Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.7749
Peer-review started: February 8, 2022
First decision: March 23, 2022
Revised: May 1, 2022
Accepted: June 27, 2022
Article in press: June 27, 2022
Published online: August 6, 2022
Processing time: 164 Days and 0.2 Hours
Iron plays an important role in neurodevelopmental functions in the brain. Serum ferritin levels are different in children with attention deficit hyperactivity disorder and tic disorder than in healthy children.
To explore the current status of iron deficiency in children with neurodevelopmental disorders and its sex and age effects.
A total of 1565 children with attention deficit hyperactivity disorder (ADHD), 1694 children with tic disorder (TD), 93 children with ASD and 1997 healthy control children were included between January 1, 2020, and December 31, 2021 at Beijing Children's Hospital. We describe the differences in age levels and ferritin levels between different disease groups and their sex differences. The differences between the sexes in each disease were analyzed using the t test. The incidence rate of low serum ferritin was used to describe the differences between different diseases and different age groups. A chi-square test was used to analyze the difference in the incidence of low serum ferritin between the disease group and the control group. Analysis of variance was used for comparisons between subgroups, and regression analysis was used for confounding factor control.
A total of 1565 ADHD patients aged 5-12 years were included in this study, and the average serum ferritin levels of male and female children were 36.82 ± 20.64 μg/L and 35.64 ± 18.56 μg/L, respectively. A total of 1694 TD patients aged 5-12 years were included in this study, and the average serum ferritin levels of male and female children were 35.72 ± 20.15 μg/L and 34.54 ± 22.12 μg/L, respectively. As age increased, the incidence of low serum ferritin in ADHD and TD first decreased and then increased, and 10 years old was the turning point of rising levels. The incidence of ADHD with low serum ferritin was 8.37%, the incidence of TD with low serum ferritin was 11.04%, and the incidence of the healthy control group with low serum ferritin was 8.61%, among which male children with TD accounted for 9.25% and female children with TD accounted for 11.62%. There was a significant difference among the three groups (P < 0.05). In addition, there were 93 children with ASD with an average serum ferritin level of 30.99 ± 18.11 μg/L and a serum ferritin incidence of 15.05%.
In conclusion, low serum ferritin is not a risk factor for ADHD or TD. The incidence of low serum ferritin levels in children with ADHD and TD between 5 and 12 years old decreases first and then increases with age.
Core Tip: By investigating the status of iron deficiency in children with neurodevelopmental disorders and its influence on gender and age, it is suggested to check the serum ferritin level and related hematological indexes of children with neurodevelopmental disorders at the age of 5-10 years, and make necessary iron supplementation.
- Citation: Tang CY, Wen F. Serum ferritin levels in children with attention deficit hyperactivity disorder and tic disorder. World J Clin Cases 2022; 10(22): 7749-7759
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/7749.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.7749
Iron deficiency in early childhood can lead to developmental abnormalities in gene expression, neurotransmitter function, neurometabolism and other aspects related to brain development, which in turn affects children’s sensorimotor functions, growth and development, cognitive language, social emotions, the development of learning and memory, and so on[1]. Iron deficiency can also lead to a decrease in iron content in the brain, which can cause central nervous system dysfunction and ultimately lead to neuropsychiatric symptoms[2]. Iron participates in several basic biochemical functions in the brain, is a cofactor for many metabolic processes and material synthesis and has important effects on neurodevelopmental functions such as myelination, transmitter transmission, and gene expression[3].
Notably, it has been reported that iron deficiency in children is associated with neurodevelopmental diseases, leading to abnormalities in growth and development, learning behavior, motor function, social emotion, intellectual development, cognitive ability, language function, sleep cycle, etc.[4-6]. Ferritin is an objective and sensitive indicator for the investigation and study of iron deficiency because serum ferritin is an important iron storage protein that is crucial for iron homeostasis and participates in a variety of physiological and pathological processes. Serum ferritin is one of the most reliable and widely used markers of iron storage status in the body[7]. The variability of serum ferritin levels is lower than that of serum iron levels, and when iron reserves are depleted, the decline of serum ferritin precedes the decline of serum iron[8].
