Patients. Gastroenterology Res. 2016;9:47-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Mohammad Alizadeh AH, Abbasinazari M, Hatami B, Abdi S, Ahmadpour F, Dabir S, Nematollahi A, Fatehi S, Pourhoseingholi MA. Comparison of rectal indomethacin, diclofenac, and naproxen for the prevention of post endoscopic retrograde cholangiopancreatography pancreatitis. Eur J Gastroenterol Hepatol. 2017;29:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Li L, Liu M, Zhang T, Jia Y, Zhang Y, Yuan H, Zhang G, He C. Indomethacin down-regulating HMGB1 and TNF-α to prevent pancreatitis after endoscopic retrograde cholangiopancreatography. Scand J Gastroenterol. 2019;54:793-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Katoh T, Kawashima K, Fukuba N, Masuda S, Kobatake H, Masaki K, Araki Y, Kawano K, Nishi K, Takenaka M, Ishihara S, Kinoshita Y. Low-dose rectal diclofenac does not prevent post-ERCP pancreatitis in low- or high-risk patients. J Gastroenterol Hepatol. 2020;35:1247-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Sudhindran S, Bromwich E, Edwards PR. Prospective randomized double-blind placebo-controlled trial of glyceryl trinitrate in endoscopic retrograde cholangiopancreatography-induced pancreatitis. Br J Surg. 2001;88:1178-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Moretó M, Zaballa M, Casado I, Merino O, Rueda M, Ramírez K, Urcelay R, Baranda A. Transdermal glyceryl trinitrate for prevention of post-ERCP pancreatitis: A randomized double-blind trial. Gastrointest Endosc. 2003;57:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Kaffes AJ, Bourke MJ, Ding S, Alrubaie A, Kwan V, Williams SJ. A prospective, randomized, placebo-controlled trial of transdermal glyceryl trinitrate in ERCP: effects on technical success and post-ERCP pancreatitis. Gastrointest Endosc. 2006;64: 351-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Hao JY, Wu DF, Wang YZ, Gao YX, Lang HP, Zhou WZ. Prophylactic effect of glyceryl trinitrate on post-endoscopic retrograde cholangiopancreatography pancreatitis: a randomized placebo-controlled trial. World J Gastroenterol. 2009;15: 366-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Nøjgaard C, Hornum M, Elkjaer M, Hjalmarsson C, Heyries L, Hauge T, Bakkevold K, Andersen PK, Matzen P; European Post-ERCP Pancreatitis Preventing Study Group. Does glyceryl nitrate prevent post-ERCP pancreatitis? Gastrointest Endosc. 2009;69: e31-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Bhatia V, Ahuja V, Acharya SK, Garg PK. A randomized controlled trial of valdecoxib and glyceryl trinitrate for the prevention of post-ERCP pancreatitis. J Clin Gastroenterol. 2011;45:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Sotoudehmanesh R, Eloubeidi MA, Asgari AA, Farsinejad M, Khatibian M. A randomized trial of rectal indomethacin and sublingual nitrates to prevent post-ERCP pancreatitis. Am J Gastroenterol. 2014;109:903-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 2037] [Article Influence: 59.9] [Reference Citation Analysis (1)] |
| 35. | Márta K, Gede N, Szakács Z, Solymár M, Hegyi PJ, Tél B, Erőss B, Vincze Á, Arvanitakis M, Boškoski I, Bruno MJ, Hegyi P. Combined use of indomethacin and hydration is the best conservative approach for post-ERCP pancreatitis prevention: A network meta-analysis. Pancreatology. 2021;21: 1247-1255.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 36. | Ishiwatari H, Urata T, Yasuda I, Matsusaki S, Hisai H, Kawakami H, Ono M, Iwashita T, Doi S, Kawakubo K, Hayashi T, Sonoda T, Sakamoto N, Kato J. No Benefit of Oral Diclofenac on Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis. Dig Dis Sci. 2016;61:3292-3301. [PubMed] [DOI] [Full Text] |
| 37. | Geraci G, Palumbo VD, D’Orazio B, Maffongelli A, Fazzotta S, Lo Monte AI. Rectal Diclofenac administration for prevention of post-Endoscopic Retrograde Cholangio-Pancreatography (ERCP) acute pancreatitis. Randomized prospective study. Clin Ter. 2019;170:e332-e336. [PubMed] [DOI] [Full Text] |
| 38. | Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Gross V, Leser HG, Heinisch A, Schölmerich J. Inflammatory mediators and cytokines—new aspects of the pathophysiology and assessment of severity of acute pancreatitis? Hepatogastroenterology. 1993;40:522-530. [PubMed] |
| 40. | Staritz M, Poralla T, Ewe K, Meyer zum Büschenfelde KH. Effect of glyceryl trinitrate on the sphincter of Oddi motility and baseline pressure. Gut. 1985;26:194-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 1.7] [Reference Citation Analysis (0)] |