Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.7674
Peer-review started: March 16, 2022
First decision: April 11, 2022
Revised: May 5, 2022
Accepted: June 30, 2022
Article in press: June 30, 2022
Published online: August 6, 2022
Processing time: 127 Days and 20.4 Hours
Inflammatory bowel disease (IBD) is a complex chronic IBD that is closely associated with risk factors such as environment, diet, medications and lifestyle that may influence the host microbiome or immune response to antigens. At present, with the increasing incidence of IBD worldwide, it is of great significance to further study the pathogenesis of IBD and seek new therapeutic targets. Traditional Chinese medicine (TCM) treatment of diseases is characterized by multiple approaches and multiple targets and has a long history of clinical application in China. The mechanism underlying the effect of zedoary turmeric-trisomes on inducing mucosal healing in IBD is not clear.
To explore the effective components and potential mechanism of zedoary turmeric-trisomes in the treatment of IBD with intestinal fibrosis using network pharmacology and molecular docking techniques.
The chemical constituents and targets of Rhizoma zedoary and Rhizoma sanarum were screened using the TCMSP database. The GeneCards database was searched to identify targets associated with intestinal fibrosis in IBD. The intersection of chemical component targets and disease targets was obtained using the Venny 2.1 online analysis platform, and the common targets were imported into the STRING 11.0 database to construct a protein interaction regulatory network. A “zedoary turmeric-trisomes-chemical composition-target-disease” network diagram was subsequently constructed using Cytoscape 3.7.2 software, and the topological properties of the network were analyzed using the “Network Analysis” plug-in. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses of the common targets were performed using the DAVID 6.8 database to elucidate the mechanism of zedoary turmeric-trisomes in the treatment of IBD. Subsequently, molecular docking of the compounds and targets with the highest intermediate values in the “zedoary turmeric-trisomes-chemical composition-target-disease” network was performed using Sybyl-x 2.1.1 software.
A total of 5 chemical components with 60 targets were identified, as well as 3153 targets related to IBD and 44 common targets. The protein-protein interaction network showed that the core therapeutic targets included JUN, MAPK14, CASP3, AR, and PTGS2. The GO enrichment analysis identified 759 items, and the KEGG enrichment analysis yielded 52 items, including the cancer pathway, neuroactive ligand-receptor interaction, hepatitis B, and the calcium signaling pathway, reflecting the complex biological processes of the multicomponent, multitarget and multipathway treatment of diseases with zedoary turmeric-trisomes. Molecular docking showed that the compound bonded with the target through hydrogen bond interactions and exhibited good docking activity.
This study identified the potential mechanism of action of zedoary turmeric-trisomes in the treatment of inflammatory bowel fibrosis using network pharmacology and molecular docking technology, providing a scientific basis for further expansion of their clinical use.
Core Tip: Intestinal fibrosis is one of the common complications of inflammatory bowel disease (IBD). Finding effective drug treatment is an important issue that needs to be solved at the moment. The mechanism of zedoary turmeric-trisomes in the treatment of IBD with intestinal fibrosis can be predicted through network pharmacology and molecular docking, so as to provide theoretical reference for it to better play its therapeutic role.
- Citation: Zheng L, Ji YY, Dai YC, Wen XL, Wu SC. Network pharmacology and molecular docking reveal zedoary turmeric-trisomes in Inflammatory bowel disease with intestinal fibrosis. World J Clin Cases 2022; 10(22): 7674-7685
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/7674.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.7674
Intestinal fibrosis is a common complication of inflammatory bowel disease (IBD)[1]. In severe cases, intestinal obstruction leads to a decrease in the quality of life of patients; most patients require surgical intervention, which poses a major challenge for clinicians[2]. Fibrosis forms after repeated stimulation of intestinal tissue by long-term chronic inflammation. Similar to fibrosis in other organs, the potential mechanism of action is complex and may be related to the interactions between a variety of cells and cytokines[3]. Due to the destruction of the epithelial barrier observed in IBD, intestinal bacterial products penetrate the stroma, and the epithelial gap induced by immune cells and immune cell activation mediates the innate immune response[4]. Changes in environmental factors and chronic inflammation due to repeated stimulation result in obvious changes in intestinal extracellular matrix components and, through their mechanical properties, facilitate the formation of fibrosis[5]. The traditional view that intestinal fibrosis is an inevitable and irreversible process is gradually changing based on studies of its underlying pathological, cellular and molecular mechanisms[6]. In addition, clinical studies have shown that stenosis formation is reversible in patients undergoing septoplasty[7]. Previously prescribed drugs, such as mesalazine and risadomide, cannot prevent or improve intestinal fibrosis, and their side effects are obvious[8]. Therefore, the use of traditional Chinese medicine (TCM) for the treatment of intestinal fibrosis is gradually increasing due to the advantages being safe and having stable efficacy and no obvious side effects.
Zedoary turmeric-trisomes are from the three-rib-pill prescription written by Yao Jun in the Qing dynasty. Studies have shown that zedoary turmeric-trisomes can inhibit or reverse fibrosis changes in multiple organs[9,10], but the specific active components and their interactions remain unclear. In this study, the mechanism of action of zedoary turmeric-trisomes in the treatment of IBD intestinal fibrosis was systematically studied using a network pharmacology approach. The virtual binding of compounds in zedoary turmeric-trisomes to receptor molecules was analyzed by molecular docking to identify the targets and binding sites, and the potential mechanism of action of zedoary turmeric-trisomes in the treatment of IBD intestinal fibrosis was elucidated. This study provides a theoretical and scientific basis for further research.