Neurodevelopmental outcomes include attention deficit disorder hyperactivity (ADHD), tic disorders (TD) and autism spectrum disorders (ASD). The incidence of neurodevelopmental disorders in children is also increasing year by year[8]. The following will introduce iron deficiency-related research on these three diseases. First, a study of ADHD confirmed that the serum ferritin level of children with ADHD was significantly lower than that of the control group[9]. Mahmoud et al studied 58 untreated children with ADHD and found that the serum ferritin level of children with ADHD was significantly lower than that of the control group[10]. There are also studies on the serum ferritin level and the Conners Parental Rating Scale (CPRS) total score. There is a significant negative correlation between serum ferritin levels and scores on the Conners Teacher Rating Scale (CTRS)[11,12]. This suggests that iron deficiency may indicate more severe ADHD symptoms. It should be emphasized that iron supplementation can also improve ADHD symptoms[13,14]. Studies have also found that iron supplementation is related to the increase in serum ferritin levels and the parallel reduction in the severity of ADHD symptoms assessed by the parents' Conners score[15]. Second, studies on TD have shown that children and adults with mature Tourette syndrome have reduced serum ferritin[3]. A large-scale epidemiological study showed that children with iron deficiency anemia are at increased risk of tic disorder, iron storage status may be related to children’s tics, and low ferritin levels may be the result of pediatric tic risk factors for the development of the disease or predictors of the severity of tics in children[16]. A study of 107 children and adults found that ferritin levels were significantly reduced. Compared with the control group, the caudate nucleus and putamen nucleus of TS patients were larger[17]. Lower iron reserves may help to reduce the size of the caudate and putamen nuclei, thereby increasing the susceptibility to tics[18]. Third, research on ASD has shown that anemia diagnosed earlier in pregnancy was associated with an increased risk of the development of ASD, ADHD, and particularly ID in offspring[19]. In a study that investigated iron levels in ASD patients aged 19 mo to 13 years, 52% of ASD children developed ID[20]. Another study showed that 8.3% of autistic children aged 1 to 2 years, 14.2% of autistic children aged 3 to 5 years, and 20% of autistic children aged 6 to 10 years had below-normal serum ferritin levels[21]. Other studies have shown that children with ASD have significantly lower serum hemoglobin, hematocrit, iron, and mean corpuscular volume levels than healthy children, but these are not enough to cause anemia[22]. However, meta-analyses have shown that the available evidence is inconsistent with regard to whether iron levels are lower in children with ASD[23].
There is a lack of cohort studies on the status of iron deficiency and iron supplementation in children with ADHD, TD and ASD among Chinese children and adolescents. The sex and age effects of iron deficiency and the critical period of iron supplementation are unclear. In conclusion, the level of iron deficiency in neurodevelopmental disorders of ADHD, TD and ASD and the status of sex-age effects are still unclear, and data from Chinese samples are lacking. This study will adopt the thinking of retrospective research and investigation by enrolling ADHD, TD and ASD children in the psychiatric department of Beijing Children's Hospital as the sample. The sample size is large, and more attention can be paid to the comparison of the difference in serum ferritin levels of ADHD, TD and ASD children with respect to gender and age than previous studies. This study will explore the current status of iron deficiency in children with neurodevelopmental disorders and its influence on sex and age, providing an important reference for the correlation between neurodevelopmental disorders and ferritin and necessary iron supplementation.
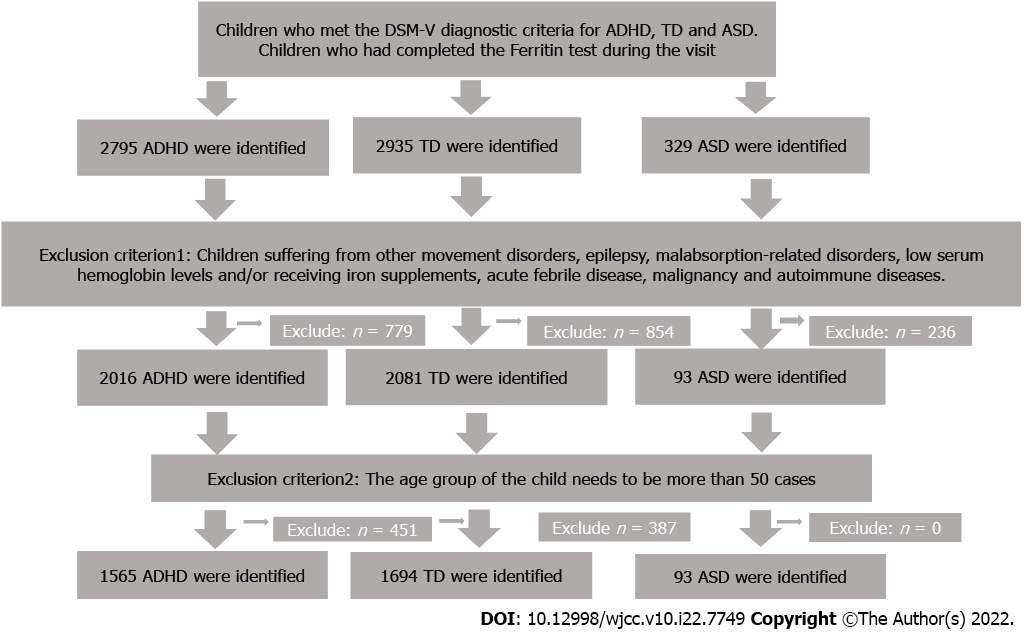
We retrospectively reviewed data from Beijing Children's Hospital for consecutive diagnoses of ADHD, TD, and ASD between January 1, 2020, and December 31, 2021. This study was in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Beijing Children's Hospital (No. IEC-C-006-A04-V.06). We confirmed that all patient data were anonymous in this study. Additionally, children with normal physical examinations were selected as a healthy control group. The possible influencing factors of ferritin results should be strictly controlled, and drug use and comorbidities of patients should be thoroughly analyzed to prevent drugs and other diseases from affecting the results of ferritin examination of patients. There were also strict admission and exclusion criteria. The diagnosis of neurodevelopmental disorders was carried out by professional psychiatrists. More details are provided in Figure 1.
