Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.7794
Peer-review started: January 27, 2022
First decision: March 11, 2022
Revised: April 4, 2022
Accepted: June 3, 2022
Article in press: June 3, 2022
Published online: August 6, 2022
Processing time: 175 Days and 16.9 Hours
Advanced chronic kidney disease (CKD) is a common complication for people with type 1 and 2 diabetes and can often lead to glucose instability. Continuous glucose monitoring (CGM) helps users monitor and stabilize their glucose levels. To date, CGM and intermittent scanning CGM are only approved for people with diabetes but not for those with advanced CKD.
To compare the performance of Dexcom G5 and FreeStyle Libre sensors in adults with type 1 or 2 diabetes and advanced CKD.
This was a non-randomized clinical trial that took place in two outpatient clinics in western Sweden. All patients with type 1 or 2 diabetes and an estimated glomerular filtration rate (eGFR) of < 30 mL/min per 1.73 m2 were invited to participate. Forty patients (full analysis set = 33) carried the Dexcom G5 sensor for 7 d and FreeStyle Libre sensor for 14 d simultaneously. For referencing capillary blood glucose (SMBG) was measured with a high accuracy glucose meter (HemoCue®) during the study period. At the end of the study, all patients were asked to answer a questionnaire on their experience using the sensors.
The mean age was 64.1 (range 41-77) years, hemoglobin A1c was 7.0% [standard deviation (SD) 3.2], and diabetes duration was 28.5 (SD 14.7) years. A total of 27.5% of the study population was on hemodialysis and 22.5% on peritoneal dialysis. The mean absolute relative difference for Dexcom G5 vs SMBG was significantly lower than that for FreeStyle Libre vs SMBG [15.2% (SD 12.2) vs 20.9% (SD 8.6)], with a mean difference of 5.72 [95% confidence interval (CI): 2.11-9.32; P = 0.0036]. The mean absolute difference was also significantly lower for Dexcom G5 than for FreeStyle Libre, 1.21 mmol/L (SD 0.78) and 1.76 mmol/L (SD 0.78), with a mean diffrenec of 0.55 (95%CI: 0.27-0.83; P = 0.0004).The mean difference (MD) was -0.107 mmol/L and -1.10 mmol/L (P = 0.0002), respectively. In all, 66% of FreeStyle Libre values were in the no risk zone on the surveillance error grid compared to 82% of Dexcom G5 values.
Dexcom G5 produces more accurate sensor values than FreeStyle Libre in people with diabetes and advanced CKD and is likely safe to be used by those with advanced CKD.
Core Tip: This study bridges a needed gap within the diabetes device area for people with diabetes and advanced chronic kidney disease and was done in a home setting for analyses as close to real life as possible. The study found that Dexcom G5 showed greater accuracy both in relation to the mean absolute relative difference and on a surveillance error grid, but participants rated their user experience for FreeStyle Libre higher but rated no difference in feeling safe.
- Citation: Ólafsdóttir AF, Andelin M, Saeed A, Sofizadeh S, Hamoodi H, Jansson PA, Lind M. Performance of Dexcom G5 and FreeStyle Libre sensors tested simultaneously in people with type 1 or 2 diabetes and advanced chronic kidney disease. World J Clin Cases 2022; 10(22): 7794-7807
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/7794.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.7794
For people with diabetes, good glycemic control is essential to avoid problems due to diabetes complications[1]. To reach recommended glucose levels, it is important to monitor glucose levels and for several years, self-measurement of blood glucose (SMBG) with capillary measurements has been the best way to do this[1,2]. Over the last decades, continuous glucose monitoring (CGM) and intermittent glucose monitoring (isCGM) has become more common within diabetes management and for many, has replaced the multiple capillary tests. Both systems are made up of a small sensor that is inserted under the skin where it measures glucose levels in the interstitial fluid. CGM measures glucose levels continuously and every 5 min sends a glucose value to a handheld receiver or mobile telephone. It sends alarms for high and low glucose levels. The isCGM collects data, and when the user scans the sensor with a handheld receiver or a mobile phone, it sends the glucose levels to the receiver[3,4].
Within the diabetes field, there are many discussions regarding who should be given CGM and isCGM. To date, CGM and isCGM are only approved for people with diabetes but not with chronic kidney disease (CKD)[3,4] and mainly recommended for those with type 1 diabetes and who have problems with recurrent hypoglycemia[1].
Advanced CKD is a common complication in people with type 1 and 2 diabetes. It is estimated that 20%-40% of people with diabetes will develop diabetic kidney disease, and it is the leading cause of end-stage renal failure[5,6]. A recent study showed that up to 5.1% of people with type 1 diabetes in Germany and Austria had an estimated glomerular filtration rate (eGFR) below 30 mL/min, and for Sweden and United States, the corresponding figures were 1.5% and 2.1%[7]. Advanced CKD increases the risk of hypoglycemia and great glycemic variation, and therefore it can be helpful to monitor blood glucose with a CGM or isCGM[8,9]. There are very few studies available on the accuracy of CGMs or isCGMs for people with advanced CKD[10]. Two of the most common systems are Dexcom and FreeStyle Libre. Neither of these systems are approved for people in dialysis[3,4].
The aim of this study was to compare the performance of Dexcom G5 and FreeStyle Libre in adults with type 1 or 2 diabetes with CKD and an eGFR < 30 mL/min/1.73 m2, including patients on maintenance dialysis.
This study took place at NU Hospital Group and Sahlgrenska University Hospital, Sweden. It was a non-randomized, non-blinded clinical study over a 14 d period to compare the performance of FreeStyle Libre 1 and Dexcom G5 for people with diabetes and advanced CKD in an at-home situation. The protocol was approved by the regional ethics review board of Gothenburg, Sweden.
All participants provided written informed consent before the study began. The inclusion criteria were: type 1 or type 2 diabetes, between 18-years-old and 80-years-old, and eGFR < 30 mL/min per 1.73 m2 for people undergoing or not undergoing dialysis. The exclusion criteria were pregnancy, patients with severe cognitive dysfunction or other diseases that makes glucose monitoring difficult, continuous use of paracetamol, history of allergic reaction to chlorhexidine or alcohol antiseptic solution, abnormal skin at the anticipated glucose sensor attachment sites, and eGFR ≥ 30 mL/min per 1.73 m2.
After obtaining written and informed consent, a diabetes nurse inserted two different sensors in accordance with instructions from the manufacturer. Dexcom G5 was inserted in the abdomen and FreeStyle Libre on the upper arm. Participants were instructed on how they should use each monitor and instructed how to calibrate the Dexcom G5. Calibrations were done using the HemoCue® DM RD 201 (Ängelholm, Sweden). All HemoCue meters were calibrated before being assigned to participants using the absolute isotope dilution gas chromatography/mass spectrometry measurement system[11]. The total measurement error/reproducibility imprecision of HemoCue is less than 6.5%[12]. Earlier studies using HemoCue showed a strong correlation between capillary and venous HemoCue concentrations, and capillary concentrations were considered to be a suitable reference[13]. All participants were instructed by a diabetes nurse on how to use the HemoCue meter. Participants were instructed to simultaneously document their blood glucose measured by HemoCue and the value of the FreeStyle Libre and Dexcom G5 in a diary a minimum of three times per day. Participants were instructed to calibrate their Dexcom G5 twice daily in accordance with the manufacturer’s instructions and to do so after recording its value in the diary. Participants on maintenance dialysis (peritoneal dialysis or hemodialysis) were also asked to register the start and finish of each session in their diary. After 7 d, Dexcom G5 was removed by the participants but they continued to record results from the FreeStyle Libre and HemoCue. After the 14 d period, participants returned the meters to the site. The study personnel downloaded data from the meters using the Glooko-Diasend system. HemoCue measurements were manually validated by personnel going through each value and comparing to the diary. When each sensor was finished, participants rated their experience on a 10-item visual analogue scale. Similar questionnaires have been used in earlier studies[14,15].
All endpoints were predefined and registered on ClinicalTrials.gov. The primary endpoint was the difference of mean absolute relative difference (MARD) between Dexcom G5 and FreeStyle Libre using HemoCue (capillary glucose meter) as a reference. Secondary endpoints were the difference in mean absolute difference (MAD) between the Dexcom G5 and FreeStyle Libre sensors, the difference in mean difference (MD) between the Dexcom G5 and FreeStyle Libre sensors, and the correlation between the different systems. Predefined subgroup analyses for glucose ranges below 3.9 mmol/L, between 3.9 and 10 mmol/L, and above 10 mmol/L as well as for those without dialysis and undergoing dialysis.
The manufacturers of FreeStyle Libre and Dexcom G5 were not involved in the design, performance, data analysis, or publication of the article. No support was received from the manufacturers.
After sample size analysis, 40 patients were included in the study (see supplement). All main analyses between Dexcom G5 and FreeStyle Libre were performed with paired analyses. All statistical analyses were predefined in the statistical analysis plan before database lock. All participants having at least 10 matched time points, with evaluable blood glucose values from both sensors and HemoCue (reference capillary value) during the whole study period, were included in the Full Analysis Set (FAS). All matching time points were used. For paired analysis regarding continuous variables, Fisher’s non-parametric permutation test for paired observations was used and for dichotomous and ordered categorical variables sign test was used. For comparison between dialysis subjects and subjects not in dialysis, Fisher’s non-parametric permutation test was used for continuous variables.
The primary variable was MARD, which is the mean absolute relative difference between the estimated sensor glucose value of FreeStyle Libre or Dexcom G5 and blood glucose measured with HemoCue. For each individual mean of following differences from each time point was evaluated for both sensors: |(sensorі-HemoCuei)|/HemoCuei. The secondary variables were MAD and MD.
MAD is the mean absolute difference between estimated sensor glucose value of FreeStyle Libre or Dexcom G5 and blood glucose measured with HemoCue. For each individual mean of following differences from each time point was evaluated for both sensors: |sensorі-HemoCuei|. MD is the mean difference between estimated sensor glucose value of FreeStyle Libre or Dexcom G5 and blood glucose measured with HemoCue. For each individual mean of following differences from each time point was evaluated: (sensorі-HemoCuei), where i = time-point during the analyzed days in the study.
The MD between Dexcom G5 and FreeStyle Libre with 95% confidence intervals (CIs) was calculated based on Fisher’s non-parametric permutation test for paired observations for continuous variables. All analyses for different glucose ranges were based on HemoCue values within respective range.
To study the covariation between Dexcom G5/FreeStyle Libre and HemoCue Pearson correlation coefficient between each of the devices and HemoCue was calculated for each subject. These correlations were also analyzed both for Dexcom G5 and FreeStyle Libre with Fisher’s non-parametric permutation test one sample test.
Agreement between each of the devices and HemoCue were analyzed with Bland-Altman’ methods. The main result was the limit of agreement. If one got a value measured with one of the sensors, you can calculate an interval where 95% of the HemoCue values would have been. The distributions of the difference between each of the sensor and HemoCue was also given together with Intraclass correlation coefficient (ICC), Bland-Altman plots, and scatterplots.
All significance tests were two-sided and conducted at the 5% significance level. All statistical analyses were performed with SAS System Version 9.4 (Cary, NC, United States).