The TCM Systems Pharmacology (TCMSP) database and analysis platform can be used to predict the absorption, distribution, metabolism and excretion of TCM chemical components in vivo and to screen for the targets of chemical components. The TCMSP database (https://tcmspw.com/tcmsp.php)[11] was searched using the keywords “zedoary turmeric” and “trisomes” to determine the chemical composition; the criteria of an oral bioavailability (OB) of 30% or higher and a medicinal property (drug likeness, DL) of 0.18 or higher were used to filter the results. The PubChem database (https://pubchem.ncbi.nlm.nih.gov/)[12] was used to check the name of the chemical components and molecular structure, and the information for the qualified compounds was imported into Excel. Moreover, the TCMSP database was queried to retrieve the action targets of the chemical components in an Excel table, and the targets were imported into the UniProt database (https://www.UniProt.org/)[13] to correct the names of the targets for standardization purposes. The chemical components and action targets of the abovementioned drug pairs were classified, sorted and saved in Excel for future use.
The GeneCards database is a comprehensive database of human genes that integrates multiple genetic database resources. Using the keywords “inflammatory bowel diseases-intestinal fibrosis”, we searched the GeneCards (https://www.genecards.org/) database to identify associations between IBD and intestinal fibrosis target information.
The notable drug targets included Rhizoma zedoariae trisomes based on an analysis using the Venny 2.1 online platform (https://bioinfogp.cnb.csic.es/tools/venny/), IBD intestinal fibrosis target mapping, a Venn diagram and common targets. The common targets were identified as potential targets of zedoary turmeric-trisomes in the treatment of IBD intestinal fibrosis. The common targets were imported into the STRING 11.0 database (https://string-db.org/), the species was limited to Homo sapiens, the minimum required interaction score was set to ≥ 0.7, and the option “Hide disconnected nodes in the network” was selected to construct the protein-protein interaction (PPI) network. An analysis of the topological properties of the network was conducted to predict the core regulatory targets in the network.
Data files for the chemical components, corresponding targets and diseases were prepared and imported into Cytoscape 3.7.2 software to construct a network diagram of “zedoary turmeric-trisomes - chemical component - target - disease”. The “Network Analysis” plug-in was used to analyze the topological properties of the network and to measure the importance of the various nodes in the network according to the node degree value. Thus, the mechanism of action of zedoary turmeric trisomes on IBD intestinal fibrosis was predicted.
The DAVID database is an online functional annotation system based on a web server for gene function enrichment analysis and pathway enrichment analysis that includes analysis tools and biological knowledge bases and is mainly used for the identification of target function and pathway information, disease analysis and other analyses. To further understand the function of a target and its role in the signaling pathway, DAVID (https://david.ncifcrf.gov/) was used to identify 1.3 and 6.8 common targets for subsequent Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses using “Homo sapiens” as the background. To elucidate the potential mechanism of zedoary turmeric-trisomes in the treatment of IBD intestinal fibrosis, GO and KEGG pathway enrichment analyses based on biological process (BP), cellular component (CC) and molecular function (MF) were performed on the potential targets.
Molecular docking is a widely used computer virtual screening technology for predicting the interaction mode and affinity between a ligand and a receptor that is based on geometric and energy matching principles. In this study, the Chemical Book database (https://www.chemicalbook.com/Product
Using “zedoary” and “trisomes” as the keywords, 81 chemical constituents of zedoary and 30 chemical constituents of trisomes were obtained from a search of the TCMSP database. After applying the filters for OB ≥ 30% and DL ≥ 0.18 and removing compounds without targets, 5 active components were obtained, which corresponded to 60 targets, and these included 1 chemical component of zedoary and 5 chemical components of trisomes. These compounds include the components common to zedoary turmeric and trisomes. Table 1 shows the chemical composition of zedoary turmeric trisomes.
| No. | Compound ID | Compound name | Oral bioavailability (%) | Dibenzodiazepines | Herbs |
| 1 | MOL001297 | Trans-gondoic acid | 30.7 | 0.20 | Trisomes |
| 2 | MOL000358 | Beta-sitosterol | 36.91 | 0.75 | Trisomes |
| 3 | MOL000392 | Formononetin | 69.67 | 0.21 | Trisomes |
| 4 | MOL000449 | Stigmasterol | 43.83 | 0.76 | Trisomes |
| 5 | MOL000296 | Hederagenin | 36.91 | 0.75 | Trisomes, zedoary turmeric |
A total of 3153 targets related to intestinal fibrosis in IBD were retrieved from the GeneCards database using the key words “inflammatory bowel disease-intestinal fibrosis”.
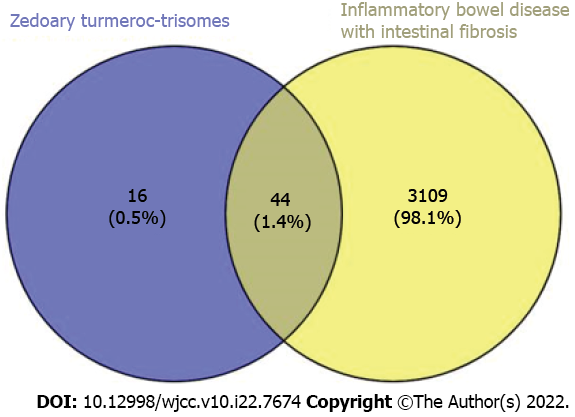
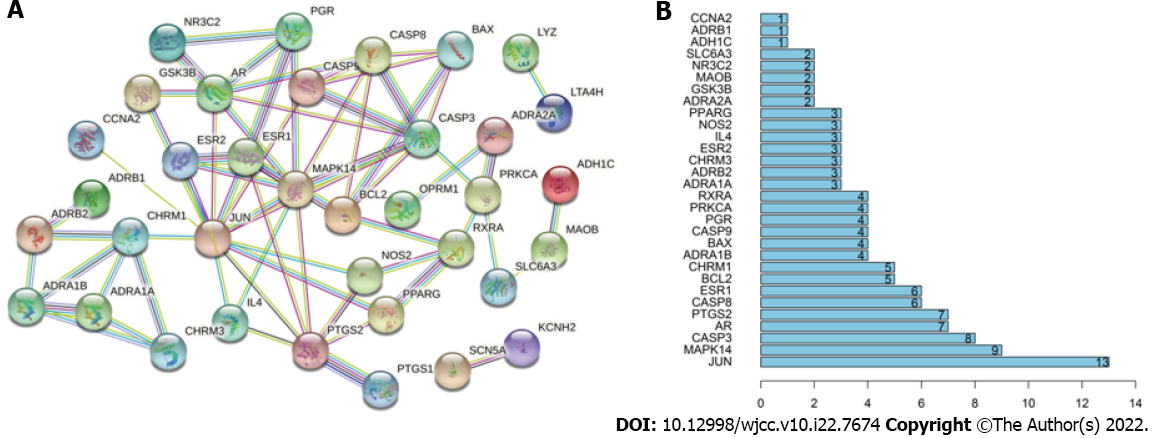
Sixty targets of the active ingredients of zedoary turmeric-trisomes and 3153 targets of enteric fibrosis in IBD were introduced into Venny 2.1 for intersection operation, and 44 common targets were obtained, reflecting the synergistic effect of zedoary turmeric-trisomes on multiple targets in the treatment of diseases (Figure 1). Forty-four common targets were imported into the STRING database, the species was set to Homo sapiens, and the minimum required interaction score was set to ≥ 0.7. After selecting the option “Hide disconnected nodes in the network”, the PPI network was generated (Figure 2A). The network involved 36 nodes with 65 edges, and the average node degree was 3.61. The node degree represents the number of edges connected to a specific node in the network. The larger the degree is, the more critical the node. Figure 2B shows the information for the top 30 targets in the PPI network based on node degree values, and the node degrees of JUN, MAPK14, CASP3, AR, PTGS2, CASP8, ESR1, BCL2, CHRM1, ADRA1B, BAX, CASP9, PGR, PRKCA and RXRA were greater than 3.61 (the average node degree value). These results indicate that these nodes interact more with other proteins in the network, which suggests that these targets play important roles in the treatment of IBD intestinal fibrosis.
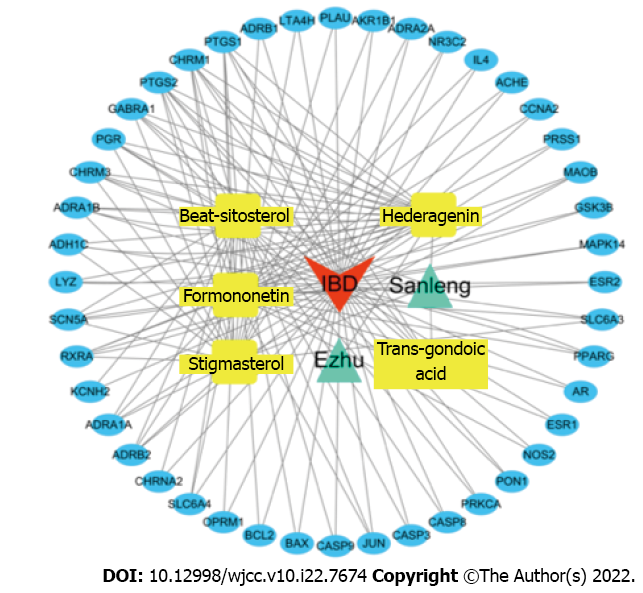
Cytoscape 3.7.2 software was used to visualize the “zedoary turmeric-trisomes - chemical composition - target - disease” regulatory network. The “Network Analyzer” plug-in was used to analyze the topological properties of the network, and the node degree was used as an important indicator describing the nodes in the network. As shown in Figure 3, the red arrow represents IBD, the green triangle represents TCM, the yellow square represents chemical components, and the blue oval represents the targets of the chemical components. The network consisted of 52 nodes, including 1 disease node, 2 TCM nodes, 5 chemical component nodes and 44 chemical component action targets. The average degree of the chemical components in the network was 19.20, and the average degree of the chemical component action targets was 3.05. An analysis of the compounds revealed that the degrees of hederagenin, beta-sitosterol, formononetin and stigmasterol were greater than 19.20. Regarding the targets, 12 had degrees greater than 3.05, and the top 5 targets were identified as PTGS1, CHRM1, PTGS2, GABRA1 and PGR, reflecting the synergistic effect of zedoary turmeric-trisomes on the treatment of IBD based on multiple components and multiple targets. The degree values of the compounds (No. 1-5) and targets (No. 6-49) are shown in Table 2.
| No. | Node | Degree | No. | Node | Degree |
| 1 | Hederagenin | 24 | 26 | OPRM1 | 2 |
| 2 | Beta-sitosterol | 23 | 27 | BCL2 | 2 |
| 3 | Formononetin | 23 | 28 | BAX | 2 |
| 4 | Stigmasterol | 21 | 29 | CASP9 | 2 |
| 5 | Trans-gondoic acid | 5 | 30 | CASP3 | 2 |
| 6 | PTGS1 | 7 | 31 | CASP8 | 2 |
| 7 | CHRM1 | 7 | 32 | PRKCA | 2 |
| 8 | PTGS2 | 7 | 33 | PON1 | 2 |
| 9 | GABRA1 | 6 | 34 | NOS2 | 2 |
| 10 | PGR | 5 | 35 | ESR1 | 2 |
| 11 | CHRM3 | 5 | 36 | AR | 2 |
| 12 | ADRA1B | 5 | 37 | ESR2 | 2 |
| 13 | SCN5A | 5 | 38 | MAPK14 | 2 |
| 14 | RXRA | 5 | 39 | GSK3B | 2 |
| 15 | ADH1C | 4 | 40 | PRSS1 | 2 |
| 16 | ADRA1A | 4 | 41 | CCNA2 | 2 |
| 17 | ADRB2 | 4 | 42 | ACHE | 2 |
| 18 | LYZ | 3 | 43 | IL4 | 2 |
| 19 | SLC6A4 | 3 | 44 | NR3C2 | 2 |
| 20 | JUN | 3 | 45 | ADRA2A | 2 |
| 21 | PPARG | 3 | 46 | AKR1B1 | 2 |
| 22 | SLC6A3 | 3 | 47 | PLAU | 2 |
| 23 | MAOB | 3 | 48 | LTA4H | 2 |
| 24 | KCNH2 | 2 | 49 | ADRB1 | 2 |
| 25 | CHRNA2 | 2 |
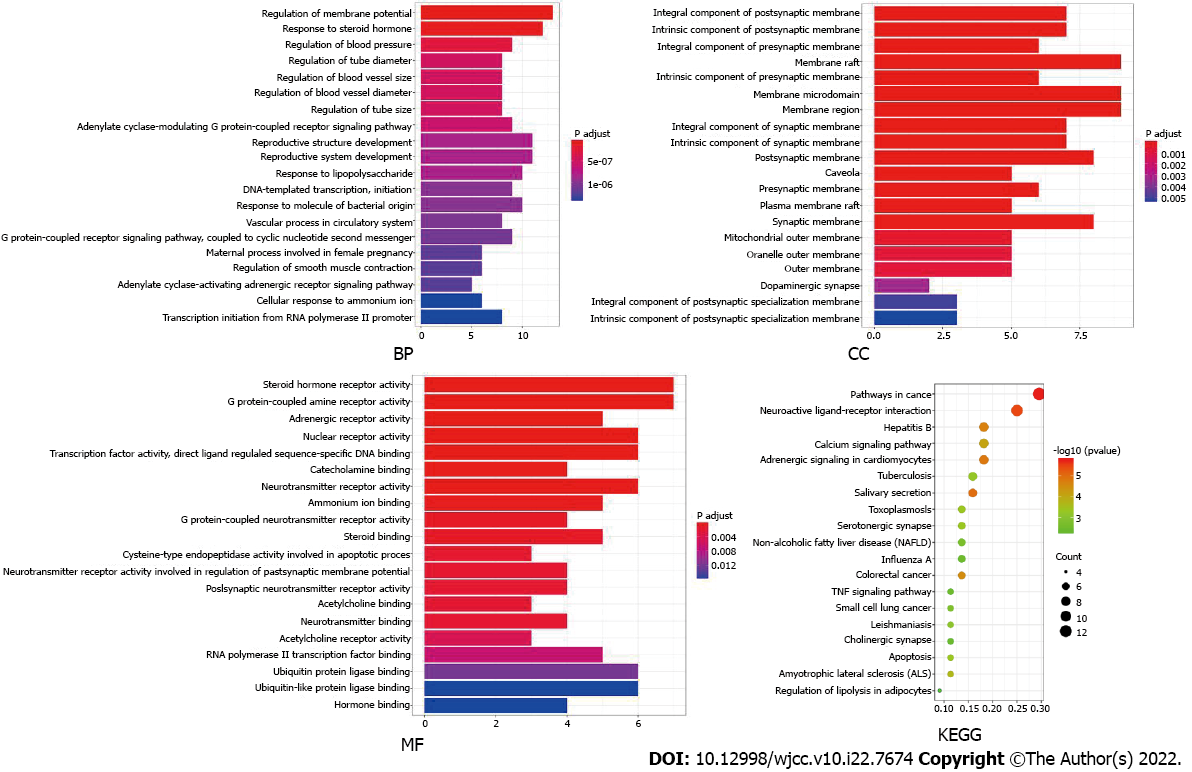
GO and KEGG enrichment analyses of the common targets were performed using the DAVID 6.8 database. A total of 759 items were obtained from the GO enrichment analysis, including 629 BP items, 41 CC items and 89 MF items. The results from the GO enrichment analysis were then sorted according to the corrected P-value, and a bar chart of the top 20 items was generated (Figure 4). The length of each bar in the figure represents the number of enriched genes, and the color difference represents the significance of the gene enrichment. The top BP terms included regulation of the membrane potential, response to steroid hormones, regulation of blood pressure, regulation of tube diameter, regulation of blood vessel size, and regulation of blood vessel diameter. The top CC terms included the intrinsic component of the postsynaptic membrane, the integral component of the postsynaptic membrane, the intrinsic component of the presynaptic membrane (membrane), the integral component of the presynaptic membrane (raft), the intrinsic component of the presynaptic membrane, and the membrane microdomain. The top MF terms included steroid hormone receptor activity and G protein-coupled amine receptor activity. Adrenergic receptor activity, nuclear receptor activity, transcription factor activity, and sequence-specific DNA binding activity are directly regulated by ligands, which also regulate sequence-specific DNA binding and catecholamine binding, among other processes.
The KEGG enrichment analysis yielded 52 items, and the top 19 items were selected to create a bubble map for visualization purposes according to the number of enriched genes, as shown in Figure 4. In the figure, the bubble size represents the number of enriched genes, and the color difference represents the significance of the gene enrichment. The KEGG enrichment results mainly involved pathways in cancer, neuroactive ligand-receptor interaction, hepatitis B, the calcium signaling pathway, adrenergic signaling in cardiomyocytes, and tuberculosis.
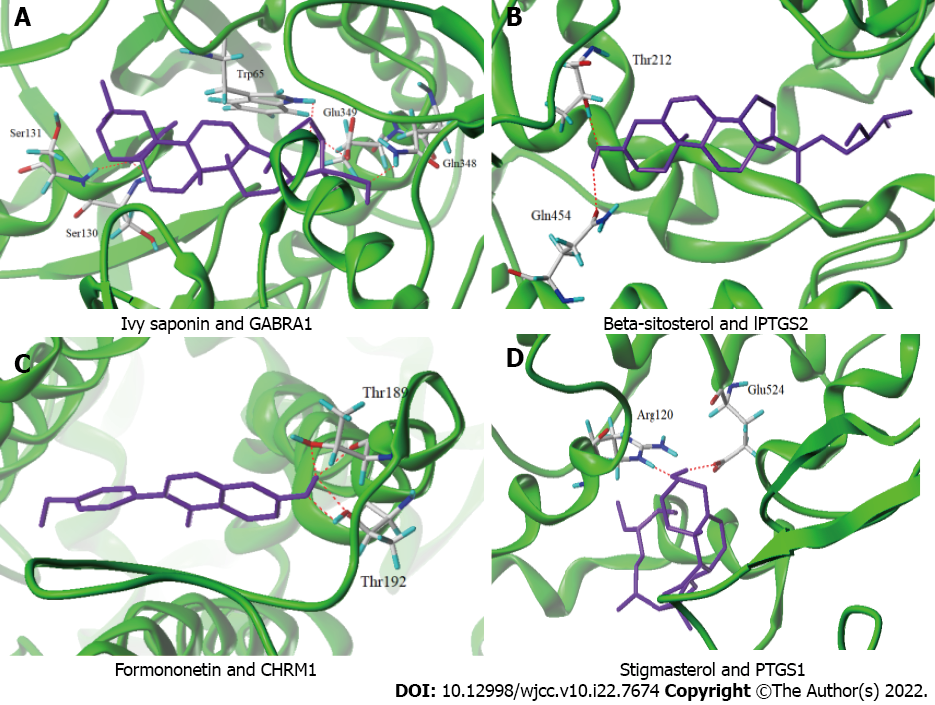
The top 4 target proteins (PTGS1, CHRM1, PTGS2 and GABRA1) in the network constructed as described in Section 2.4 were extracted. Sybyl-x 2.1.1 was used for the molecular docking of hederagenin, beta-sitosterol, formononetin and stigmasterol. In the docking results (Figure 5), the purple color represents the above four compounds, and the red dotted lines represent hydrogen bond interactions. As shown in Figure 5A, ivy saponins interact with GABRA1 through Trp65, Ser130, Ser131, Gln348 and Glu349, and as depicted in Figure 5B, β-sitosterol interacts with PTGS2 through hydrogen bonds via Thr212 and Gln454. Moreover, as indicated in Figure 5C, formononetin interacts with CHRM1 through hydrogen bond interactions between Thr189 and Thr192, and as illustrated in Figure 5D, stigmasterol interacts with PTGS1 through hydrogen bond interactions between Arg120 and Glu524. In conclusion, the compounds saponin, β-sitosterol, formononetin and stigmasterol interact with GABRA1, PTGS2, CHRM1 and PTGS1 through hydrogen bonds and thus fully bind to the active site of the target protein, exhibiting good binding activity with the target protein.
Network pharmacology aims to further clarify the interactions and mechanism of a TCM and provide new strategies for drug research and development through the construction of a “drug - target - disease” network[15]. As an auxiliary screening method, molecular docking technology has been widely used to analyze the features of pharmacodynamic substances of TCM, to search for drug targets and to explore the mechanism of action of a TCM[16]. Network pharmacology combined with molecular docking technology has shown significant advantages in basic research on the activity of a TCM[17]. In this study, through the construction of the “drug - component - target - disease” network, the 8 active ingredients with higher degree values were analyzed to provide a reference for identifying the compatibility relationship between zedoary turmeric and trisomes and were found to jointly participate in the regulation of IBD intestinal fibrosis[18]. Studies have proven that ivy saponin has anti-inflammatory, anticoagulant, antidepression, antitumor, antibacterial and other effects[19]. β-Sitosterol exerts antioxidant, cholesterol-lowering, anti-inflammatory, immunomodulatory and antitumor effects and can also enhance the secretion of IL-2 and interferon-γ, inhibit the secretion of IL-4, and exert anti-inflammatory effects by inhibiting IL-6 and tumor necrosis factor[20]. Formononetin, as an active component of flavonoids extracted naturally, was recently applied in a variety of disease models as a new inflammatory inhibitor and may have potential therapeutic value for IBD by activating Nrf2 expression[21]. Zielińska et al[22] showed that stigmasterol significantly reduced the inflammatory factors IL-1β, IL-6, and MCP-1 and the related cytokine synthase COX-2, inhibited colon shortening, and reduced the severity of IBD. These researchers also found that stigmasterol had greater anti-inflammatory activity than β-sitosterol and inhibited the symptoms of colitis[23]. In this study, the molecular docking results showed that hederagenin, β-sitosterol, formononetin and stigmasterol might be the core components of zedoary turmeric and trisomes involved in the treatment of intestinal fibrosis and might play an antifibrotic role through immune regulation and anti-inflammatory effects[24]. According to the synergistic effects of multiple components, we speculate that trisomes regulate immunity and exhibit anti-inflammatory effects in the treatment of IBD intestinal fibrosis, whereas zedoary aids the treatment of IBD intestinal fibrosis and improves body function[25].
Further analysis of the core targets in the “drug - chemical - target - disease” network and PPI network revealed that the top 5 targets were PTGS1, CHRM1, PTGS2, GABRA1 and PGR, suggesting that these genes may be the core targets in the treatment of IBD intestinal fibrosis. Among the top 30 targets in the PPI network based on the node degree value, the cancer-related targets included AR, ESR1, BCL2, CHRM1, ADRA1B, BAX, PGR, RXRA, CCNA2, ADRB1, ADH1C, SLC6A3, NR3C2, MAOB, ADRA2A, PPARG, PRKCA, NOS2, ESR2, CHRM3, ADRB2, and ADRA1A. The targets associated with hepatitis B were CCNA2, ADRB1, MAOB, and ADRB2, and the immunomodulatory targets included IL-4 and MAPK14. The targets associated with inflammation are PTGS2, PPARG and IL-4, and the targets related to the calcium signaling pathway, which include CHRM1, CHRM3, CASP3, CASP8, and CASP9, interact with the neuroactive ligand receptor. Recent studies have found that CASP activation can be observed in tumor cell apoptosis. CASP refers to a family of cysteine aspartate-specific proteases, and these proteases are the main enzymes that perform cell apoptosis and are involved in the occurrence and development of various diseases, particularly tumors and autoimmune diseases[26]. As a key protease, CASP8 participates in the transmission of exogenous apoptosis signals in mammals, and CASP9 plays a crucial role in the mitochondria-mediated endogenous apoptosis pathway[27]. CASP3 plays a central role as a key protease in apoptosis and is also known as the “death protease”, and the cascade reaction of apoptosis continues after the action of this enzyme[28]. Immune factors, including helper T cells, regulatory T cells, cytokines and autoantibodies, play an important role in the pathogenesis and progression of IBD[29]. The cytokine IL-4 plays an important role in regulating intestinal barrier function, is mainly synthesized by activated lymphocytes, and can inhibit the production of other cytokines, including IL-1, IL-6, IL-8 and TNF-α, and the generation of lymphocytes and macrophages[30]. Several studies have reported that in IBD, IL-4 significantly reduced vascular endothelial growth factor (VEGF) and inhibited the formation of blood vessels[31], but the expression of VEGF in the tumor tissues of patients with colorectal tumors was significantly increased[32]. The results of this study indicated that PRKCA was involved in the regulation of the proliferation, differentiation, survival, migration, cell polarity and cell cycle of various cancer cells; in mediating cell proliferation, differentiation, cell cycle progression and apoptosis; in regulating gene expression; and in promoting tumor formation and metastasis and participates in metabolic regulation[33]. The PTGS2 gene is induced by COX2 and is involved in the inflammatory response, cell proliferation, apoptosis and other pathological processes[34]. Further analysis of the core targets in the “drug - components - target - disease” network and PPI network revealed that the mechanism of action of zedoary turmeric-trisomes in the treatment of IBD intestinal fibrosis was primarily related to inflammatory factors and antitumor and immune regulation[35].
Compared with other organs where fibrosis occurs, the gut is the only organ where a large number of microorganisms coexist, and gut microbes have a profound impact on mucosal homeostasis between health and disease. Changes in the intestinal barrier can cause bacteria to migrate into the intestinal mucosa or portal vein circulation, which alters the host-microbe interactions that are key to intestinal inflammation[36]. In particular, gut cells can sense microbe-derived, pathogen-associated molecular patterns through pattern recognition receptors[37].
TCMs have achieved good results in the treatment of diseases through dialectical treatment and a holistic approach[38]. TCM or TCM compounds have multiple components and can have a regulatory role through multiple channels, multiple targets and multiple links[39]. The typical characteristic of IBD intestinal fibrosis is intestinal fibrosis formed by stenosis, which seriously affects the quality of life of patients[40]. This study focused on the factors and mechanisms related to fibrosis and found that trisomes and zedoary had therapeutic effects through inflammatory factor regulation, immune regulation, antitumor activity and other pathways[41,42]. Vermeire et al[43] used a San-ling pill prescription combined with Western medicine to reduce the Crohn’s disease activity index score, platelet activity index and D-dimer index of patients with Crohn’s disease and to improve the rate of endoscopic fibrosis. The underlying fibrosis mechanism in IBD is complex, involving a variety of cellular and molecular mechanisms, and the traditional view that intestinal fibrosis is an inevitable and irreversible process is gradually changing[44].
In summary, zedoary turmeric and trisomes influence each other and act together to treat IBD intestinal fibrosis through multiple pathways with their multiple components and targets, and this finding fully reflects the holistic and comprehensive characteristics of TCMs in the treatment of diseases[45]. The results of the analysis of their mechanism of action in the treatment of IBD intestinal fibrosis via a technical approach consisting of network pharmacology and molecular interconnections lay the foundation for further research and provide a new perspective for multidimensional and multilevel research on treatments with TCM compounds.
Intestinal fibrosis is a serious complications of inflammatory bowel disease (IBD), but there is no effective drug treatment. Therefore, it is important to find drug treatments with fewer side effects.
To provide an objective basis for zedoary turmeric-trisomes in the treatment of IBD with intestinal fibrosis.
To investigate the use of network pharmacology and molecular docking technology in analyzing the effective components and mechanism of zedoary turmeric-trisomes in the treatment of IBD with intestinal fibrosis.
The Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform were used to extract the active components and action targets of zedoary turmeric-trisomes.
The protein-protein interaction network showed that the core therapeutic targets included JUN, MAPK14, CASP3, AR, and PTGS2. The GO enrichment analysis identified 759 items, and the KEGG enrichment analysis yielded 52 items. Molecular docking showed that the compound bonded with the target through hydrogen bond interactions and exhibited good docking activity.
This study identified the potential mechanism of action of zedoary turmeric-trisomes in the treatment of IBD using network pharmacology and molecular docking technology.
TCM has a potential mechanism in the treatment of IBD with intestinal fibrosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Mathematical and computational biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam VA, India; Venkateswarulu T, India S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Lenti MV, Di Sabatino A. Intestinal fibrosis. Mol Aspects Med. 2019;65:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 2. | Wang J, Lin S, Brown JM, van Wagoner D, Fiocchi C, Rieder F. Novel mechanisms and clinical trial endpoints in intestinal fibrosis. Immunol Rev. 2021;302:211-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 3. | Bos S, Laukens D. Metabolic modulation during intestinal fibrosis. J Dig Dis. 2020;21:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Beraza N. Fibrosis and the intestinal microbiome; a focus on chronic liver disease. Curr Opin Pharmacol. 2019;49:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Ferretti F, Cannatelli R, Ardizzone S, Maier JA, Maconi G. Ultrasonographic Evaluation of Intestinal Fibrosis and Inflammation in Crohn's Disease. The State of the Art. Front Pharmacol. 2021;12:679924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Liu YT, Qi SL, Sun KW. Traditional Chinese medicine, liver fibrosis, intestinal flora: is there any connection? Ann Palliat Med. 2021;10:4846-4857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Yang B, Zhang G, Elias M, Zhu Y, Wang J. The role of cytokine and immune responses in intestinal fibrosis. J Dig Dis. 2020;21:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Gilchrist FJ, Green J, Carroll W. Interventions for treating distal intestinal obstruction syndrome (DIOS) in cystic fibrosis. Cochrane Database Syst Rev. 2021;12:CD012798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Kocaadam B, Şanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr. 2017;57:2889-2895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 696] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 10. | Soleimani V, Sahebkar A, Hosseinzadeh H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother Res. 2018;32:985-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 358] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 11. | Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Guo Z, Tao W, Yang Y, Xu X, Li Y, Wang Y, Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1623] [Cited by in RCA: 3148] [Article Influence: 286.2] [Reference Citation Analysis (0)] |
| 12. | Bardou P, Mariette J, Escudié F, Djemiel C, Klopp C. jvenn: an interactive Venn diagram viewer. BMC Bioinformatics. 2014;15:293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 822] [Cited by in RCA: 1544] [Article Influence: 140.4] [Reference Citation Analysis (0)] |
| 13. | Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11591] [Cited by in RCA: 22274] [Article Influence: 1713.4] [Reference Citation Analysis (0)] |
| 14. | Lohning AE, Levonis SM, Williams-Noonan B, Schweiker SS. A Practical Guide to Molecular Docking and Homology Modelling for Medicinal Chemists. Curr Top Med Chem. 2017;17:2023-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 15. | Zheng L, Wen XL, Dai YC. Mechanism of Jianpi Qingchang Huashi Recipe in treating ulcerative colitis: A study based on network pharmacology and molecular docking. World J Clin Cases. 2021;9:7653-7670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Zheng L, Zhang YL, Dai YC, Chen X, Chen DL, Dai YT, Tang ZP. Jianpi Qingchang decoction alleviates ulcerative colitis by inhibiting nuclear factor-κB activation. World J Gastroenterol. 2017;23:1180-1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Chen YL, Zheng YY, Dai YC, Zhang YL, Tang ZP. Systems pharmacology approach reveals protective mechanisms of Jian-Pi Qing-Chang decoction on ulcerative colitis. World J Gastroenterol. 2019;25:2603-2622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Dai YC, Zheng L, Zhang YL, Chen X, Chen DL, Wang LJ, Tang ZP. Jianpi Qingchang decoction regulates intestinal motility of dextran sulfate sodium-induced colitis through reducing autophagy of interstitial cells of Cajal. World J Gastroenterol. 2017;23:4724-4734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Zheng L, Wen XL, Duan SL. Role of metabolites derived from gut microbiota in inflammatory bowel disease. World J Clin Cases. 2022;10:2660-2677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Popoola TD, Guetchueng ST, Ritchie KJ, Awodele O, Dempster NM, Akinloye O, Sarker SD, Fatokun AA. Potent Nrf2-inducing, antioxidant, and anti-inflammatory effects and identification of constituents validate the anti-cancer use of Uvaria chamae and Olax subscorpioidea. BMC Complement Med Ther. 2021;21:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Dong S, Lu Y, Peng G, Li J, Li W, Li M, Wang H, Liu L, Zhao Q. Furin inhibits epithelial cell injury and alleviates experimental colitis by activating the Nrf2-Gpx4 signaling pathway. Dig Liver Dis. 2021;53:1276-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 22. | Zielińska AK, Sałaga M, Siwiński P, Włodarczyk M, Dziki A, Fichna J. Oxidative Stress Does Not Influence Subjective Pain Sensation in Inflammatory Bowel Disease Patients. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Bandara M, MacNaughton WK. Protease-activated receptor-2 activation enhances epithelial wound healing via epidermal growth factor receptor. Tissue Barriers. 2022;10:1968763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Pan Y, Ning Y, Hu J, Wang Z, Chen X, Zhao X. The Preventive Effect of Lactobacillus plantarum ZS62 on DSS-Induced IBD by Regulating Oxidative Stress and the Immune Response. Oxid Med Cell Longev. 2021;2021:9416794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Venkataraman B, Almarzooqi S, Raj V, Dudeja PK, Bhongade BA, Patil RB, Ojha SK, Attoub S, Adrian TE, Subramanya SB. α-Bisabolol Mitigates Colon Inflammation by Stimulating Colon PPAR-γ Transcription Factor: In Vivo and In Vitro Study. PPAR Res. 2022;2022:5498115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Kim C, Le D, Lee M. Diterpenoids Isolated from Podocarpus macrophyllus Inhibited the Inflammatory Mediators in LPS-Induced HT-29 and RAW 264.7 Cells. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Fukui Y, Hayano S, Kawanabe N, Wang Z, Shimada A, Saito MK, Asaka I, Kamioka H. Investigation of the molecular causes underlying physical abnormalities in Diamond-Blackfan anemia patients with RPL5 haploinsufficiency. Pathol Int. 2021;71:803-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Sargazi S, Abghari AZ, Sarani H, Sheervalilou R, Mirinejad S, Saravani R, Eskandari E. Relationship Between CASP9 and CASP10 Gene Polymorphisms and Cancer Susceptibility: Evidence from an Updated Meta-analysis. Appl Biochem Biotechnol. 2021;193:4172-4196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Huang LJ, Mao XT, Li YY, Liu DD, Fan KQ, Liu RB, Wu TT, Wang HL, Zhang Y, Yang B, Ye CQ, Zhong JY, Chai RJ, Cao Q, Jin J. Multiomics analyses reveal a critical role of selenium in controlling T cell differentiation in Crohn's disease. Immunity. 2021;54:1728-1744.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 30. | Saez A, Gomez-Bris R, Herrero-Fernandez B, Mingorance C, Rius C, Gonzalez-Granado JM. Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 31. | Cai C, Wang X, Fu Q, Chen A. The VEGF expression associated with prognosis in patients with intrahepatic cholangiocarcinoma: a systematic review and meta-analysis. World J Surg Oncol. 2022;20:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 32. | Konishi Y, Ichise H, Watabe T, Oki C, Tsukiji S, Hamazaki Y, Murakawa Y, Takaori-Kondo A, Terai K, Matsuda M. Intravital Imaging Identifies the VEGF-TXA2 Axis as a Critical Promoter of PGE2 Secretion from Tumor Cells and Immune Evasion. Cancer Res. 2021;81:4124-4132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Shen J, Rossato FA, Cano I, Ng YSE. Novel engineered, membrane-tethered VEGF-A variants promote formation of filopodia, proliferation, survival, and cord or tube formation by endothelial cells via persistent VEGFR2/ERK signaling and activation of CDC42/ROCK pathways. FASEB J. 2021;35:e22036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Zhou Y, Zhou H, Hua L, Hou C, Jia Q, Chen J, Zhang S, Wang Y, He S, Jia E. Verification of ferroptosis and pyroptosis and identification of PTGS2 as the hub gene in human coronary artery atherosclerosis. Free Radic Biol Med. 2021;171:55-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 160] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 35. | Guo FJ, Tian JY, Jin YM, Wang L, Yang RQ, Cui MH. [Retracted] Effects of cyclooxygenase2 gene silencing on the biological behavior of SKOV3 ovarian cancer cells. Mol Med Rep. 2021;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Viladomiu M, Longman RS. Decoding the matrix: multiomics reveals host-microbe biomarker for inflammatory bowel disease. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Dowdell AS, Colgan SP. Metabolic Host-Microbiota Interactions in Autophagy and the Pathogenesis of Inflammatory Bowel Disease (IBD). Pharmaceuticals (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 38. | Yu Y, Chen J, Zhang X, Wang Y, Wang S, Zhao L. Identification of anti-inflammatory compounds from Zhongjing formulae by knowledge mining and high-content screening in a zebrafish model of inflammatory bowel diseases. Chin Med. 2021;16:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Li Y, Gong Y, Zhang X, Wang J, Cheng Y, Liu F, Shi X, Xu W, Dong L. Exploring the synergistic mechanism of Gegen Qinlian Decoction on the Wnt signaling pathway using an integrated strategy of network pharmacology and RNA-seq. J Ethnopharmacol. 2021;278:114283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Qiao L, Fang L, Zhu J, Xiang Y, Xu H, Sun X, Chen H, Yang B. Total Flavone of Abelmoschus manihot Ameliorates TNBS-Induced Colonic Fibrosis by Regulating Th17/Treg Balance and Reducing Extracellular Matrix. Front Pharmacol. 2021;12:769793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Li Y, Liu XJ, Su SL, Yan H, Guo S, Qian DW, Duan JA. Evaluation of Anti-Inflammatory and Antioxidant Effectsof Chrysanthemum Stem and Leaf Extract on Zebrafish Inflammatory Bowel Disease Model. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Wang HY, Ge W, Liu SQ, Long J, Jiang QQ, Zhou W, Zuo ZY, Liu DY, Zhao HM, Zhong YB. Curcumin Inhibits T Follicular Helper Cell Differentiation in Mice with Dextran Sulfate Sodium (DSS)-Induced Colitis. Am J Chin Med. 2022;50:275-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Vermeire S, D'Haens G, Baert F, Danese S, Kobayashi T, Loftus EV, Bhatia S, Agboton C, Rosario M, Chen C, Zhang W, Kisfalvi K, Sandborn WJ. Efficacy and Safety of Subcutaneous Vedolizumab in Patients With Moderately to Severely Active Crohn's Disease: Results From the VISIBLE 2 Randomised Trial. J Crohns Colitis. 2022;16:27-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 44. | Amamou A, Rouland M, Yaker L, Goichon A, Guérin C, Aziz M, Savoye G, Marion-Letellier R. Dietary salt exacerbates intestinal fibrosis in chronic TNBS colitis via fibroblasts activation. Sci Rep. 2021;11:15055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | D'Alessio S, Ungaro F, Noviello D, Lovisa S, Peyrin-Biroulet L, Danese S. Revisiting fibrosis in inflammatory bowel disease: the gut thickens. Nat Rev Gastroenterol Hepatol. 2022;19:169-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 138] [Article Influence: 46.0] [Reference Citation Analysis (0)] |