Inclusion criteria: (1) Children who met the DSM-V diagnostic criteria for ADHD, TD and ASD; and (2) Children who had completed the ferritin test during the visit were included in the study. Exclusion criteria: Patients suffering from other movement disorders, epilepsy, malabsorption-related disorders, low serum hemoglobin levels and/or receiving iron supplements, acute febrile disease, malignancy and autoimmune diseases.
Serum ferritin was detected by a Beckman DXI800 automatic immune electrochemiluminescence analyzer and an original matching serum ferritin detection kit. After all children washed their hands with soap, 1 mL of venous blood was collected, and serum samples were separated for detection and tested by full-time staff in the biochemical room of the hospital testing center. The experimental process was strictly controlled by quality and tested in accordance with the operating procedures. The standard of low serum ferritin was 15 μg/L[23].
We extracted the identity, sex, age, diagnosis and ferritin level of subjects from the test bank based on the records of the Psychiatric Department of Beijing Children's Hospital. Considering the possible bias caused by the small sample size, our criterion for the inclusion of subjects is that when the ferritin test amount of the age group of the child needs to involve more than 50 cases, the subjects of this age group should be included.
In order to avoid the bias of retrospective study to the greatest extent, the inclusion criteria and exclusion criteria of research objects are restricted to narrow the differences between research objects. A case-control study was used to control confounding factors. The age of confounding factors was stratified and then treated with corresponding statistical methods. Analysis of variance was used for comparisons between subgroups. Linear regression and logistic regression models were used to control the confounding bias.
SPSS version 23 was used for statistical analysis. We used the mean, standard deviation, and 95% confidence intervals to describe age levels, ferritin levels, and sex differences between disease groups. A test was used to analyze the sex difference in each disease, and the chi-square test was used to analyze the difference in the low incidence of serum ferritin between the disease group and the control group, with P = 0.05 considered as significant. Analysis of variance was used for comparisons between subgroups, and regression analysis was used for confounding factor control. The low incidence of serum ferritin was used to describe differences between disease and age groups.
A total of 1565 children with ADHD, 1694 TD children, 93 ASD children and 1997 healthy control children were included in this study. The age range was from 5 to 12 years old. In the ADHD group, 1317 children (84.15%) were male, and 248 children (15.85%) were female. The average age of the children was 7.92 ± 1.85 years. The average serum ferritin of the children was 36.63 ± 20.32 μg/L. The average serum ferritin levels of male and female children were 36.82 ± 20.64 μg/L and 35.64 ± 18.56 μg/L, respectively. There was no significant difference in the average age of male and female children (P > 0.05). In the TD group, 1282 children (64.2%) were male, and 412 children (35.8%) were female. The average age of the children was 7.61 ± 2.03 years. The average serum ferritin of the children was 35.43 ± 20.64 μg/L. The average serum ferritin levels of male and female children were 35.72 ± 20.15 μg/L and 34.54 ± 22.12 μg/L, respectively. There was no significant difference in the average age of male and female children (P > 0.05). In the ASD group, 83 children (89.25%) were male, and 10 children (10.75%) were female. The average age of the children was 6.14 ± 2.88 years. The average serum ferritin of the children was 30.99 ± 18.11 μg/L. The average serum ferritin levels of male and female children were 31.42 ± 18.58 μg/L and 27.42 ± 13.73 μg/L, respectively. There was no significant difference in the average age of male and female children (P > 0.05). In the healthy control group, 979 children (49.02%) were male, and 1018 children (50.98%) were female. The average age of the children was 7.61 ± 2.03 years. The average serum ferritin of the children was 71.66 ± 51.99 μg/L. The average serum ferritin levels of male and female children were 74.34 ± 51.19 μg/L and 69.08 ± 52.64 μg/L, respectively. There was a significant difference in the average age of male and female children (P < 0.05).
The results showed that the incidence of ADHD with low serum ferritin was 8.37% (131/1565), the incidence of TD with low serum ferritin was 11.04% (182/1649), and the incidence of ASD with low serum ferritin was 15.05% (14/93). The incidence in the healthy control group with low serum ferritin was 8.61% (172/1997). There was a significant difference among the four groups (P < 0.05). More details are provided in Tables 1 and 2.