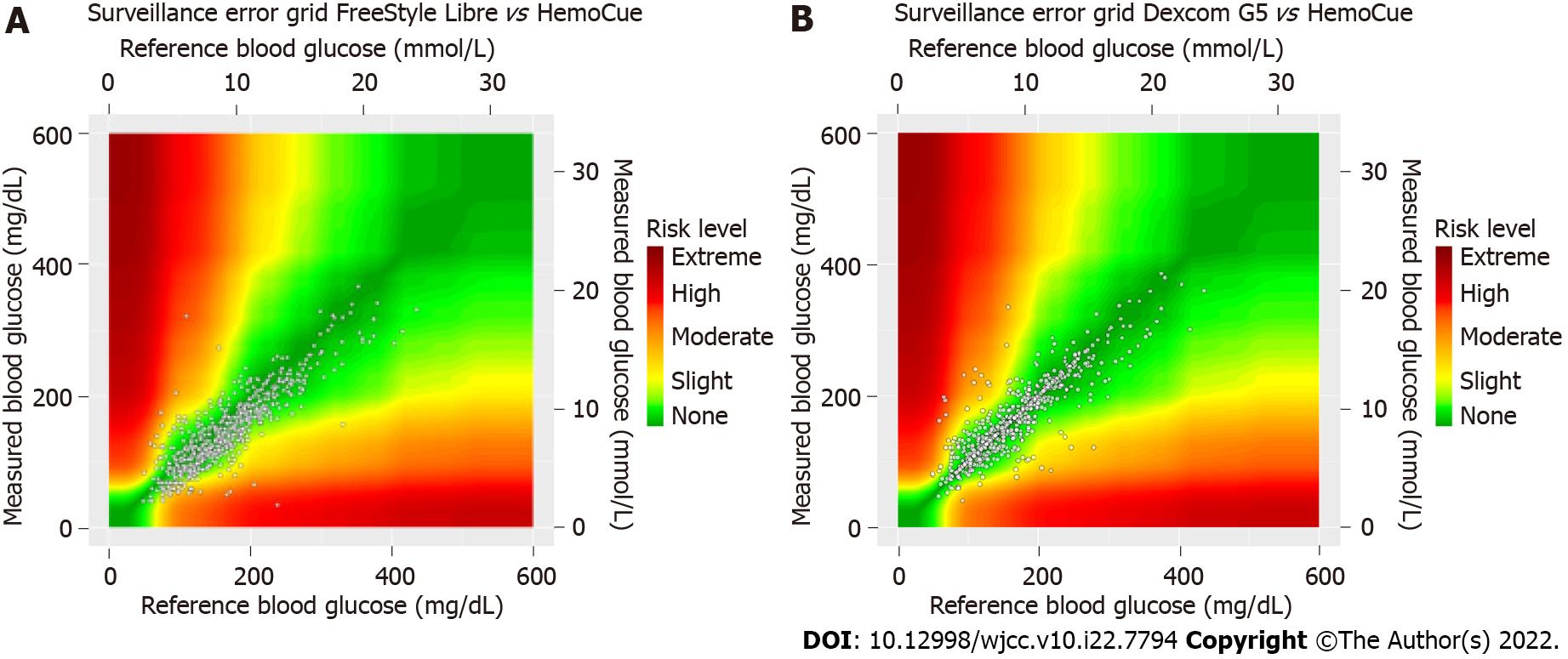
The surveillance error grid graph for Dexcom G5/FreeStyle Libre vs HemoCue was calculated by using https://www.diabetestechnology.org/seg/. The proportion of sensor values within 15%, 20%, and 30% of reference values HemoCue for blood glucose > 100 mg/dL (5.6 mmol/L) or within 15, 20, and 30 mg/dL (0.8, 1.1, 1.7 mmol/L) of reference values for blood glucose ≤ 100 mg/dL (5.6 mmol/L), respectively, was calculated (%15/15, %20/20, %30/30). MARD FreeStyle Libre vs HemoCue the first week was compared with the second week with the same requirements as main study with Fisher’s nonparametric permutation test for paired observations.
The study included 40 participants with type 1 and 2 diabetes and advanced CKD; 33 (FAS) met the criteria for data analysis and at least 10 time points with evaluable values from both systems and the HemoCue within 5 min during the whole study period (June 2016-March 2019). Of the 7 patients who were not included in FAS, 2 chose not to participate after starting the study and 5 did not meet the criteria for data analysis described above; that is, they did not have 10 matched time points for both sensors. Mean hemoglobin A1c (HbA1c) was 7.0%, 25.6% were women, mean age was 64.1 (range 41-77), and 50% were on dialysis. Additional baseline characteristics are shown in Table 1.
| Variable | All, n = 40 | FAS population, n = 33 |
| Age in yr | 64.1 (9.2) | 63.2 (9.2) |
| 66 (41; 77) | 65.5 (41; 77) | |
| n = 39 | n = 32 | |
| HbA1c in mmol/mol | 53.1 (11.0) | 53.9 (11.5) |
| 53 (31; 75) | 54 (31; 75) | |
| n = 40 | n = 33 | |
| HbA1c % | 7 (3.2) | 7.1 (3.2) |
| 53 (5; 9) | 7.1 (5; 9) | |
| n = 40 | n = 33 | |
| Dialysis | ||
| Not in dialysis | 20 (50.0%) | 17 (51.5%) |
| Hemodialysis | 11 (27.5%) | 7 (21.2%) |
| Peritoneal dialysis | 9 (22.5%) | 9 (27.3%) |
| Diabetes duration | 28.5 (14.7) | 29.2 (15.4) |
| 27.5 (5.3; 64.5) | 28.5 (5.3; 64.5) | |
| n = 32 | n = 26 | |
| Sex | ||
| Man | 29 (74.4%) | 22 (68.8%) |
| Woman | 10 (25.6%) | 10 (31.3%) |
| Smoking | ||
| No | 25 (71.4%) | 19 (67.9%) |
| Yes | 3 (8.6%) | 3 (10.7%) |
| Do not know | 7 (20.0%) | 6 (21.4%) |
| Systolic blood pressure in mmHg | 145.6 (24.2) | 146.5 (24.7) |
| 145 (95; 213) | 142.5 (95; 213) | |
| n = 37 | n = 30 | |
| Diastolic blood pressure in mmHg | 77.4 (13.7) | 78.6 (13.8) |
| 80 (52; 103) | 80 (52; 103) | |
| n = 37 | n = 30 | |
| Insulin delivery | ||
| Basal insulin only | 12 (32.4%) | 10 (33.3%) |
| Mix insulin | 2 (5.4%) | 2 (6.7%) |
| MDI | 24 (64.9%) | 20 (66.7%) |
| Other glucose lowering treatment | 5 (13.5%) | 3 (10.0%) |
| Total insulin dose per day | 73.0 (60.3) | 65.7 (63.8) |
| 45 (8; 277) | 41 (8; 277) | |
| n = 25 | n = 20 | |
| Type of diabetes | ||
| Type 1 | 11 (30.6%) | 10 (33.3%) |
| Type 2 | 25 (69.4%) | 20 (66.7%) |
The MARD analyzed for all participants for Dexcom G5 was significantly lower than that for FreeStyle Libre vs SMBG (15.2% [SD 12.2] vs 20.9% [SD 8.6]), respectively, with mean difference of 5.72 (95%CI: 2.11-9.32; P = 0.0036). The MAD was also significantly lower for Dexcom G5 than for FreeStyle Libre, 1.21 mmol/L (SD 0.78) and 1.76 mmol/L (SD 0.78), with a mean difference of 0.55 (95%CI: 0.27-0.83; P = 0.0004). There was also a significant difference between the MD of the systems. There was a systematic MD between FreeStyle Libre and HemoCue of -1.10 mmol/L (95%CI: -1.55 to -0.66 mmol/L; P < 0.0001) but no systematic MD between Dexcom G5 and HemoCue -0.107 (95%CI: -0.439 to 0.225; P = 0.052) (Table 2).
| Variable | FreeStyle Libre (isCGM) | Dexcom G5 CGM | Difference (isCGM-CGM) | P value1 |
| Mean MARD | 20.9 (8.6) | 15.2 (12.2) | 5.72 (10.17) | 0.0036 |
| 19.8 (8.5; 43.1) | 11.9 (2.2; 60.5) | 6.5 (-26.75; 24.68) | ||
| n = 33 | n = 33 | (2.11; 9.32) | ||
| n = 33 | ||||
| Mean MAD | 1.76 (0.78) | 1.21 (0.78) | 0.548 (0.795) | 0.0004 |
| 1.65 (0.48; 4.48) | 0.95 (0.23; 3.12) | 0.679 (-1.662; 2.11) | ||
| n = 33 | n = 33 | (0.267; 0.830) | ||
| n = 33 | ||||
| Mean MD | -1.10 (1.24) | -0.107 (0.937) | -0.998 (1.278) | 0.0002 |
| -1.46 (-4.48; 1.63) | -0.229 (-2.47; 3.007) | -1.1 (-3.586; 2.431) | ||
| n = 33 | n = 33 | (-1.451; -0.545) | ||
| n = 33 | ||||
| Persons in dialysis | ||||
| Mean MARD | 19.3 (7.4) | 15.5 (14.8) | 3.80 (11.09) | 0.19 |
| 17.7 (8.5; 33.7) | 8.6 (4.1; 60.5) | 6.51 (-26.75; 15.75) | ||
| n = 16 | n = 16 | (-2.11; 9.71) | ||
| n = 16 | ||||
| Mean MAD | 1.74 (0.91) | 1.26 (0.85) | 0.489 (0.828) | 0.035 |
| 1.65 (0.77; 4.48) | 0.99 (0.43; 3.12) | 0.611 (-1.236; 1.65) | ||
| n = 16 | n = 16 | (0.048; 0.931) | ||
| n = 16 | ||||
| Mean MD | -1.29 (1.29) | 0.056 (1.232) | -1.34 (1.30) | 0.0019 |
| -1.32 (-4.48; 0.96) | -0.252 (-2.47; 3.007) | -1.46 (-3.59; 1.07) | ||
| n = 16 | n = 16 | (-2.03; -0.65) | ||
| n = 16 | ||||
| People not in dialysis | ||||
| Mean MARD | 22.5 (9.5) | 15.0 (9.6) | 7.53 (9.19) | 0.0033 |
| 19.8 (8.8; 43.1) | 12.5 (2.2; 38.5) | 6 (-15.59; 24.68) | ||
| n = 17 | n = 17 | (2.80; 12.25) | ||
| n = 17 | ||||
| Mean MAD | 1.77 (0.65) | 1.16 (0.72) | 0.604 (0.784) | 0.0057 |
| 1.67 (0.48; 3.22) | 0.95 (0.23; 2.77) | 0.679 (-1.662; 2.11) | ||
| n = 17 | n = 17 | (0.201; 1.007) | ||
| n = 17 | ||||
| Mean MD | -0.934 (1.211) | -0.260 (0.529) | -0.673 (1.209) | 0.037 |
| -1.482 (-2.586; 1.632) | -0.223 (-1.323; 0.932) | -0.855 (-2.348; 2.431) | ||
| n = 17 | n = 17 | (-1.295; -0.052) | ||
| n = 17 | ||||
| Glucose values < 3.9 mmol/L | ||||
| Mean MARD | 53.0 (37.1) | 89.8 (66.8) | -36.9 (42.0) | 0.027 |
| 35.3 (12.8; 115.2) | 66.7 (7.7; 197.4) | -44.7 (-127.2; 7.1) | ||
| n = 9 | n = 9 | (-69.2; -4.5) | ||
| n = 9 | ||||
| Mean MAD | 1.81 (1.33) | 3.10 (2.47) | -1.29 (1.52) | 0.027 |
| 1.2 (0.47; 3.9) | 1.8 (0.27; 7.2) | -1.3 (-4.65; 0.2) | ||
| n = 9 | n = 9 | (-2.46; -0.12) | ||
| n = 9 | ||||
| Mean MD | 1.18 (1.96) | 3.03 (2.54) | -1.85 (1.42) | 0.0078 |
| 1.2 (-1.2; 3.9) | 1.8 (0.27; 7.2) | -1.8 (-4.65; 0.2) | ||
| (-2.94; -0.76) | ||||
| n = 9 | n = 9 | n = 99 | ||
| Glucose values 3.9-10.0 mmol/L | ||||
| Mean MARD | 22.6 (8.9) | 14.8 (10.6) | 7.83 (9.88) | < 0.0001 |
| 21 (8.7; 44.1) | 11.9 (1.5; 40.5) | 8.05 (-13.18; 28.95) | ||
| n = 33 | n = 33 | (4.32; 11.33) | ||
| n = 33 | ||||
| Mean MAD | 1.60 (0.62) | 1.03 (0.71) | 0.568 (0.689) | < 0.0001 |
| 1.45 (0.5; 2.98) | 0.82 (0.13; 2.59) | 0.633 (-0.918; 2.045) | ||
| n = 33 | n = 33 | (0.323; 0.812) | ||
| n = 33 | ||||
| Mean MD | -0.868 (1.183) | 0.136 (0.859) | -1.00 (1.19) | < 0.0001 |
| -1.067 (-2.9; 2.508) | -0.017 (-2.1; 2.445) | -1.07 (-3.57; 1.38) | ||
| n = 33 | n = 33 | (-1.43; -0.58) | ||
| n = 33 | ||||
| Glucose values > 10.0 mmol/L | ||||
| Mean MARD | 16.6 (11.1) | 12.3 (11.6) | 4.22 (8.63) | 0.010 |
| 15.3 (1.9; 59) | 7.9 (1.9; 50.4) | 4.69 (-25.08; 16.94) | ||
| n = 31 | n = 31 | (1.06; 7.39) | ||
| n = 31 | ||||
| Mean MAD | 2.06 (1.29) | 1.54 (1.31) | 0.520 (1.139) | 0.016 |
| 1.9 (0.2; 6.45) | 1.04 (0.2; 5.5) | 0.533 (-3.4; 2.18) | ||
| n = 31 | n = 31 | (0.102; 0.938) | ||
| n = 31 | ||||
| Mean MD | -1.82 (1.56) | -0.944 (1.633) | -0.875 (1.777) | 0.0087 |
| -1.89 (-6.45; 1.16) | -0.563 (-5.5; 2.7) | -0.95 (-4.1; 5.72) | ||
| n = 31 | n = 31 | (-1.527; -0.223) | ||
| n = 31 | ||||
| Hemodialysis | ||||
| Mean MARD | 21.4 (9.1) | 20.3 (18.9) | 1.13 (14.27) | 0.86 |
| 20.7 (8.5; 33.7) | 17 (5.6; 60.5) | 2.88 (-26.75; 15.75) | ||
| n = 7 | n = 7 | (-12.07; 14.33) | ||
| n = 7 | ||||
| Mean MAD | 1.79 (0.69) | 1.44 (0.87) | 0.354 (1.012) | 0.38 |
| 1.89 (0.77; 2.73) | 1.28 (0.56; 3.12) | 0.211 (-1.236; 1.614) | ||
| n = 7 | n = 7 | (-0.582; 1.290) | ||
| n = 7 | ||||
| Mean MD | -0.905 (1.451) | 0.378 (1.185) | -1.28 (1.47) | 0.063 |
| -1.606 (-2.733; 0.96) | 0.056 (-0.433; 3.007) | -1.99 (-2.54; 1.07) | ||
| n = 7 | n = 7 | (-2.65; 0.08) | ||
| n = 7 | ||||
| Peritoneal dialysis | ||||
| Mean MARD | 17.6 (5.9) | 11.7 (10.4) | 5.88 (8.16) | 0.078 |
| 14.9 (12.1; 28.8) | 6.9 (4.1; 36.7) | 7.18 (-14.64; 13.35) | ||
| n = 9 | n = 9 | (-0.39; 12.15) | ||
| n = 9 | ||||
| Mean MAD | 1.71 (1.09) | 1.11 (0.86) | 0.595 (0.700) | 0.043 |
| 1.4 (0.96; 4.48) | 0.77 (0.43; 2.83) | 0.736 (-0.89; 1.65) | ||
| n = 9 | n = 9 | (0.057; 1.132) | ||
| n = 9 | ||||
| Mean MD | -1.58 (1.15) | -0.193 (1.278) | -1.39 (1.23) | 0.0039 |
| -1.25 (-4.48; -0.8) | -0.442 (-2.47; 2.195) | -1 (-3.59; -0.33) | ||
| n = 9 | n = 9 | (-2.34; -0.44) | ||
| n = 9 | ||||
We found that for glucose values that were in range (3.9-10.0 mmol/L) and above range (> 10 mmol/L), there was a significantly lower MARD, MAD, and MD for Dexcom G5 than for FreeStyle Libre (Table 2). For glucose values in range, the MARD was 14.8% (SD 10.6) for Dexcom G5 and 22.6% (SD 8.9) for the FreeStyle Libre, with a mean difference of 7.83 (95%CI: 4.32-11.33; P < 0.0001). The MARD for hyperglycemic values were 12.3% (SD 11.6) and 16.6% (SD 11.1), respectively, with a mean difference of 4.22 (95%CI: 1.06-7.39; P = 0.010). There were few values below range (< 3.9 mmol/L), 14 values from 9 individuals (Table 2).
Subgroup analyses for MARD, MAD, and MD were done for people requiring and not requiring dialysis. The MARD for FreeStyle Libre for people in dialysis was 19.3% (SD 7.4) compared to 22.5% (SD 9.5) for those not in dialysis (P = 0.29). The corresponding values for Dexcom G5 were 15.5% (SD 14.8) and 15.0% (SD 9.6), respectively (P = 0.91). For people not in dialysis, there was a significant difference between the sensors MARD and MAD (P = 0.0033 and P = 0.0057, respectively). For people in dialysis, there was a significant difference between the systems MAD (P = 0.035), whereas a numerical difference was found between the sensors MARD, although not statistically significant (Table 2). Further subgroup analysis with people on peritoneal dialyses showed numerically lower MARD and MAD for Dexcom G5 compared to FreeStyle Libre as in the total population, and there was a significant systematic difference between FreeStyle Libre and HemoCue -1.58 (P = 0.01). There were 7 people on hemodialysis and Dexcom G5 showed a numerically lower MARD and MAD compared to FreeStyle Libre in this subgroup, but the differences were less (Table 2).
Analyses were done to see how well the systems correlated with the capillary reference system. Values obtained by Dexcom G5 and FreeStyle Libre significantly correlated with those obtained by the HemoCue capillary reference system [r = 0.784, (SD 0.29) P < 0.0001, and 0.777, (SD 0.34) P < 0.0001, respectively]. Interclass correlation coefficient (ICC) was 0.68 for FreeStyle Libre and 0.88 for Dexcom G5 and limits of agreement (-3.54- 1.34) for FreeStyle Libre and (-1.94- 1.73) for Dexcom G5 (Supplementary Table 1). This could clearly be seen on the Bland-Altman plot in Figure 1 and Supple
After using the systems, participants evaluated their experience (Table 3). Participants were significantly more positive towards FreeStyle Libre than Dexcom G5 in all factors except feeling safe, for which there was no significance between the two systems. FreeStyle Libre scored 7.94 of 10 and Dexcom G5 scored 7.19 of 10 (P = 0.32; Table 3).
| Variable | FreeStyle Libre isCGM, n = 31 | Dexcom G5 CGM, n = 31 | Change from FreeStyle Libre isCGM to Dexcom G5 CGM | |
| P value | ||||
| My experience of the system was very positive | 8.35 (2.03) | 6.84 (2.70) | -1.52 (3.41) | 0.023 |
| 9.00 (3.00; 10.00) | 8.00 (2.00; 10.00) | -2.00 (-7.00; 7.00) | ||
| (7.61; 9.10) | (5.85; 7.83) | (-2.77; -0.26) | ||
| n = 31 | n = 31 | n = 31 | ||
| The insertion of the sensor was easy | 9.03 (1.71) | 8.27 (2.29) | -0.767 (1.455) | 0.0084 |
| 10.00 (2.00; 10.00) | 9.00 (1.00; 10.00) | 0.000 (-5.000; 2.000) | ||
| (8.39; 9.67) | (7.41; 9.12) | (-1.310; -0.223) | ||
| n = 30 | n = 30 | n = 30 | ||
| I felt safe during my time using the system | 7.94 (2.67) | 7.19 (2.46) | -0.742 (3.916) | 0.32 |
| 9.00 (0.00; 10.00) | 8.00 (3.00; 10.00) | -1.000 (-7.000; 9.000) | ||
| (6.96; 8.91) | (6.29; 8.09) | (-2.178; 0.694) | ||
| n = 31 | n = 31 | n = 31 | ||
| It was easy to use the system | 9.52 (0.96) | 7.60 (2.62) | -1.93 (2.21) | < 0.0001 |
| 10.00 (6.00; 10.00) | 9.00 (2.00; 10.00) | -1.00 (-7.00; 0.00) | ||
| (9.16; 9.87) | (6.62; 8.58) | (-2.76; -1.11) | ||
| n = 31 | n = 30 | n = 30 | ||
| It was easy to interpret the information on the receiver screen | 9.42 (0.99) | 7.97 (2.46) | -1.45 (1.98) | < 0.0001 |
| 10.00 (7.00; 10.00) | 9.00 (1.00; 10.00) | -1.00 (-7.00; 1.00) | ||
| (9.06; 9.78) | (7.07; 8.87) | (-2.18; -0.73) | ||
| n = 31 | n = 31 | n = 31 | ||
| I was not in pain or had discomfort in connection to my use of the system | 9.74 (0.73) | 8.48 (2.78) | -1.26 (2.68) | 0.0078 |
| 10.00 (7.00; 10.00) | 10.00 (0.00; 10.00) | 0.00 (-10.00; 0.00) | ||
| (9.47; 10.01) | (7.46; 9.50) | (-2.24; -0.27) | ||
| n = 31 | n = 31 | n = 31 | ||
| I experienced no problem scanning/contact with the system | 9.55 (0.93) | 7.19 (3.29) | -2.35 (3.23) | < 0.0001 |
| 10.00 (7.00; 10.00) | 9.00 (0.00; 10.00) | -1.00 (-10.00; 2.00) | ||
| (9.21; 9.89) | (5.99; 8.40) | (-3.54; -1.17) | ||
| n = 31 | n = 31 | n = 31 | ||
| The sensor was comfortable to have on my body in my daily life | 9.20 (1.40) | 7.23 (2.69) | -1.90 (2.34) | < 0.0001 |
| 10.00 (4.00; 10.00) | 8.00 (1.00; 10.00) | -1.00 (-8.00; 1.00) | ||
| (8.68; 9.72) | (6.24; 8.21) | (-2.77; -1.03) | ||
| n = 30 | n = 31 | n = 30 | ||
| The system did not disturb my daily life | 9.33 (1.09) | 7.74 (2.62) | -1.50 (2.43) | 0.0024 |
| 10.00 (6.00; 10.00) | 9.00 (3.00; 10.00) | 0.00 (-7.00; 4.00) | ||
| (8.93; 9.74) | (6.78; 8.70) | (-2.41; -0.59) | ||
| n = 30 | n = 31 | n = 30 | ||
| I would like to use the system in my daily life | 8.45 (2.86) | 5.42 (3.49) | -2.66 (4.98) | 0.0096 |
| 10.00 (0.00; 10.00) | 6.00 (0.00; 10.00) | -3.00 (-10.00; 9.00) | ||
| (7.36; 9.54) | (4.14; 6.70) | (-4.55; -0.76) | ||
| n = 29 | n = 31 | n = 29 | ||
| It was easy to calibrate the Dexcom G5 | 8.40 (2.21) | |||
| 10.00 (3.00; 10.00) | ||||
| (7.58; 9.22) | ||||
| n = 30 | ||||
| The alarms did not disturb my daily life | 7.97 (2.74) | |||
| 9.00 (0.00; 10.00) | ||||
| (6.95; 8.99) | ||||
| n = 30 | ||||
For Dexcom G5, %20/20 = 79.6, which indicates that 79.6% of the values above 5.6 mmol/L were within 20% of the reference instrument and within 1.11 mmol/L (20 mg/dL) for values below 5.6 mmol/L. The corresponding figure for FreeStyle Libre was 61.3%. For %15/15 the values were 70.3% for Dexcom G5 and 43.9% for FreeStyle Libre. For %30/30 the corresponding figures were 89.1% and 84.6% respectively. The surveillance error grid (Figure 2) showed that 82% of the values for Dexcom G5 were within the no risk zone (green color) compared to 66.3% of the values for FreeStyle Libre. Data from the second week of Libre showed that there was a greater MARD during this week, 24.8% (95%CI: 20.4-29.2 mmol/L) compared to the first week when it was 19.4%, P = 0.0042. MARD for participants with type 1 diabetes was 11.8 % (SD 10.0) for Dexcom G5 and 17.4% (SD 5.7) for FreeStyle Libre with a mean difference of 5.6 (95%CI: (-0.4-11.8, P = 0.068). Corresponding results for participants with type 2 diabetes were 16.2 % (SD 12.7) for Dexcom G5 and 21.6% (SD 8.6) for FreeStyle Libre with a mean difference of 5.4 (95%CI: (0.25-10.49, P = 0.042).
Dexcom G5 showed greater overall accuracy than FreeStyle Libre. Dexcom G5 also showed greater accuracy for glucose values within range (3.9-10 mmol/L) and above range (> 10 mmol/L). Furthermore, in a subgroup analysis, Dexcom G5 showed greater accuracy for people not in dialysis. However, for people in dialysis, Dexcom G5 had a numerically lower MARD and a significantly lower MAD compared with FreeStyle Libre. On the surveillance error grid, Dexcom G5 had 82% of values within the no risk zone compared to 66% for FreeStyle Libre. Glucose values from both sensors correlated well with the reference instrument, HemoCue. FreeStyle Libre showed a greater systematic deviation than Dexcom G5. Participants rated their user experience of FreeStyle Libre higher after a 2 wk period than Dexcom G5 but did not experience a difference in safety.
Earlier studies with similar methodology and the same reference instrument showed that the FreeStyle Libre had a MARD of 13.2% and an earlier Dexcom sensor (Dexcom 4G) had a MARD of 13.8% when tested in people with type 1 diabetes[14,15]. A recent study analyzed how well FreeStyle Libre correlates with capillary measurements (Medisafe® Fit) during hemodialysis in people with type 2 diabetes, and showed that the FreeStyle Libre had a MARD between 13% and 22% depending on the glycemic range and that it showed a 18.4 mg/dL (1.0 mmol/L) lower value than the capillary reference instrument. The same study found that the Medtronic iPro Enlite sensor had a MARD between 5% and 30% depending on the glycemic value and showed a 4.7 mg/dL (0.3 mmol/L) lower value than the reference instrument[10]. It was previously shown that the FreeStyle Libre deviates systematically by -0.5 mmol/L in people with type 1 diabetes using HemoCue capillary measurements as a reference[15]. The Dexcom G5 was found to have a MARD of 7.1%-15.7% when tested in people with type 1 diabetes and using a Yellow Spring Instrument as a reference[16].
People with advanced CKD more frequently experience glycemic excursions[15]. During hemo
This study showed that even people undergoing peritoneal dialysis, which can have high glucose fluctuations, had a MARD which is similar to previous systems. The peritoneal dialysis fluids did not seem to affect the MARD.
FreeStyle Libre had a higher MARD and MAD than Dexcom G5 and there was a greater percentage of values within the safe zone for Dexcom G5. This can partly be explained by the fact that the FreeStyle Libre showed a systematic deviation of -1.1 mmol/L. It is important that users of the system are aware of the systems tendency of reporting lower glucose values. This systematic deviation is not only evident when the sensor is used by people with advanced CKD although it seems to be greater for this patient group[15]. The surveillance error grid showed that only 66% of FreeStyle Libre values were in the no risk zone whilst 82% of Dexcom G5 values were within the no risk zone.
Participants rated the user experience of the FreeStyle Libre significantly higher than for the Dexcom G5. They found the system easier to use and easier to interpret the data on the receiver. The sensor was more comfortable, and it was less painful to insert. There was a greater interest to use the system in their daily life. This might be different with Dexcom's latest sensors which do not require calibration by the user. It is important to note that the users did not experience any difference of safety when using the system.
The strength of this study is that it was done independently from the manufacturers of this study. The study was done in a real-life environment as patients used the sensors in their daily life. All analyses were predefined. The limitations of this study were the short duration the participants used the sensors, and the evaluation of the user experience might change if the users become more comfortable and confident in the use of the sensors, and the questionnaire used is not validated. For certain subgroup analysis the number of participants or values obtained was low, therefore these analyses have to be interpreted with caution. It should be noted that Dexcom G5 was calibrated with the same capillary method as the reference system, and it cannot be excluded that more novel generations of Dexcom sensors which do not need calibrations may have a greater systematic deviation from HemoCue. Neither Dexcom G5 nor FreeStyle Libre are approved to be used by people with advanced chronic kidney disease. Another limitation is that the most novel sensors often used today were not evaluated. However, these data must be viewed in the light that CGM accuracy data are overall lacking in people with Diabetes and advanced CKD and data are therefore urgently needed.
In conclusion, this study supports that Dexcom G5 has a similar accuracy in people with diabetes and advanced CKD as in people with diabetes without advanced CKD. The FreeStyle Libre system showed similar correlations between sensor value and blood glucose values as Dexcom, but a lower number of values in the no risk zone indicating that greater caution should be taken to use it in the current population. The FreeStyle Libre showed a systematic deviation at least partly explaining the lower accuracy.
People with diabetes and advanced chronic kidney disease (CKD) often have fluctuating blood glucose levels and today no blood glucose sensors are approved to be used in this patient group.
It is of great importance to give the best possible care to all people with diabetes. This is a patient group with difficult complications due to their diabetes and need all the help they can get.
The objective of this study was to see if the sensors FreeStyle Libre and Dexcom G5 were accurate when used by people with advanced CKD.
This was a non-randomized clinical study. The results were evaluated by using mean absolute relative difference as a main analysis. Mean absolute difference and mean difference was also calculated. A surveillance error grid was even used for accuracy evaluations.
The main analysis found that the Dexcom G5 had a mean absolute relative difference of 15.2% while it was 20.9% for the FreeStyle Libre. There was no significant difference if the patients were on maintenance dialysis or not. There was no significant difference between those with type 1 or 2 diabetes. The surveillance error grid showed that Dexcom G5 had 82% of its values within the safe zone while FreeStyle Libre had 66% within the safe zone.
The study concludes that the Dexcom G5 produces more accurate values than the FreeStyle Libre.
This study is a great start for evaluating how we can use glucose sensors for this patient group, but further studies have to be done with more novel glucose sensors.
We would like to thank Lena Heijdenberg, Mary Dana, and Anders Bergdahl for their involvement in the study and examining participants. We would also like to thank Nils-Gunnar Pehrsson for his assistance in the data analyses and interpretation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Sweden
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Shen Q, China; Wang W, China A-Editor: Sun XD, China S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | American Diabetes Association. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S85-S99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 2. | Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17510] [Cited by in RCA: 16280] [Article Influence: 508.8] [Reference Citation Analysis (3)] |
| 3. | Abbott. Freestyle Libre Bruksanvisning 2020. Available from: https://freestyleserver.com/Payloads/IFU/2021/q1/ART40989-105_rev-A-Web.pdf. |
| 4. | Inc D. Dexcom G5 - System för kontinuerlig glukosmätning Användarhandbok. 2020. Available from: https://s3-us-west-2.amazonaws.com/dexcompdf/Downloads+and+Guides+Updates/LBL013367+G5+Mobile+UG+OUS+SV+mmol.pdf. |
| 5. | American Diabetes Association. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S151-S167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 246] [Article Influence: 61.5] [Reference Citation Analysis (1)] |
| 6. | Njurregister S. Svenskt Njuregister Årsrapport 20202020. 2021.05.27. |
| 7. | Dena M, Svensson AM, Olofsson KE, Young L, Carlson A, Miller K, Grimsmann J, Welp R, Mader JK, Maahs DM, Holl RW, Lind M. Renal Complications and Duration of Diabetes: An International Comparison in Persons with Type 1 Diabetes. Diabetes Ther. 2021;12:3093-3105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98:S1-S115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 686] [Article Influence: 137.2] [Reference Citation Analysis (0)] |
| 9. | Galindo RJ, Beck RW, Scioscia MF, Umpierrez GE, Tuttle KR. Glycemic Monitoring and Management in Advanced Chronic Kidney Disease. Endocr Rev. 2020;41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 10. | Matoba K, Hayashi A, Shimizu N, Moriguchi I, Kobayashi N, Shichiri M. Comparison of accuracy between flash glucose monitoring and continuous glucose monitoring in patients with type 2 diabetes mellitus undergoing hemodialysis. J Diabetes Complications. 2020;34:107680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Hannestad U, Lundblad A. Accurate and precise isotope dilution mass spectrometry method for determining glucose in whole blood. Clin Chem. 1997;43:794-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Kos S, van Meerkerk A, van der Linden J, Stiphout T, Wulkan R. Validation of a new generation POCT glucose device with emphasis on aspects important for glycemic control in the hospital care. Clin Chem Lab Med. 2012;50:1573-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Andelin M, Kropff J, Matuleviciene V, Joseph JI, Attvall S, Theodorsson E, Hirsch IB, Imberg H, Dahlqvist S, Klonoff D, Haraldsson B, DeVries JH, Lind M. Assessing the Accuracy of Continuous Glucose Monitoring (CGM) Calibrated With Capillary Values Using Capillary or Venous Glucose Levels as a Reference. J Diabetes Sci Technol. 2016;10:876-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Matuleviciene V, Joseph JI, Andelin M, Hirsch IB, Attvall S, Pivodic A, Dahlqvist S, Klonoff D, Haraldsson B, Lind M. A clinical trial of the accuracy and treatment experience of the Dexcom G4 sensor (Dexcom G4 system) and Enlite sensor (guardian REAL-time system) tested simultaneously in ambulatory patients with type 1 diabetes. Diabetes Technol Ther. 2014;16:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Ólafsdóttir AF, Attvall S, Sandgren U, Dahlqvist S, Pivodic A, Skrtic S, Theodorsson E, Lind M. A Clinical Trial of the Accuracy and Treatment Experience of the Flash Glucose Monitor FreeStyle Libre in Adults with Type 1 Diabetes. Diabetes Technol Ther. 2017;19:164-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 16. | Link M, Kamecke U, Waldenmaier D, Pleus S, Garcia A, Haug C, Freckmann G. Comparative Accuracy Analysis of a Real-time and an Intermittent-Scanning Continuous Glucose Monitoring System. J Diabetes Sci Technol. 2021;15:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Gai M, Merlo I, Dellepiane S, Cantaluppi V, Leonardi G, Fop F, Guarena C, Grassi G, Biancone L. Glycemic pattern in diabetic patients on hemodialysis: continuous glucose monitoring (CGM) analysis. Blood Purif. 2014;38:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Skubala A, Zywiec J, Zełobowska K, Gumprecht J, Grzeszczak W. Continuous glucose monitoring system in 72-hour glucose profile assessment in patients with end-stage renal disease on maintenance continuous ambulatory peritoneal dialysis. Med Sci Monit. 2010;16:CR75-CR83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Joubert M, Fourmy C, Henri P, Ficheux M, Lobbedez T, Reznik Y. Effectiveness of continuous glucose monitoring in dialysis patients with diabetes: the DIALYDIAB pilot study. Diabetes Res Clin Pract. 2015;107:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |