INTRODUCTION
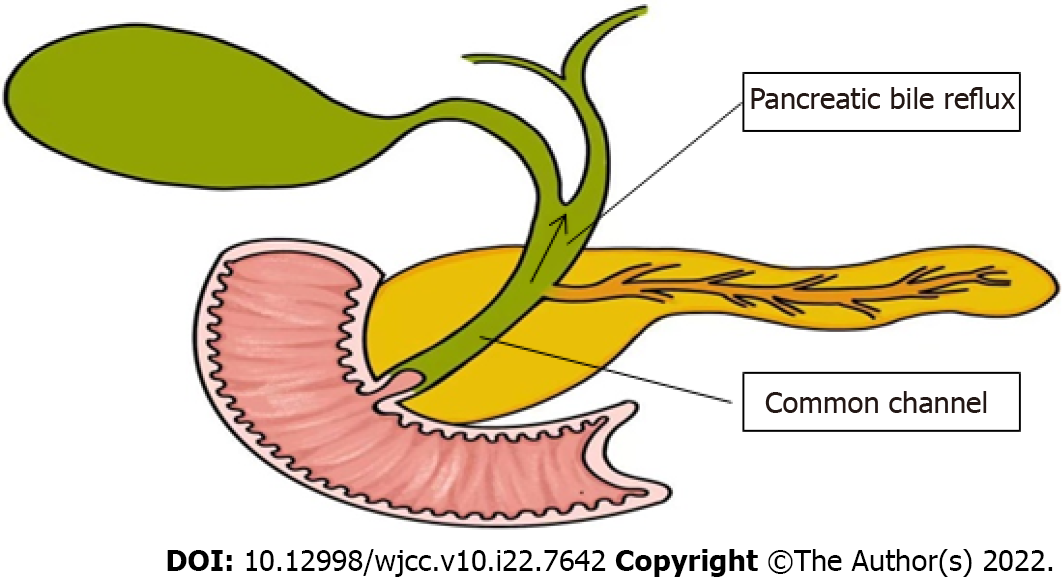
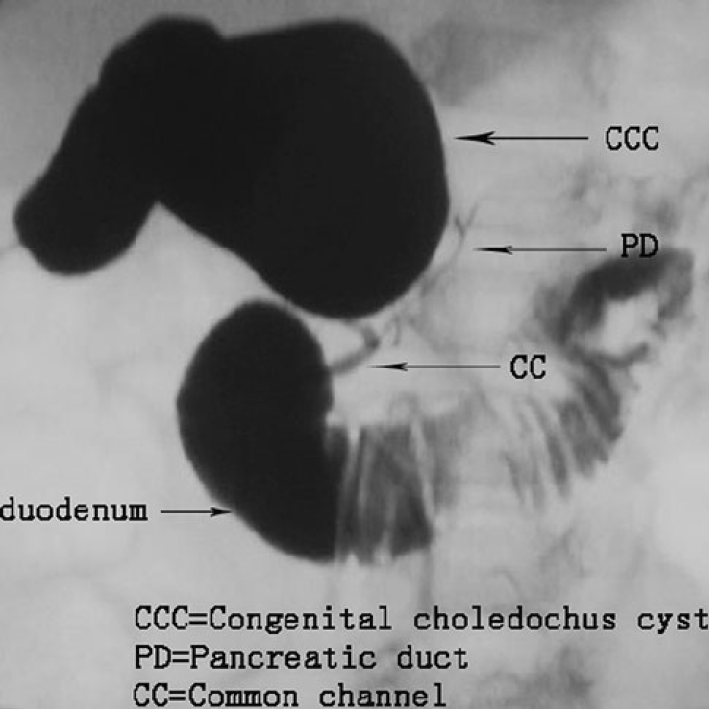
Pancreaticobiliary maljunction (PBM) was first recorded in the early 20th century, and officially named in 1969, which is referred to as congenital malfuntional[1]. The main anatomical feature of PBM is that the bile duct and pancreatic duct join out of the duodenal wall, forming a lengthy common duct (Figure 1)[2,3], often combined with sphincter of Oddi dysplasia. As a result, the pancreatic duct and bile duct lose control, causing reflux[4]. Due to this anatomical abnormality, PBM is often associated with certain diseases, such as cholelithiasis, cholangitis, pancreatitis, and increased risk of cholangiocarcinoma[5]. PBM is often reported in Asian countries and is one of the main reasons for biliary tract cancer. In PBM patients, the pressure in the pancreatic duct is usually higher than that in the bile duct. Pancreatic juice often flows back to the bile duct[6] and mixes with bile to produce cytotoxic substances, such as lysophosphatides. Due to the persistent pancreatic juice reflux to the bile duct, the mucosa of the bile duct and gallbladder is continuously damaged. The repeated repair and damage process of the biliary tract and gallbladder mucosa is related to DNA mutation, thus contributing to various gene mutations. This results in histological variety, for instance, hyperplasia, metaplasia, and dysplasia, and finally leads to biliary and gallbladder carcinogenesis. Kamisawa et al[7] reported that prevalence of biliary tract cancer is 21.6%–42.4% among adult PBM patients[8]. The theory of the hyperplasia-dysplasia-carcinoma sequence seems to explain the carcinogenesis of PBM[9]. This is different from the general principle of carcinogenesis that arises from the adenoma-carcinoma sequence. Rungsakulkij et al[10] set forth that there is evidence that gene mutations are accompanied with carcinogenesis, such as in the K-ras and p53 genes, which are involved in the carcinogenesis of gallbladder cancer in this condition. Thus, patients with PBM have higher rates of stones and tumors within the biliary tract and gallbladder[11]. The characteristic of pathological change seen in PBM patients is epithelial hyperplasia of the gallbladder and biliary tract due to longstanding continuous stasis of the bile intermixed with refluxed pancreatic juice. In summary, PBM is greatly associated with pancreatic biliary disease[12]. The early diagnosis of PBM is very important. The treatment of choice for PBM is prophylactic surgery before malignant changes can take place, which is heavily dependent on imaging techniques. Endoscopic retrograde cholangiopancreatography (ERCP) is the most effective way to detect PBM, which could show the connection structure clearly. On ERCP, there is communication between the pancreas and the bile duct despite the contraction of the sphincter, and therefore PBM is diagnosed. Magnetic resonance cholangiopancreatography (MRCP) and computed tomography (CT) can diagnose PBM, based on findings of an abnormal combination between the common bile duct (CBD) and the pancreatic duct, in addition to a long common channel. Thickening of the gallbladder wall and expansion of the bile duct on conventional ultrasound (US) are clues to the diagnosis of PBM. Intraoperative cholangiography (IOC) is used during surgery to observe the anatomy of the pancreaticobiliary system and the function of Oddi sphincter, which therefore has great value in both diagnosis and treatment. For patients with PBM, early diagnosis and timely treatment are highly important, which are largely dependent on imaging techniques. The following imaging features can be used for the diagnosis of PBM: Abnormal long pancreatic bile duct confluence common channel and a morphological anomaly of the confluence[13]. In this paper, we review the current literature about imaging techniques for PBM, in order to help clinicians make early diagnosis and timely treatment of the disease.
Figure 1 Pathophysiology of pancreaticobiliary maljunction.
DIAGNOSTIC IMAGING TECHNIQUES
ERCP
ERCP is the most effective way to detect PBM, which could show the connection structure clearly. ERCP has been widely applied for the diagnosis of biliary and pancreatic diseases. On ERCP, there is communication between the pancreas and the bile duct despite the contraction of the sphincter, by which PBM is diagnosed (Figure 2). ERCP has a sensitivity of 90%-100% in the diagnosis of PBM[14]. ERCP is often referred as the gold standard diagnostic imaging technique for PBM. ERCP can use a side-viewing duodenoscope in order to assess the masses in the distal bile duct and the main pancreatic duct. It plays an important role in detecting biliary tract cancer. Patients with a long common channel in which communication between the pancreatic and bile ducts was maintained in both relaxation and contraction of the sphincter under serial observation during ERCP were diagnosed as having PBM[15]. Whereas, considering that this technique is invasive, other diagnostic imaging techniques are preferred in patients with PBM. Before ERCP, all patients underwent initial tests, including trypsin test, liver function test, US, and MRCP to preliminarily evaluate the basic condition of the patients[16]. If MRCP or US indicates some diseases, such as biliary pancreatitis, obstructive jaundice, cholangitis, CBD dilatation, bile duct stones, or pancreatic obstruction, these diseases will increase the risk of surgery. Therefore, doctors and patients can consider ERCP[16]. Before surgery, ERCP can improve drainage, solve complications, and allow subsequent safe surgery. Post-ERCP pancreatitis was the main complication that was considered[17-19]. Zeng et al[16] reported that it was diagnosed according to the following criteria: Presence of pancreatic pain persisting for at least 24 h and serum amylase level at least 3 times higher than the normal level after ERCP. Weng et al[20] set forth that it is worrisome that ERCP could yield false positive findings (5.35%), with a specificity of 94.65%, when the contrast does not fill the CBD. During ERCP operation, bile can be extracted by fine needle to detect the amylase level in bile. If the bile amylase level is higher than the upper limit of serum amylase, PBR can be suspected after excluding some cases, such as enterobiliary reflux. Enterobiliary reflux (flow of pancreatic juice into the biliary tract) usually occurs in patients with PBM. For these patients, further examination and verification are needed. ERCP is often used as the golden standard for the diagnosis of PBM, but requires anesthesia. For some patients, the procedure is difficult. In addition, Paris et al[21] reported that the incidence of complications after ERCP in children was as high as 13.5%, thus demonstrating a certain risk of complications, for example, pancreatitis and bleeding[22]. The characteristics of invasive examination and postoperative complications mean that we should carefully consider ERCP as a diagnostic examination.
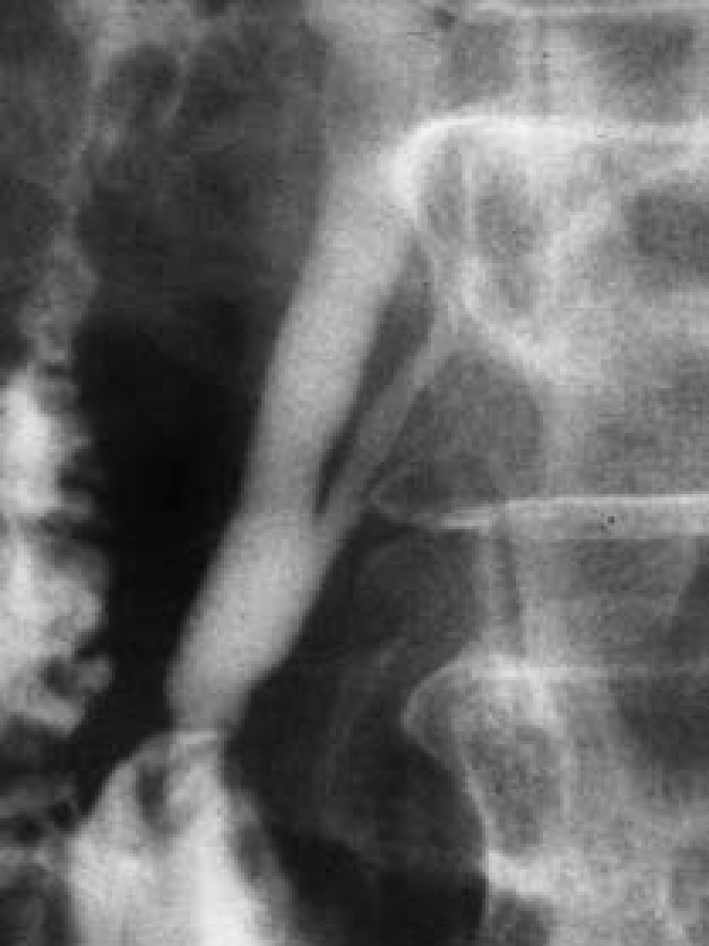
Figure 2 Endoscopic retrograde cholangiopancreatography.
Endoscopic retrograde cholangiopancreatography in a patient with pancreaticobiliary maljunction showed a long common channel[22]. Citation: Wang CL, Ding HY, Dai Y, Xie TT, Li YB, Cheng L, Wang B, Tang RH, Nie WX. Magnetic resonance cholangiopancreatography study of pancreaticobiliary maljunction and pancreaticobiliary diseases. World J Gastroenterol 2014; 20: 7005-7010. Copyright © The Authors 2022. Published by Baishideng Publishing Group Inc.
MRCP
MRCP is a noninvasive and low-risk cholangiopancreatography technique, which is widely used in the diagnosis of pancreatic and biliary abnormalities. It was rapidly applied in clinical trials in the 1990s. MRCP can reliably measure the length of the pancreaticobiliary channel[23]. For MRCP, half Fourier acquisition single shot turbo spin echo was used with multilayer thin coronal and axial T2-weighted imaging [repetition time (TR): 1200 ms; echo time (TE): 80 ms; slice thickness: 4 mm]. Oblique thick slabs were acquired in the planes of the CBD and pancreatic duct. For multi-angle imaging, TR was 4500 ms, TE 950 ms, and slice thickness 60 mm. It provides high-resolution three-dimensional images of the CBD and pancreatic duct at multiple locations and angles. MRCP can clearly show the pancreaticobiliary junction. Compared with other imaging techniques (such as US and CT), MRCP can better display the unexpanded pancreatic duct in PBM with common channel protein plug in the case of unclear body and tail of the pancreas. MRCP should be performed on individuals who show gallbladder wall thickening on US for further examination, in order to detect PBM without bile duct dilatation early before the onset of gallbladder cancer. Sometimes, measuring the common channel is difficult to achieve, either because it is too narrow or because the CBD prevents the connection with the pancreatic duct from being evaluated. In addition, in patients with cholangitis, motion artifacts will reduce the imaging quality. Some researchers found that lemon/orange juice can improve the view of the pancreatic duct. When secretin stimulates pancreatic exocrine secretion, dynamic MRCP can observe the backflow of pancreatic juice into the bile duct. Diagnostic accuracy can be upgraded with tridimensional MRCP or dynamic MRCP with secretin stimulation. For common short channels, such as infant PBM imaging, the effect of ordinary MRCP is not as good as that of ERCP, and there are image quality defects such as motion artifacts. The disadvantages of MRCP are the potentially poor definition of the pancreatic duct branch and peripheral biliary tree and the inherent poor spatial resolution compared with ERCP. In some cases, the display of the pancreatic body and tail is not satisfactory[24]. The accuracy of diagnostic MRCP can be increased through using 3D or dynamic MRCP with secretin stimulation. An overwhelming amount of evidence shows that the information provided by MRCP is almost equivalent to that by ERCP. To some extent, MRCP can be used as an image alternative to ERCP. Compared with invasive ERCP, this is a non-invasive imaging technique. When the pancreatic duct and bile duct merge in the duodenal wall to form an abnormally long common channel, the diagnosis of PBM can be made (Figure 3). In a way, this imaging technique allows ducts to be visualized, although the ducts are as narrow as 1 mm in diameter. It also proved to provide other findings not found on ERCP, and the consistency with ERCP was 81%. MRCP allows for a detailed visualization of the CBD and PBM, with detection rates of PBM between 82% and 100%[25]. MRCP plays key role in the early relative accuracy diagnosis and preventive treatment of pancreaticobiliary diseases. Overall, MRCP, as a non-invasive method, has become the first choice for the diagnosis of pancreaticobiliary diseases. This technique has certain limitations, high price, and limited availability, and is related to the subjectivity of long-time exploration and imaging interpretation due to its dependence on operators. Compared with ERCP, MRCP has limitations in the diagnosis of PBM, even if pancreatin is used. However, for diagnosing patients with anatomical maljunction, ERCP remains the gold standard. When ERCP is used as the reference standard, the detectability rate of MRCP for this anomaly has been reported to be 82%[26].
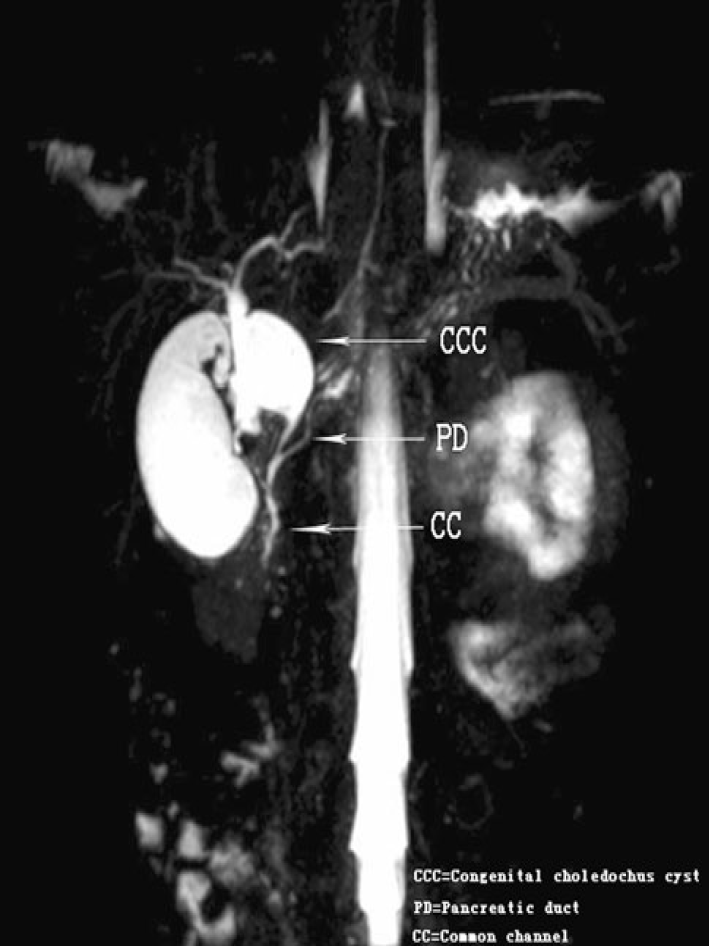
Figure 3 Magnetic resonance cholangiopancreatography.
Coronal 4 mm-thick half fourier acquisition single shot turbo spin echo image shows the pancreatic duct joining the common bile duct outside the duodenal wall[24]. Citation: Guo WL, Huang SG, Wang J, Sheng M, Fang L. Imaging findings in 75 pediatric patients with pancreaticobiliary maljunction: a retrospective case study. Pediatr Surg Int 2012; 28: 983-988. Copyright © The Authors 2022. Published by Springer Nature Switzerland AG.
US
As a safe, rapid, accurate, and economical routine examination tool, US can be used to detect gallbladder wall thickening and intrahepatic and extrahepatic bile duct dilatation (Figure 4A and B). In addition, it can also show pancreatic duct dilatation and other features for the diagnosis of pancreatitis. Although US can observe common pancreaticobiliary channels, this method is limited because it cannot provide accurate measurement of common channels because the coronal plane is not visible. PBM patients without biliary dilatation rarely have some symptoms such as acute abdominal pain and vomiting hyperamylasemia, hyperbilirubinemia, and abnormal liver function (ASD), so most patients are not diagnosed before advanced gallbladder cancer. Kamisawa et al[27] reported that gallbladder wall thickening is a diagnostic clue for PBM patients without bile duct dilatation. US can detect gallbladder wall thickening and bile duct dilatation, so US plays a key role in the pre-diagnosis of PBM. Although gallbladder wall thickening has its own limitations, it is a key factor of PBM. Patients need further examinations, such as MRCP, CT, ERCP, and IOC, to further clarify. In most cases, US is often used as a screening modality. We did not find the literature describing the detection rate of PBM by ultrasound. Endoscopic ultrasound (EUS) is useful for obtaining high-resolution images of pancreaticobiliary diseases. In the normal gallbladder wall, EUS shows a two layered structure consisting of an inner hypoechoic layer composed of the mucosa and the muscular layer, and an outer hyperechoic layer composed of the subserosal layer and the serosa. On EUS, the gallbladder wall of PBM patients showed two layers of thickening, showing epithelial hyperplasia and subserosal fibrosis, or three layers of thickening, one of which was medium and low echo layer, showing hypertrophic muscle layer. In PBM, EUS can detect the confluence of the pancreatic duct and bile duct in the proximal portion of the duodenal wall, the so-called common channel (Figure 4C)[28]. However, it is not a routine test, because it requires dedicated endoscopes (e.g., endoscopes for radial and linear EUS). The imaging capability of EUS is generally good, but the technique of EUS imaging is sometimes difficult, and the diagnostic performance of EUS is operator-dependent. In fact, several investigators have reported that EUS could confirm the PBM in 4 (2.9%) of 137 patients who underwent screening US[28]. When ERCP is used as the reference standard, the detectability rate of EUS for this anomaly has been reported to be 88%. Intraductal ultrasonography (IDUS) during ERCP is highly helpful to describe pancreaticobiliary confluence. However, IDUS also has its own limitations, such as weak penetration and poor operability in exploring narrow biliary tract[29]. Increasing the number of ultrasonic examinations is also necessary to facilitate the early diagnosis of pancreaticobiliary diseases. Because most PBM patients without biliary dilatation are not easy to find in the early stage and are in a bad situation once found, a new method for early diagnosis and treatment of PBM should be developed to improve the prognosis. Despite its own limitations, US can raise questions as soon as possible and suggests us to make further diagnosis. Compared with other imaging methods, US has its unique advantages: Noninvasiveness, no radiation, and moderate price. Compared with other imaging techniques, it uses a more comprehensive method to screen all patients with recognized PBM risk factors because it is fast, flexible, and easy to operate[30].
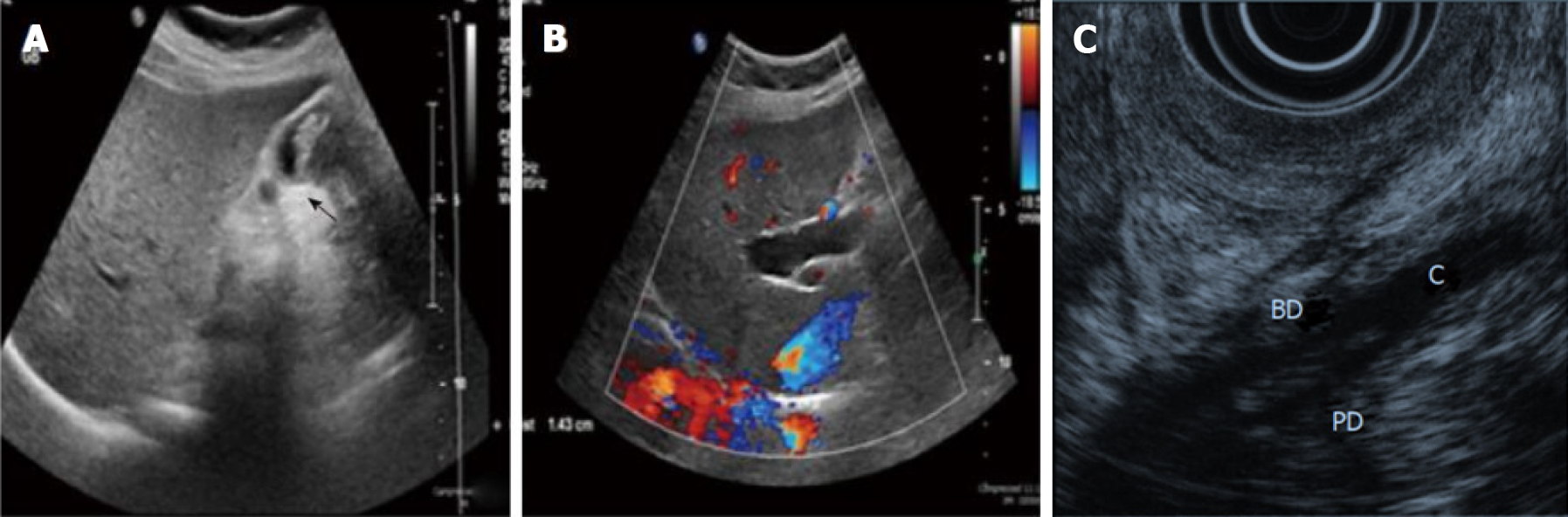
Figure 4 Ultrasound imaging and endoscopic ultrasound.
A: Ultrasound showing a thick-walled gallbladder and intraluminal mass (arrow); B: Ultrasound showing intrahepatic bile duct dilation[10]. Citation: Rungsakulkij N, Boonsakan P. Synchronous gallbladder and pancreatic cancer associated with pancreaticobiliary maljunction. World J Gastroenterol 2014; 20: 14500-14504. Copyright © The Authors 2022. Published by Baishideng Publishing Group Inc; C: The confluence of pancreatic duct and bile duct in the proximal portion of the duodenal wall[19]. Citation: Kamisawa T, Takuma K, Itokawa F, Itoi T. Endoscopic diagnosis of pancreaticobiliary maljunction. World J Gastrointest Endosc 2011; 3: 1-5. Copyright © The Authors 2022. Published by Baishideng Publishing Group Inc.
CT
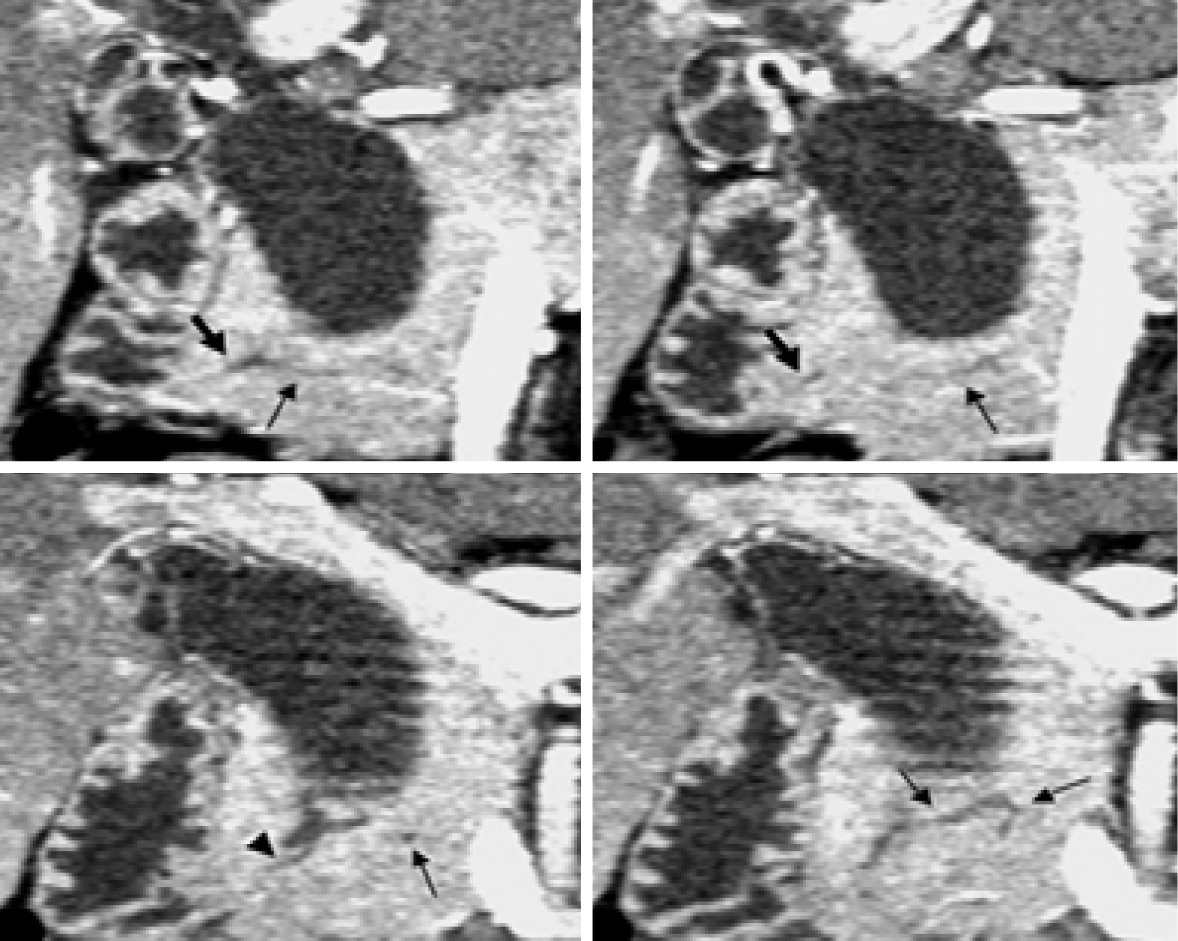
CT has a higher resolution even than MRCP. CT is widely used in pancreaticobiliary diseases. Traditional examination can observe the relationship between the size of lesion area and surrounding tissues, and further formulate the operation plan. The thickening of the gallbladder wall on US can be used as an indication for further examination of CT. Wall thickening at the stricture site may suggest malignancy because primary ductal stricture lacks wall thickening. Enhanced CT has been strengthened in the diagnosis and treatment of irregular biliary nodules. Postoperative follow-up screening and canceration monitoring have certain value. Because of its own characteristics, CT is more used for the differentiation of pancreaticobiliary diseases than used for PBM. Conventional CT scan is axial scanning, which cannot show the overall shape and cavity of the biliary tract, so it is not satisfied with the observation of the biliary system[31]. Some researchers reported that it is difficult to accurately measure the length of the common channel on CT due to the following reasons: First, the whole process of the common channel is not visible on CT, because in many cases, the common channel part is involved by the area showing less contrast enhancement between the pancreatic head and duodenum. Second, in some cases, the whole process of common channel is not described on the single-layer multiplane reconstructed (MPR) image. Third, public channels are tortuous in some cases. Recent advances in spiral CT techniques, such as multi-detector and sub-second rotation, make it possible to scan the pancreas and pancreas biliary system with a collimation of 1.25 mm and less. This will lead to further improvements, in the mass of multi-plane reformatting (multiplanar reconstruction) due to the high-resolution and MPR images on the z-axis enable us to select the best section to evaluate the pancreas and bile duct and their function confluence. By using high-resolution MPR images, Modified Discrete Cosine Transform (MDCT) allows us to diagnose abnormal pancreaticobiliary connections according to the relationship between catheter confluence and pancreatic parenchyma (Figure 5). The sensitivity of MPR images of MDCT in the diagnosis of abnormal pancreaticobiliary confluence was 78%[32]. It has been reported that perfusion cholangiography spiral CT (spiral CT during drip cholangiography; DIC-CT) is used in the diagnosis of PBM[33]. High resolution images were obtained by intravenous hepatobiliary drugs (lipperamide meglumine) and three-dimensional CT. Pancreaticobiliary channels and bidirectional reflux of pancreatic juice and bile were clearly visible. Due to exposure to ionizing radiation, this technique has not been widely used in patients. Another major drawback of DIC-CT is that injection of contrast agents (meglumine iodoacetate) may produce adverse reactions, which occur in 0.8%-3.4% of patients, although the main symptom is rash and usually does not need treatment[34]. At present, it is only used as a second-line diagnostic tool for PBM.
Figure 5 Computed tomography.
Coronal images generated from pancreatic phase scanning show that the pancreatic and biliary ducts join within pancreatic parenchyma. Furthermore, these images make it possible to visualize common channel (arrowhead) and ventral pancreatic duct (thin arrows), which is narrow and tortuous. Thick arrows indicate dorsal pancreatic duct[51]. Citation: Itoh S, Fukushima H, Takada A, Suzuki K, Satake H, Ishigaki T. Assessment of anomalous pancreaticobiliary ductal junction with high-resolution multiplanar reformatted images in MDCT. AJR Am J Roentgenol 2006; 187: 668-675. Copyright © The Authors 2022. Published by American Roentgen Ray Society.
IOC
IOC has become an indispensable technique in hepatobiliary surgery, which provides an intuitive understanding of the anatomy of the biliary system, especially suitable for judging bile duct stenosis, stones and protein embolism, and can safely guide the operation in real time[34]. During the operation, IOC was used to observe the anatomical structure of the pancreaticobiliary system and the function of Oddi sphincter. The diagnosis of PBM can be determined according to the cholangiography results (Figure 6). It can also be used to evaluate the patency of the distal bile duct. IOC is usually divided into intraoperative total cholangiography (ITCP) and intraoperative selective pancreatangiography (ISCP). The method of IOC is chosen according to the patient's condition and the degree of disease development. If the total bile duct cyst is huge, the duodenum is displaced forward. Using ITCP contrast is not easy to show the distal end of the bile duct and bile and pancreatic duct confluence[35]. ITCP can display the intrahepatic and external bile duct and the pancreatic duct, to understand the pathological morphology of all the biliary ducts and pancreatic ducts. ISCP is suitable to focus on the distal intrahepatic or extrahepatic biliary tract and the pancreatic duct. Intraoperative biliary structure shadow combined with preoperative MRCP can reduce the image occlusion of large cyst and is conducive to the imaging of PBM patients with proximal total bile duct stenosis. IOC is a diagnostic and therapeutic method with surgical and anesthetic risks. During IOC, due to the increased pressure in the bile duct, this technique can lead to slight dilatation of the bile duct, which is similar to ERCP[36]. The clinical application of IOC faces several challenges. These challenges include the need for detailed anatomical understanding before IOC, which may be technically difficult (in acute or chronic inflammatory diseases), the need to insert short, thin, or curved cystic duct, and the risk of avulsion when inserting inflammatory cystic duct. In addition, intraoperative cholangiography has some disadvantages, such as being time-consuming, the need for additional equipment and technicians, the risk of radiation exposure to staff and patients, and the need to inject contrast agent into the bile duct, which may increase the risk of bile duct injury[37,38]. Compared with traditional ERCP, MRCP, US, CT, and other imaging techniques, IOC has the advantages of intraoperative navigation, high sensitivity, simple operation, and low price[39]. We firmly believe that this imaging technique provides a new source of real-time anatomical information for hepatobiliary surgery[40]. Intravenous injection of indocyanine green (ICG) has potential advantages over conventional radiographic cholangiography in saving time and avoiding bile duct injury associated with the catheterization required for injection of contrast materials[41,42]. ICG fluorescence imaging has been gradually applied to hepatobiliary surgery, and there are no relevant reports on the application of ICG fluorescence imaging to PBM-related diseases. We can boldly imagine that fluorescence imaging technique will also be suitable for pancreatic bile system anatomy in the near future. We searched the literature for intraoperative cholangiography. Most of the literature was related to endoscopic cholecystectomy, and few were related to PBM. Therefore, the accuracy of intraoperative cholangiography in the diagnosis of PBM could not be retrieved.
Figure 6 Intraoperative cholangiography.
Intraoperative cholangiography shows the junction of the bile and pancreatic ducts located outside the duodenal wall. The common bile duct joins the pancreatic duct[24]. Citation: Guo WL, Huang SG, Wang J, Sheng M, Fang L. Imaging findings in 75 pediatric patients with pancreaticobiliary maljunction: a retrospective case study. Pediatr Surg Int 2012; 28: 983-988. Copyright © The Authors 2022. Published by Springer Nature Switzerland AG.
TREATMENT OF PBM
PBM can be divided into PBM with biliary dilatation and PBM without[43]. It is agreed that preventive surgery should be performed on PBM patients as soon as possible after diagnosis[44]. For bile duct dilatory PBM, current cholecystectomy, extrahepatic cholbectomy, and hepatic tube-jejunal Roux-en-Y reconstruction is the standard surgical modality. New complications may also occur later, such as narrow anastomosis, reflux cholangitis, intra-hepatic duct stones, and bile duct tumors. There are some PBM patients with obstructive jaundice or acute pancreatitis or other diseases[45,46]. The operation of these patients is considered to increase the postoperative risk. With the continuous progress of imaging techniques, ERCP is not only the standard technique for diagnosing PBM, but also used to improve drainage and solve complications[47,48]. ERCP is also an effective therapeutic option for patients with PBM. ERCP can improve drainage, solve complications, and allow subsequent safe surgery. The indications for ERCP (one case may involve one or more indications) are pancreatitis, pancreaticobiliary calculi, biliary obstruction, and stent displacement. Endoscopic treatments for PBM primarily include endoscopic sphincteropapillotomy (EST), stent insertion, and endoscopic nasobiliary drainage (ENBD)/endoscopic nasal and pancreatic duct drainage (ENPD). If pancreaticobiliary stones or protein plugs are detected, stone removal treatment is required. ERCP needs to be performed under general anesthesia. Two experienced endoscopists intubate the trachea in the prone position and use duodenoscopy. After successful intubation of the CBD, 10 mL of bile samples are taken to measure bile amylase (PBM is indicated when bile amylase level is higher than the upper limit of serum amylase level). EST can help bile and pancreatic juice flow into the duodenum regularly. Endoscopic hemostatic clip is also used to treat high-risk patients with bleeding after EST. Endoscopic papillary balloon dilatation is used if there are large CBD stones. Finally, ENBD/ENPD or endoscopic retrograde cholangiopancreatic drainage is performed when necessary to prevent complications. Jin et al[40] reported that the total effective rate of ERCP in the treatment of PBM was 60.7% (34/56). It is useful to plan the timing and choice of the appropriate surgical procedure.
For PBM patients without biliary dilatation, prophylactic cholecystectomy is recommended to prevent gallbladder cancer. Nevertheless, the risk of developing cancer in the remnant biliary tract is still high, so careful follow-up is needed for such patients in the future. During laparoscopic cholecystectomy, in order to perform accurate resection and prevent bile duct and vascular injury, it is necessary to understand the anatomy of the bile duct and vessels[49]. Laparoscopic ultrasound (LUS) during cholecystectomy allows minimal invasive study of the biliary tract and has excellent ability to identify anatomical structures. LUS, which is cheap, fast and non-irradiated, can be repeated as needed during laparoscopic surgery. Adjacent organs can also be explored[49]. LUS can be a valuable adjunct and can be performed before dissection, and repeated as needed to guide the surgeon. LUS can be performed before Calot’s triangle dissection, which facilitates the mapping of biliary and hilar structures during difficult scenarios such as severe inflammation and fibrosis. Conventional abdominal ultrasound and CT examination are usually performed before operation to provide a reference for operation. To some extent, imaging techniques is also of great value in the treatment of PBM. With the continuous progress of imaging techniques, the relationship between diagnosis and treatment of the disease is becoming closer and more inseparable. In the diagnosis and treatment of PBM, imaging techniques run through the whole process and play an important role in clinical practice.
CONCLUSION
In summary, the clinical features of PBM are atypical and usually characterized by pancreaticobiliary diseases. PBM is one of the pathogenic factors of pancreaticobiliary diseases such as cholangitis, pancreatitis, cholangiocarcinoma, and gallbladder cancer. Early diagnosis and timely treatment are very important. Early diagnosis of PBM can improve the prognosis of PBM, which is closely related to the development of various imaging techniques such as ERCP, MRCP, CT, US, and IOC. If imaging shows that the bile duct and pancreatic duct outside the duodenal wall are connected through a long common tube, a diagnosis of PBM can be made[50]. The imaging techniques mentioned in this paper (ERCP, MRCP, US, CT, and IOC) are valuable for the diagnosis and treatment of PBM patients. ERCP can be used as the gold standard for the diagnosis of PBM and can also be used to alleviate PBM related complications, but this technique is invasive and its application needs careful consideration. As a noninvasive imaging technique, MRCP can clearly show the junction of the pancreaticobiliary duct. It is the first choice for the diagnosis of PBM in most patients. US can be used to detect gallbladder wall thickening and congenital biliary dilatation, which can play a warning role and need further examination. CT has high resolution in the diagnosis of PBM. IOC provides an intuitive understanding of the anatomy of the biliary system and guides the operation in real time. Each imaging technique has its unique advantages and disadvantages. No paper has been found to report the official golden standard. So, further research is needed on golden diagnosing imaging techniques for PBM. Appropriate techniques should be chosen according to the actual situation to achieve the desired effect. The imaging techniques mentioned in this paper guide various diagnostic and treatment procedures, help surgeons accurately perform some surgical operation, and reduce the risk of intraoperative and postoperative complications. They can achieve complete and correct diagnosis and real staging, and help to establish an appropriate treatment attitude. We believe that the imaging technique with all the above characteristics is of great value in the diagnosis and treatment of PBM.
In conclusion, imaging techniques allow us to diagnose an anomalous pancreaticobiliary ductal junction on the basis of findings regarding the relationship between the duct confluence and the pancreatic parenchyma. The detection rates of ERCP, MRCP, EUS, and MDCT for PBM are 90%-100%, 82%-100%, 88%, and 78%[51,52], respectively. Various imaging techniques also play a great role in the treatment of PBM. In order to achieve the purpose of early diagnosis and timely treatment, imaging techniques for PBM should be used more and more clinically.