| HC | ADHD | TD | ASD | |
| n | 1997 | 1565 | 1694 | 93 |
| (M/F) | (979/1018) | (1317/248) | (1282/412) | (83/10) |
| % | 49.02/50.98 | 84.15/15.85 | 64.2/35.8 | 89.25/10.75 |
| Age | 8.57 ± 2.28 | 7.92 ± 1.85 | 7.61 ± 2.03 | 6.14 ± 2.88 |
| Sex | ||||
| Male | 74.34 ± 51.19a | 36.82 ± 20.64 | 35.72 ± 20.15 | 31.42 ± 18.58 |
| Female | 69.08 ± 52.64a | 35.64 ± 18.56 | 34.54 ± 22.12 | 27.42 ± 13.73 |
| SF | 71.66 ± 51.99 | 36.63 ± 20.32 | 35.43 ± 20.64 | 30.99 ± 18.11 |
| F (%) | 8.61a | 8.37a | 11.04a | 15.05a |
| Grouping | Mini | Max | Mean | SD | Skewness | Kurtosis | 95%CI lower | 95%CI upper |
| HC age | 5.00 | 12.00 | 8.57 | 2.28 | -0.03 | -1.26 | 8.47 | 8.67 |
| HC SF | 1.30 | 200.00 | 71.66 | 51.99 | 0.76 | -0.51 | 69.38 | 50.63 |
| ADHD age | 5.00 | 12.00 | 7.92 | 1.85 | 0.47 | -0.65 | 7.83 | 8.01 |
| ADHD SF | 2.60 | 148.60 | 36.63 | 20.32 | 1.53 | 3.42 | 35.62 | 37.64 |
| TD age | 5.00 | 12.00 | 7.61 | 2.03 | 0.49 | -0.76 | 7.52 | 7.71 |
| TD SF | 2.90 | 262.70 | 35.43 | 20.64 | 2.66 | 15.31 | 34.45 | 36.41 |
| ASD age | 1 | 14.00 | 6.14 | 2.88 | 0.47 | 0.35 | 5.55 | 6.73 |
| ASD SF | 4.7 | 89.6 | 30.98 | 18.11 | 1.25 | 1.41 | 27.26 | 34.71 |
In the liner regression using the fitting least square method, ADHD (β = -0.110, P < 0.001) and TD (β = -0.114, P < 0.001) were both associated with lower level of serum ferritin even after age and sex (see Table 3). In logistic regression analyses, ADHD and TD were not significantly associated with to low serum ferritin concentration in univariate models. And the associations were still not statistically significant in multivariate models. Besides, we found sex 2 was related to the increased risk of low serum ferritin concentration with adjusted OR of 1.38 (95%CI, 1.08-1.75) (see Table 4).
| Model 1 | Model 2 | Model 3 | ||||
| β | P | β | P | β | P | |
| Among children with ADHD | ||||||
| ADHD | -0.107 | < 0.001 | -0.107 | < 0.001 | -0.110 | < 0.001 |
| Age | 0.011 | < 0.001 | 0.011 | < 0.001 | ||
| Sex | 0.021 | < 0.001 | ||||
| Among children with TD | ||||||
| TD | -0.113 | < 0.001 | -0.108 | < 0.001 | -0.114 | < 0.001 |
| Age | 0.001 | < 0.001 | 0.010 | < 0.001 | ||
| Sex | 0.021 | < 0.001 | ||||
| Model 1, OR (95%CI) | Model 2, OR (95%CI) | Model 3, OR (95%CI) | |
| Among children with ADHD | |||
| ADHD | 0.94 (0.74-1.19) | 0.90 (0.71-1.15) | 0.82 (0.63-1.06) |
| Age | 0.68 (0.46-1.01) | 0.68 (0.46-1.01) | |
| Sex | 1.22 (0.94-1.58) | ||
| Among children with TD | |||
| TD | 0.97 (0.77-1.22) | 0.91 (0.72-1.15) | 0.99 (0.78-1.28) |
| Age | 0.65 (0.93-1.56) | 0.65 (0.95-1.53) | |
| Sex | 1.38 (1.08-1.75) | ||
To better present the ferritin levels of children with ADHD and TD in different age groups, we first conducted analysis of variance for different subgroups of different disease groups according to age and found that ferritin levels in different age groups of different disease groups were statistically significant. ASD data were not included in this analysis because of the small sample size of ASD patients grouped by age. More details are provided in Table 5.
| Age (yr) | HC | ADHD | TD |
| 5 | 63.15 ± 52.54 | 28.77 ± 17.54 | 29.73 ± 21.55 |
| 6 | 65.05 ± 48.64 | 31.66 ± 16.38 | 32.68 ± 16.54 |
| 7 | 75.24 ± 55.18 | 33.78 ± 19.16 | 33.99 ± 19.48 |
| 8 | 79.05 ± 53.37 | 37.93 ± 20.34 | 36.63 ± 16.76 |
| 9 | 77.88 ± 52.77 | 43.27 ± 22.41 | 41.92 ± 23.44 |
| 10 | 77.10 ± 49.96 | 43.59 ± 21.15 | 40.74 ± 18.28 |
| 11 | 68.65 ± 49.32 | 42.36 ± 24.06 | 38.17 ± 25.55 |
| 12 | 66.85 ± 52.22 | 32.66 ± 18.77 | 39.16 ± 28.08 |
| F | 3.651 | 14.206 | 9.381 |
| P | 0.001 | 0.000 | 0.000 |
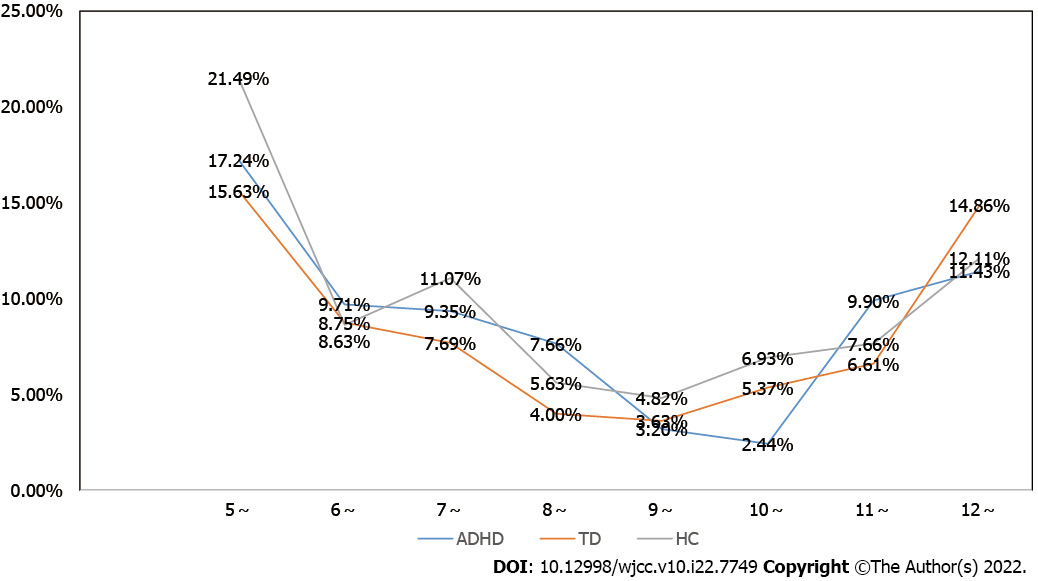
Next, we calculated the incidence of low serum ferritin in different age groups of 5-12 years old and found that in ADHD, TD and healthy controls, as age increased, the incidence of low serum ferritin first decreased and then increased. The high trend, at 10 years old, was the turning point of rising levels. ASD data were not included in this analysis because of the small sample size of ASD patients grouped by age. More details are provided in Figure 2.
This study mainly explores the level of ferritin in children with ADHD and TD and its effects on sex and age. This study is currently the largest sample size study in China to explore ADHD and TD iron deficiency. This study shows that serum ferritin levels are significantly correlated with sex, age and disease type, which is inconsistent with most previous research results on ADHD and TD ferritin levels at home and abroad[9,12,24]. This may be due to the further research results obtained on the basis of fully controlling confounding factors by using more scientific statistical methods. The results showed that the incidence of ADHD with low serum ferritin was 8.37%, and the incidence of TD with low serum ferritin was 11.04%. The incidence of low serum ferritin in ASD was 15.05%, but low serum ferritin was not a risk factor for ADHD or TD. The results are consistent with previous studies[25-27]. At the same time, we reported ferritin levels of children of different ages and sexes, which can provide an important reference for follow-up studies of iron deficiency in children with ADHD and TD.
The results of this study show that the incidence of low serum ferritin deficiency in children 5-12 years old with ADHD is 8.37%, and the incidence of low serum ferritin deficiency in children 5-12 years old with TD is 11.04%, which is related to the normal control group. This is lower than the results of similar studies at home and abroad[12,28]. Low serum ferritin was not a risk factor for ADHD or TD. The possible reason is that the reference value ranges used in different studies are different. This study uses the World Health Organization guidelines and expert consensus on the diagnosis and treatment of iron deficiency in China. The reference value limit is 15 μg/L[29], and the reference value range used in domestic studies on ADHD serum ferritin is 24 μg/L or 30 μg/L[25,27]. In related foreign studies, the limit of ADHD serum ferritin is set higher[12]. Thus, when the cutoff value of serum ferritin levels in children with ADHD is different, the incidence of low serum ferritin deficiency is also different. This highlights the lack of ferritin norms in children with ADHD. Therefore, in future studies, it is necessary to establish serum ferritin standards based on Chinese ADHD samples to provide a more detailed and substantial basis and recommendations for the supplementation of ferritin in children with ADHD.
The results of this study showed that ferritin levels were different at different ages of ADHD and TD, and the incidence of low serum ferritin levels in children with ADHD and TD between 5 and 12 years old decreases first and then increases with age. Ten years old is the turning point. Generally, the age range of 5-10 years old is a period of high incidence for ADHD and TD visits, as well as a period of high incidence of iron deficiency. This suggests that the relationship between iron deficiency and ADHD and TD still needs to be further explored. Although there are many qualitative studies on the relationship between the two[12], there are few studies on the levels of serum ferritin in different age groups. Therefore, based on this research, this study recommends that at the age of 5-10 years, special attention should be given to the assessment of serum ferritin levels in children with ADHD and TD. It is necessary to pay attention to differences in different age groups and determine whether it is possible to establish reference intervals for different age groups of ADHD and TD based on different serum ferritin levels to facilitate more sensitive detection of these levels and timely supplementation. Achieving the greatest improvement in ADHD and TD symptoms will be an important research direction for future ADHD and TD ferritin deficiency investigations and follow-up interventions.
The etiological and physiological mechanisms of ADHD and TD caused by iron deficiency are as follows: First, this metal plays an active role in the anabolism of neurotransmitters, the activity of dopamine D2 receptors, and the concentration of basal ganglia (especially the Globus pallidus). Iron is a cofactor of enzymes necessary for the synthesis and catabolism of monoaminergic neurotransmitters[12,30]. Monoamine neurotransmitters mainly include epinephrine and norepinephrine, 5-hydroxytryptamine, and dopamine. Dopamine neurotransmitters act on the prefrontal lobe and striatum, and dysfunction of the prefrontal striatum plays an important role in the pathogenesis of ADHD. Iron deficiency is related to a decrease in dopamine transporter expression[18], the gene of dopamine transporter is related to the genetic susceptibility of ADHD[31]. Second, iron is a part of neuron development, myelination, DNA synthesis/repair and phospholipid metabolism[1], which is the basis for the neurodevelopmental disease ADHD in children. Third, iron deficiency leads to residual structural defects and the neurological function-related gene imbalance hypothesis[6]. Early nutrient intake (such as malnutrition) during the critical period of life will lead to abnormal structural development, ranging from overall structural abnormalities to fine ultrastructural changes. Therefore, in future research, we need to further explore the inner link between iron deficiency and the occurrence of ADHD symptoms and provide a new perspective for the exploration of the pathophysiological mechanism of ADHD and TD[32].
Future research directions of ADHD and TD iron deficiency are discussed in the following. After combining ADHD and TD ferritin research, ADHD and TD iron deficiency research can be carried out from the following three aspects in the future. First, the sensitivity index and cutoff value of ADHD and TD iron deficiency, or the establishment of a cutoff value of serum ferritin levels in different age groups, can be used to formulate specific guidelines for screening and appropriate iron supplementation. Second, most studies evaluating iron status in ADHD and TD are based on measurements of serum ferritin levels, but there is no strong evidence that serum ferritin is a highly reliable marker of brain iron. Brain iron affects nerve function and white matter myelination. The degree of correlation between serum ferritin and brain iron levels is unclear[29,33]. Therefore, in addition to evaluating the surrounding iron markers, the evaluation of brain iron levels is essential to determine the possible role of iron deficiency in the pathophysiology of ADHD and TD. Third, regarding whether iron supplementation can alleviate the symptoms of ADHD, related research results are inconsistent, and some studies have shown that iron supplementation can improve ADHD and TD[13]. Studies have also found that iron supplementation is related to the increase in serum ferritin levels and the parallel reduction in the severity of ADHD and TD symptoms as assessed by the parents' Conner’s score[15]. These aspects need to be validated further. Therefore, future investigations should include iron-supplemented ADHD and TD cohort studies to provide a new perspective for ADHD and TD intervention research. In terms of iron supplementation, the course, safety and compliance of iron supplementation are also very important references[32,33].
The advantage of our research lies in the extraction of large samples of data. At the same time, the average serum ferritin levels of children with ADHD and TD at 5-12 years of age were analyzed, which is a measure of the serum ferritin levels of children with ADHD and TD at different ages. These measurements can provide a reference for iron supplementation. Some limitations of our study need to be pointed out. First, neurodevelopmental disorders were studied in outpatient cases, and only individuals who used medical resources to seek psychiatric care were identified. Our sampling method involves convenience sampling. There may be some selection bias in the sample; however, the patients included in our study were diagnosed by professional psychiatrists, and the diagnoses were more reliable than self-reported diagnoses. Second, serum ferritin concentrations may be related to inappropriate dietary habits, and the link between neurodevelopmental disorders and altered dietary patterns remains unclear. Third, we did not have personal information that would help us understand patients' risk of mental disorders, such as environmental factors (long-term life stress, traumatic experiences) and a family history of mental disorders. Fourth, our study cannot prove a causal relationship between low serum ferritin and neurodevelopmental disease, although our results suggest a significant association between neurodevelopmental disease and serum ferritin.
Neurodevelopmental disorders (ADHD, TD and ASD) are heterogeneous diseases. The relationship between ADHD and TD and serum ferritin needs further exploration. We found that the incidence of low serum ferritin levels in children with ADHD and TD between 5-12 years old was 8.37% and 11.04%, respectively. The incidence of ASD with low serum ferritin was 15.05%. It is recommended to routinely check the serum ferritin levels and related hematological indicators of children with ADHD, TD and ASD and to perform necessary iron supplementation. In particular, children with ADHD and TD aged 5-10 years were diagnosed. In the future, we need to conduct cohort studies to further consolidate the evidence of iron deficiency in children with ADHD, TD and ASD and carry out necessary iron intervention studies to explore the underlying mechanisms.
Iron deficiency in early childhood can lead to developmental abnormalities in gene expression, neurotransmitter function, neurometabolism and other aspects related to brain development. it has been reported that iron deficiency in children is associated with neurodevelopmental diseases. Serum ferritin is one of the most reliable and widely used markers of iron storage status in the body. This study will explore the current status of iron deficiency in children with neurodevelopmental disorders and its influence on sex and age, providing an important reference for the correlation between neurodevelopmental disorders and ferritin and necessary iron supplementation.
The level of iron deficiency in neurodevelopmental disorders of attention deficit disorder hyperactivity (ADHD), tic disorder (TD) and autism spectrum disorders (ASD) and the status of sex-age effects are still unclear, and data from Chinese samples are lacking. This study will adopt the thinking of retrospective research and investigation by enrolling ADHD, TD and ASD children in the psychiatric department of Beijing Children's Hospital as the sample. The sample size is large, and more attention can be paid to the comparison of the difference in serum ferritin levels of ADHD, TD and ASD children with respect to gender and age than previous studies.
This study will explore the current status of iron deficiency in children with neurodevelopmental disorders and its influence on sex and age, providing an important reference for the correlation between neurodevelopmental disorders and ferritin and necessary iron supplementation.
A total of 1565 children with ADHD, 1694 children with TD, 93 children with ASD and 1997 healthy control children were included between January 1, 2020, and December 31, 2021 at Beijing Children's Hospital. We describe the differences in age levels and ferritin levels between different disease groups and their sex differences. T test, Chi-square analysis, variance analysis and regression analysis were used for statistical processing of the data.
The average serum ferritin levels of male and female children were 36.82 ± 20.64 μg/L and 35.64 ± 18.56 μg/L in 1565 ADHD patients. The average serum ferritin levels of male and female children were 35.72 ± 20.15 μg/L and 34.54 ± 22.12 μg/L in 1694 TD patients. As age increased, the incidence of low serum ferritin in ADHD and TD first decreased and then increased, and 10 years old was the turning point of rising levels. The incidence of ADHD with low serum ferritin was 8.37%, the incidence of TD with low serum ferritin was 11.04%, and the incidence of the healthy control group with low serum ferritin was 8.61% (P < 0.05). There may be some selection bias and confounding factors such as diet, environmental factors and family history in the sample, and our study cannot prove a causal relationship between low serum ferritin and neurodevelopmental disease.
Neurodevelopmental disorders (ADHD, TD and ASD) are heterogeneous diseases. We found that the incidence of low serum ferritin levels in children with ADHD and TD between 5-12 years old was 8.37% and 11.04%, respectively. The incidence of ASD with low serum ferritin was 15.05%. It is recommended to routinely check the serum ferritin levels and related hematological indicators of children with ADHD, TD and ASD and to perform necessary iron supplementation. In particular, children with ADHD and TD aged 5-10 years were diagnosed.
In the future, we need to conduct cohort studies to further consolidate the evidence of iron deficiency in children with ADHD, TD and ASD and carry out necessary iron intervention studies to explore the underlying mechanisms.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Salmanian M, Iran; Singh P, India S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Bakoyiannis I, Gkioka E, Daskalopoulou A, Korou LM, Perrea D, Pergialiotis V. An explanation of the pathophysiology of adverse neurodevelopmental outcomes in iron deficiency. Rev Neurosci. 2015;26:479-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Berglund S, Domellöf M. Meeting iron needs for infants and children. Curr Opin Clin Nutr Metab Care. 2014;17:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Avrahami M, Barzilay R, HarGil M, Weizman A, Watemberg N. Serum Ferritin Levels Are Lower in Children With Tic Disorders Compared with Children Without Tics: A Cross-Sectional Study. J Child Adolesc Psychopharmacol. 2017;27:192-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Chan A, Karpel H, Spartz E, Willett T, Farhadian B, Jeng M, Thienemann M, Frankovich J. Hypoferritinemia and iron deficiency in youth with pediatric acute-onset neuropsychiatric syndrome. Pediatr Res. 2021;89:1477-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Mattei D, Pietrobelli A. Micronutrients and Brain Development. Curr Nutr Rep. 2019;8:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Barks A, Hall AM, Tran PV, Georgieff MK. Iron as a model nutrient for understanding the nutritional origins of neuropsychiatric disease. Pediatr Res. 2019;85:176-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 843] [Article Influence: 93.7] [Reference Citation Analysis (4)] |
| 8. | Sayers D, Hu Z, Clark LL. Attrition Rates and Incidence of Mental Health Disorders in an Attention-Deficit/Hyperactivity Disorder (ADHD) Cohort, Active Component, U.S. Armed Forces, 2014-2018. MSMR. 2021;28:2-8. [PubMed] |
| 9. | Juneja M, Jain R, Singh V, Mallika V. Iron deficiency in Indian children with attention deficit hyperactivity disorder. Indian Pediatr. 2010;47:955-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Mahmoud MM, El-Mazary AA, Maher RM, Saber MM. Zinc, ferritin, magnesium and copper in a group of Egyptian children with attention deficit hyperactivity disorder. Ital J Pediatr. 2011;37:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Calarge C, Farmer C, DiSilvestro R, Arnold LE. Serum ferritin and amphetamine response in youth with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2010;20:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Wang Y, Huang L, Zhang L, Qu Y, Mu D. Iron Status in Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis. PLoS One. 2017;12:e0169145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Sever Y, Ashkenazi A, Tyano S, Weizman A. Iron treatment in children with attention deficit hyperactivity disorder. A preliminary report. Neuropsychobiology. 1997;35:178-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 78] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Parisi P, Villa MP, Donfrancesco R, Miano S, Paolino MC, Cortese S. Could treatment of iron deficiency both improve ADHD and reduce cardiovascular risk during treatment with ADHD drugs? Med Hypotheses. 2012;79:246-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Konofal E, Lecendreux M, Deron J, Marchand M, Cortese S, Zaïm M, Mouren MC, Arnulf I. Effects of iron supplementation on attention deficit hyperactivity disorder in children. Pediatr Neurol. 2008;38:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Chen MH, Su TP, Chen YS, Hsu JW, Huang KL, Chang WH, Chen TJ, Bai YM. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: a nationwide population-based study. BMC Psychiatry. 2013;13:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Ghosh D, Burkman E. Relationship of serum ferritin level and tic severity in children with Tourette syndrome. Childs Nerv Syst. 2017;33:1373-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Adisetiyo V, Jensen JH, Tabesh A, Deardorff RL, Fieremans E, Di Martino A, Gray KM, Castellanos FX, Helpern JA. Multimodal MR imaging of brain iron in attention deficit hyperactivity disorder: a noninvasive biomarker that responds to psychostimulant treatment? Radiology. 2014;272:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Paus T. Investigating the Role of Micronutrients in Brain Development and Psychiatric Disorders via Magnetic Resonance Imaging. JAMA Psychiatry. 2018;75:880-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Latif A, Heinz P, Cook R. Iron deficiency in autism and Asperger syndrome. Autism. 2002;6:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Dosman CF, Drmic IE, Brian JA, Senthilselvan A, Harford M, Smith R, Roberts SW. Ferritin as an indicator of suspected iron deficiency in children with autism spectrum disorder: prevalence of low serum ferritin concentration. Dev Med Child Neurol. 2006;48:1008-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Gunes S, Ekinci O, Celik T. Iron deficiency parameters in autism spectrum disorder: clinical correlates and associated factors. Ital J Pediatr. 2017;43:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Tseng PT, Cheng YS, Chen YW, Stubbs B, Whiteley P, Carvalho AF, Li DJ, Chen TY, Yang WC, Tang CH, Chu CS, Liang HY, Wu CK, Yen CF, Lin PY. Peripheral iron levels in children with autism spectrum disorders vs controls: a systematic review and meta-analysis. Nutr Res. 2018;50:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Millichap JG, Yee MM, Davidson SI. Serum ferritin in children with attention-deficit hyperactivity disorder. Pediatr Neurol. 2006;34:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Topal Z, Tufan AE, Karadag M, Gokcen C, Akkaya C, Sarp AS, Bahsi I, Kilinc M. Evaluation of peripheral inflammatory markers, serum B12, folate, ferritin levels and clinical correlations in children with autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD). Nord J Psychiatry. 2022;76:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Gorman DA, Zhu H, Anderson GM, Davies M, Peterson BS. Ferritin levels and their association with regional brain volumes in Tourette's syndrome. Am J Psychiatry. 2006;163:1264-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | De Giacomo A, Medicamento S, Pedaci C, Giambersio D, Giannico OV, Petruzzelli MG, Simone M, Corsalini M, Marzulli L, Matera E. Peripheral Iron Levels in Autism Spectrum Disorders vs. Other Neurodevelopmental Disorders: Preliminary Data. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Cortese S, Konofal E, Bernardina BD, Mouren MC, Lecendreux M. Sleep disturbances and serum ferritin levels in children with attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2009;18:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Red Cell Diseases (Anemia) Group; Chinese Society of Hematology; Chinese Medical Association. Multidisciplinary expert consensus on the diagnosis, treatment and prevention of iron deficiency and iron deficiency anemia. Zhonghua Yixue Zazhi. 2018;98:2233-2237. |
| 30. | Cortese S, Angriman M, Lecendreux M, Konofal E. Iron and attention deficit/hyperactivity disorder: What is the empirical evidence so far? Expert Rev Neurother. 2012;12:1227-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Lozoff B, Castillo M, Clark KM, Smith JB. Iron-fortified vs low-iron infant formula: developmental outcome at 10 years. Arch Pediatr Adolesc Med. 2012;166:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | Degremont A, Jain R, Philippou E, Latunde-Dada GO. Brain iron concentrations in the pathophysiology of children with attention deficit/hyperactivity disorder: a systematic review. Nutr Rev. 2021;79:615-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Donfrancesco R, Parisi P, Vanacore N, Martines F, Sargentini V, Cortese S. Iron and ADHD: time to move beyond serum ferritin levels. J Atten Disord. 2013;17:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |