Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7599
Peer-review started: March 12, 2022
First decision: May 11, 2022
Revised: May 19, 2022
Accepted: June 15, 2022
Article in press: June 15, 2022
Published online: July 26, 2022
Processing time: 120 Days and 17.4 Hours
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) occurs in approximately 9% of non-Hodgkin B-cell lymphomas. The gastrointestinal tract is the most commonly affected site of the extranodal forms of primary non-Hodgkin’s lymphomas. However, it rarely occurs within the rectum, and at present, there is no consensus on its diagnosis and treatment at this site.
We report a rare laterally spreading tumour-like rectal MALT lymphoma case in which the diagnosis and the depth of infiltration were determined by magnifying endoscopy and ultrasonic endoscopy. Then, the lesion was en bloc resected by endoscopic submucosal dissection (ESD) alone. The lesion was confirmed as MALT lymphoma by haematoxylin and eosin staining, immunohistochemical staining and gene arrangement analysis. Surveillance exams have indicated a 2-year disease-free survival for this patient.
We report a rare primary rectal MALT lymphoma that was curable with resection by ESD. ESD is a safe and effective therapeutic option for rectal MALT lymph
Core Tip: Primary rectal extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) is a rare clinical entity. The clinical symptoms and endoscopic findings are nonspecific. The patient in this case was diagnosed with low-grade rectal MALT lymphoma, which was limited to the mucosa based on magnifying endoscopy, histopathological features, immunohistochemical tests, and gene rearrangement analyses. The lesion was en bloc resected by endoscopic submucosal dissection alone.
- Citation: Tao Y, Nan Q, Lei Z, Miao YL, Niu JK. Rare primary rectal mucosa-associated lymphoid tissue lymphoma with curative resection by endoscopic submucosal dissection: A case report and review of literature. World J Clin Cases 2022; 10(21): 7599-7608
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7599.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7599
Primary colorectal lymphoma is a rare condition. The incidence has varied from 0.1% to 0.9% of all colorectal malignancies in many series[1]. Lymphoma can be roughly divided into two subtypes: indolent and aggressive. The former has a long survival period and responds to multiple treatments, while the latter progresses rapidly. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) is an indolent, low-grade, mature small B-cell non-Hodgkin lymphoid neoplasm. MALT-type lymphoma was first defined by Isaacson and Wright in 1983[2]. It has subsequently been defined as a specific indolent B-cell lymphoma with the ability to develop extranodal manifestations in mucosa-associated lymphoid tissues throughout the whole body. MALT lymphomas arise from mucosa-associated lymphoid tissue and can involve various organs, including the gastrointestinal tract, lung, salivary glands, thyroid, and unusual sites, such as the dura[3]. The most common site for MALT lymphoma is the stomach. MALT lymphoma of the colon and rectum is the least common gastrointestinal location. Due to the rarity of rectal MALT lymphoma, the treatment strategies for rectal MALT lymphoma have not been systematically determined[4]. Here, we present a male patient with rectal MALT lymphoma who was diagnosed by magnifying endoscopy and successfully treated with endoscopic submucosal dissection without adjuvant therapy or empiric Helicobacter pylori (H. pylori) eradication.
A 46-year-old male presented to the Digestive Department of our hospital for further diagnosis and treatment of a 3.0 cm x 3.5 cm laterally spreading tumour-like elevated lesion observed in the rectum.
The patient was initially evaluated for a screening colonoscopy as part of a routine health examination. He was asymptomatic, with no weight loss, bleeding, fever, abdominal pain, or adenopathy. Colonoscopy showed a rectal 3.0 cm x 3.5 cm lesion, which was 10 cm from the anus. The biopsy specimen had been confirmed histologically as mucosa-associated lymphoid tissue lymphoma.
The patient had no previous medical history.
The patient had no smoking and drinking history.
Physical examination upon admission showed that the patient had a blood pressure of 112/84 mmHg with a heart rate of 87 beats/min. There were no obvious signs of the cardiopulmonary system or abdomen during physical examination.
Laboratory results of lactate dehydrogenase, hemoglobin, plasmacytic differentiation and soluble interleukin-2 receptor showed no obvious abnormalities. The 13C-urea breath test tested negative for H. pylori.
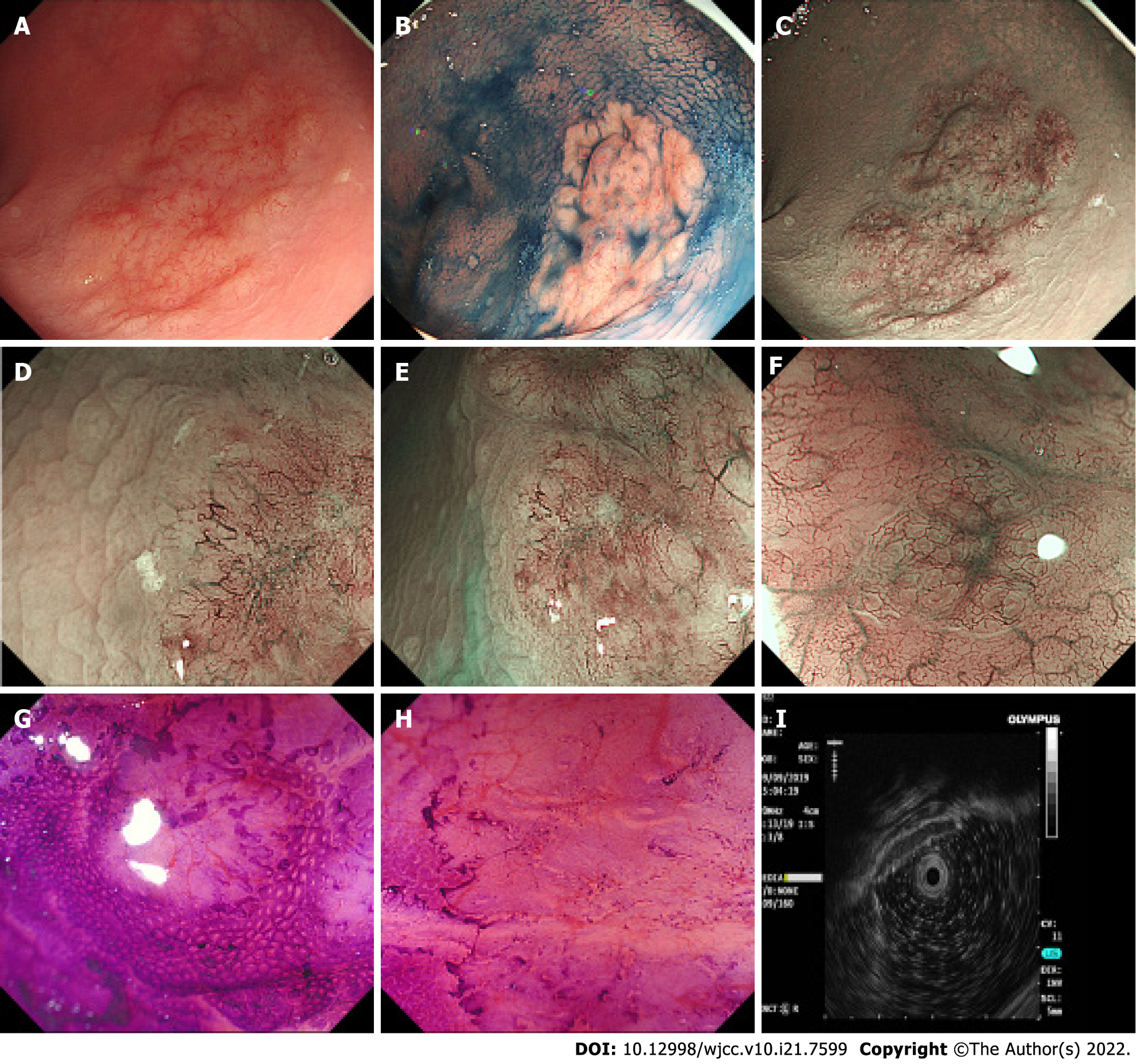
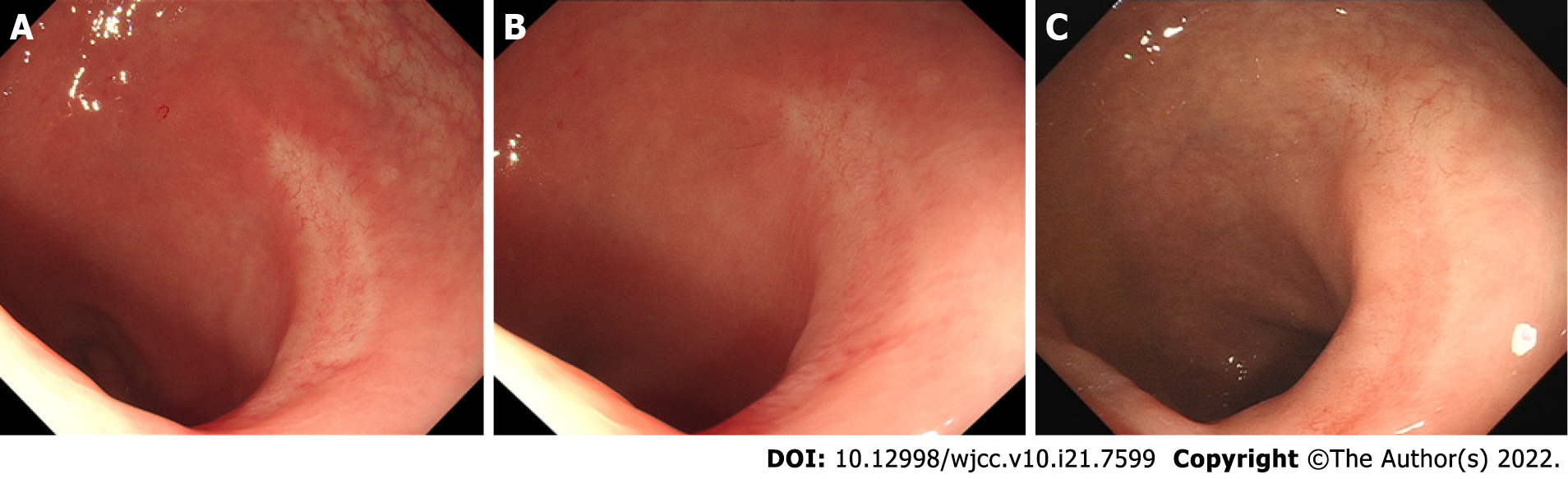
A 3.0 cm x 3.5 cm laterally spreading tumour-like elevated lesion was observed in the rectum approximately 10 cm away from the anus under white light endoscopy. Its surface was uneven, congested, and oedematous (Figure 1A). The lesion margin became clearer and more uneven after indigo carmine staining (Figure 1B). To observe the microstructure and capillaries of the lesion, magnifying endoscopy with narrow-band imaging (NBI) was carried out. NBI showed a clear margin with a dark brown background and enlarged branch-like vessels on the surface of the lesion. The lesion was classified as NBI international colorectal endoscopic (NICE) type 3 and Japanese NBI expert team (JNET) type 3.
Magnifying endoscopy showed dendritic and grid-like irregular microvessels scattered in the lesion, which had a clear boundary with normal mucosa (Figure 1C-F). Crystal violet staining magnifying endoscopy showed that the pit pattern structure disappeared on the surface of the lesion, classified as Kudo Pit Patterns type VN (Figure 1G and H). Hypoechoic thickening of the mucosal layer was detected by endoscopic ultrasound (EUS) (Figure 1I). On the basis of dendritic and grid-like irregular microvessels and pit pattern structure disappeared on the surface of the lesion in magnifying endoscopy, the rectal lesion was diagnosised as lymphoma. At the same time, biopsy specimen had been taken to pathological examination and confirmed histologically as MALT lymphoma. Other examinations, including enhanced computed tomography, positron emission tomography/computed tomography (PET-CT), bone marrow biopsy and gastroscopy, showed no obvious abnormalities.
In summary, the lesion was confirmed to be MALT lymphoma by HE, immunohistochemical staining and gene arrangement analysis.
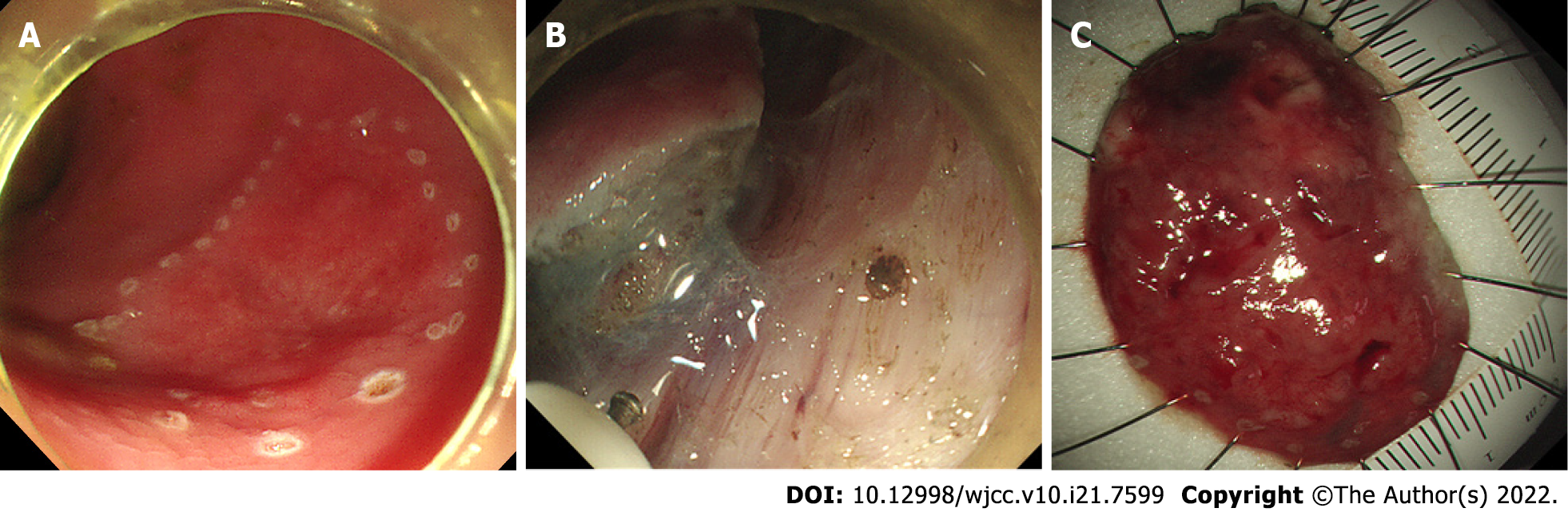
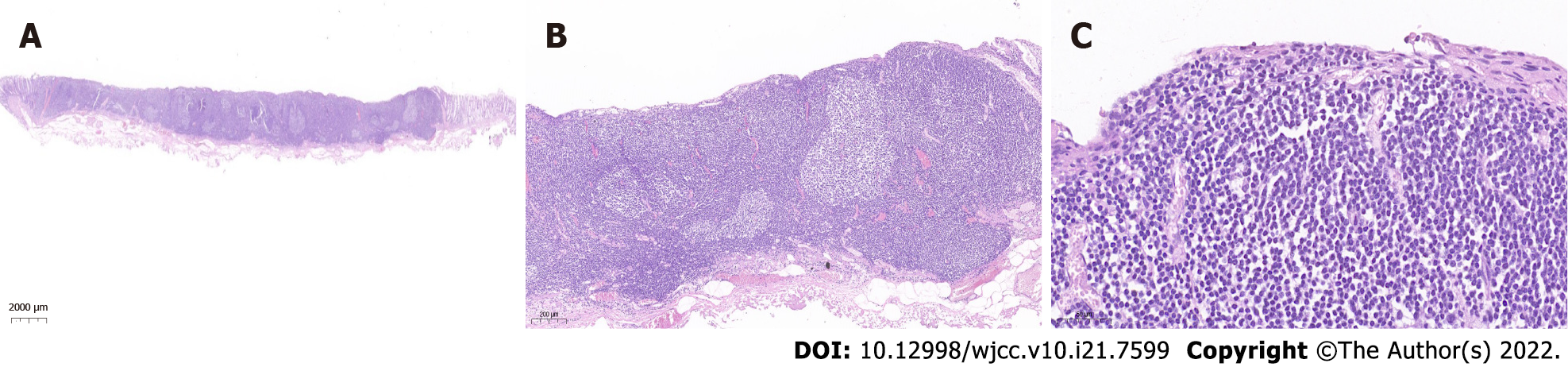
To treat the lesion, the patient underwent curative endoscopic submucosal dissection (ESD). The mucosal layer clinging to the muscularis propria were completely resected. No obvious adhesions were found during the ESD procedure (Figure 2). The histopathological findings of the ESD specimen showed diffusely hyperplastic lymphoid tissue with a lymphoid follicle structure surrounded by an abundance of diffusely infiltrated lymphoid cells that exhibited clear cytoplasm and similar sizes. The lymphoma had invaded the mucosal layer inside (Figure 3).
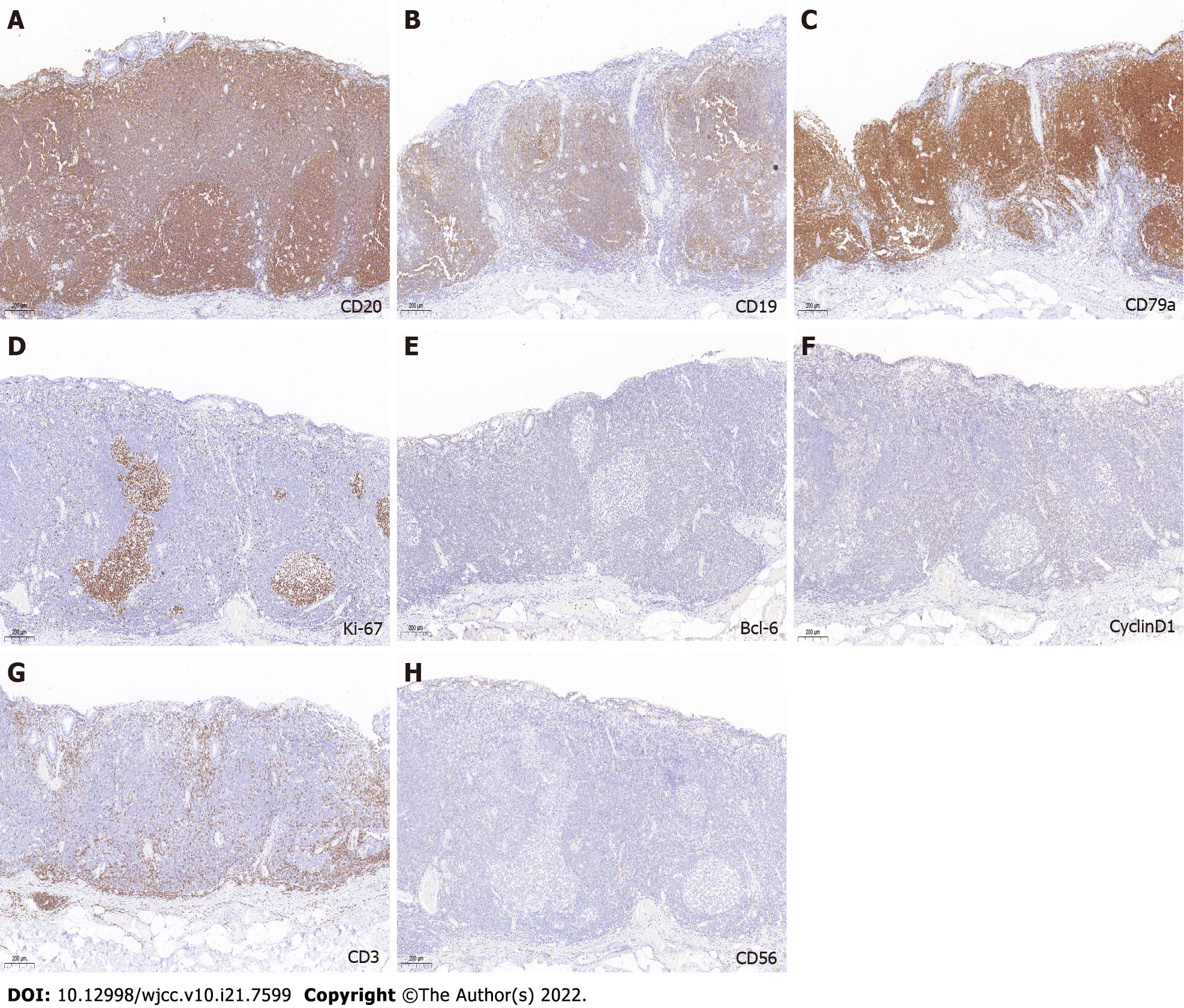
Immunohistochemical staining was positive for CD20, CD19, and CD79 (Figure 4A-C). The Ki-67 Labeling index was 3% (Figure 4D). Immunohistochemical staining was negative for BCL-6, cyclin D1, CD3, and CD 56 (Figure 4E-H).
Gene arrangement analysis showed that the specific immunoglobulin (IG) genes in the original B lymphocytes were rearranged.
The patient was subjected to regular clinical and colonoscopic follow-up at 6 mo, 1 year, and 2 years after ESD (Figure 5). Considering that this MALT lymphoma case is a low-grade malignant tumour and has been completely resected, a reasonable follow-up strategy was made after discussion with hematologists, pathologists and the patient. Endoscopy and lymph node ultrasound review at 6 and 12 mo and PET-CT at 12 mo after ESD was carried out. When no suspicious lymphoma lesions were found, we decided to perform colonoscopy, EUS, and lymph node ultrasound annually. To date, for 2 years, endoscopy, lymph node ultrasound, and PET-CT have revealed no disease recurrence.
Primary rectal extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) is a rare clinical entity. In a review of the literature, Dionigi et al[5] found primary colorectal lymphoma to account for only 0.2% of all malignant tumours of the colorectum. The colon and rectum are the least common gastrointestinal locations for primary lymphoma, accounting for < 10% of gastrointestinal lymphomas. Colorectal MALT lymphoma is an even rarer disease[6]. Colorectal MALT lymphoma comprises only 2.5% of MALT lymphomas[7]. This case is even more exceptional, as laterally spreading tumour-like elevated lesions are even rarer. MALT lymphoma has the highest incidence between the ages of 50 and 60 years, with no obvious difference between males and females[8]. The pathogenesis of MALT lymphoma has been characterized as a dynamic process driven by lymphoma cell dependency on T-cell signalling, chronic antigenic stimulation of marginal zone B cells and activation of the NF-kappa B signalling pathway[9]. MALT lymphoma and its tumour microenvironment suggest a potentially complex pathogenesis. Pathogenic mechanisms include Borrelia burgdorferi in cutaneous lymphomas, Chlamydia psittani in orbital lymphomas, Campylobacter jejuni in small intestinal lymphomas, and Achromobacter xylosoxidans in pulmonary lymphomas[10]. In addition, autoimmune disorders, especially Sjögren syndrome and chronic autoimmune thyroiditis, are also associated with MALT lymphomas[11]. H. pylori infection is now considered a major cause of gastric MALT lymphomas[12]. However, the pathogenesis of colorectal MALT lymphomas is still not clearly understood. In this case, the patient did not have an H. pylori infection. MALT lymphomas usually maintain an indolent course with 10-year recurrence-free rates of 57% to 76% and survival rates of 79% to 87%[13]. For colorectal MALT lymphoma, the reported overall survival at 5 years ranges from 50% to 94%[4,7,15]. Patients who underwent surgery had the highest 5-year survival[16].
The clinical symptoms of gastrointestinal MALT lymphomas include gastrointestinal bleeding, abdominal pain, perforation, and intussusception. Gastrointestinal bleeding is the major symptom, but the amount of bleeding varies[17]. Some patients can be completely asymptomatic[18], which was the case for our patient. He had no obvious symptoms, and the lesion was found only by routine endoscopy examination.
To establish an accurate diagnosis and staging of this heterogeneous group of lymphomas, different procedures have been used, including EUS, endoscopic biopsies, computed tomography (CT), magnetic resonance imaging (MRI), PET-CT, and laboratory testing for molecular cancer markers. In many instances, diagnoses rely on morphology and immunophenotype, but there is an increasing need to incorporate molecular genetic markers. It is also important to take into consideration the endoscopic and clinical presentations[19]. Endoscopic findings are nonspecific, ranging from a single polypoid lesion to a subepithelial tumour type, an epithelial mass type, and an ileitis type[14]. EUS is an accurate technique for evaluating the extent and invasion of the lesion. Pathology is used to accurately determine whether the lesion is confined to the mucosal layer. The depth of lymphoma infiltration and the presence of perigastric lymph nodes can be detected by EUS and are important for treatment planning[20].
Magnifying endoscopy is of great value in the diagnosis of gastrointestinal tract MALT lymphoma. This is because lymphocytes show infiltrative growth and destroy the mucosal layer, which usually presents as elevated lesions. Magnifying endoscopy with NBI can identify the typical branch-like vessels, and magnifying chromoendoscopy can identify the disappearance of glandular structures at the lesion[21,22]. Our patient presented with the typical endoscopic findings mentioned above.
Immunohistochemical analysis is performed in almost all patients to distinguish MALT lymphomas from other low-grade lymphomas. MALT lymphomas present with B-cell phenotypes that are positive for CD19, CD20, CD79a, and Bcl-2 and negative for CD3, CD5, CD10, cyclin D1, Bcl-6, and the Ki-67 marker index[7].
Genome rearrangement is an important oncogenic mechanism of human tumours. IG gene rearrangement is a specific marker of B lymphocyte cloning and can be used in the diagnosis of B-cell lymphoma. The detection of IG gene rearrangement can help determine the nature of the lesion and distinguish between benign and malignant lymphocytes. Our case met the above immunohistochemical and gene rearrangement criteria[23].
In this case, the colonoscopy revealed an uneven surface lesion in the rectum. The patient was diagnosed with low-grade rectal MALT lymphoma, which was limited to the mucosa based on magnifying endoscopy, histopathological features, immunohistochemical tests, and gene rearrangement analyses.
For patients with gastric MALT lymphoma, eradication of H. pylori can improve prognosis[24]. However, there does not appear to be a relationship between H. pylori and extragastric MALT lymphoma[25]. Due to the rare occurrence of rectal MALT lymphoma, the therapeutic strategies are largely based on case reports. The treatment modalities include surgical resection, etiopathogenetic therapies, chemotherapy, chemoimmunotherapies, radiation, and endoscopic resection, while most cases use surgery or chemotherapy as the first-line treatment. The stage of disease is a relevant parameter[11]. Approximately 33% of patients with colorectal MALT lymphoma receive endoscopic mucosal resection[7]. There are few reports about ESD in the treatment of colorectal MALT lymphoma. A review of 51 cases of the MALT variant of primary rectal lymphoma revealed significant differences in treatment modalities. A complete response was achieved in 12 of 19 cases treated with H. pylori eradication therapy, 5 of 6 cases treated with radiation, 2 of 4 cases treated with chemotherapy, 2 of 4 cases treated with endoscopic resection, 6 of 8 cases treated with surgical resection, and all 8 cases treated with combination therapies[26]. In most cases, tumour resection or chemotherapy is used as the primary treatment. The remission rates of resection and chemotherapy were higher than 90%. Radical surgery or local excision showed 5.3% treatment failure as a first-line treatment[27]. Although rectal MALT lymphoma is a rare disease, the appropriate evaluation and proper treatment option might benefit the patients. First, H. pylori eradication was ineffective for treatment of extra-gastric MALT lymphomas[28]. Anti-H. pylori therapy seems unnecessary for those patients who test negative for H. pylori, as seen in the present case. Second, radiotherapy has been reported as highly active therapy for MALT lymphoma limited to a focal area of the gastrointestinal tract, including the rectum. However, radiotherapy in conventional doses is associated with side effects particularly in radiation-sensitive tissues like the gastrointestinal mucosa (strictures, gastroparesis)[29]. Endoscopic resection can be attempted as a priority for localized lesions to avoid unnecessary surgery or radiotherapy. When endoscopic resection of the lesion is not possible, surgical resection may be further considered. As local treatments, the effectiveness of surgical or endoscopic tumor resection and radiotherapy has been reported for primary colorectal MALT lymphoma[30,31]. Lastly, a large case analysis of colorectal MALT lymphoma reported that chemotherapy is most commonly performed for advanced disease. Chemotherapy is not recommended for patients with localized MALT lymphomas as these tumors are less responsive to standard chemotherapy than aggressive lymphomas[32]. Chemotherapy and/or immunotherapy is given when patients fail to respond to localized therapy or develop metastasis.
Patients with MALT lymphoma mostly present with local disease, and distant metastases are rare[33]. Because postsurgical sequelae and organ dysfunction are more injurious than lymphoma itself[11], local treatment is a reasonable approach. Thus, endoscopic resection is a more feasible treatment method than surgery for gastrointestinal tract MALT lymphoma as it is effective and minimally invasive. At present, based on the depth and size of the lesion, endoscopic treatment of rectal MALT lymphoma is mostly endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD)[15,34]. Compared to EMR, ESD is superior because it allows en bloc resection and accurate histological examination[35]. EUS could be of great help in detecting submucosal lesions and evaluating the depth and size of the lesion. It is also used in remission follow-up, because it can evaluate the extraluminal extent of the disease and the extension in the lymph nodes[8,36].
Ahlawat et al[37] reported 30 patients with rectal MALT lymphomas, including 5 patients managed solely with EMR. Deeper lesions have usually been treated surgically[16]. In our case, EUS was used to accurately determine the size and depth of the lesion, and ESD was used to resect the lesion completely. Pathological sections confirmed that the horizontal and vertical resection margins were negative, and curative resection was achieved. In our case, no obvious adhesions were found during the ESD procedure. Before resection, the characterization of lesions and their architecture should be fully evaluated with the help of abdominal CT, NBI, EUS and other techniques. EUS and submucosal injection of normal saline may be helpful to evaluate the infiltration depth of the lesion. The tumour lesion above the muscularis propria should be completely resected during ESD. To avoid or reduce the occurrence of bleeding and perforation, the stripping speed should be controlled and a clear surgical field should be created, risk factors for perforation should be accurately evaluated before surgery, and the operation time should be shortened. Preoperative examination to evaluate whether the lesion can be resected in whole is very important for rectal MALT lymphoma. During the implementation of colorectal ESD, the lesion should be carefully treated to reduce the probability of fragment resection. The patient showed no recurrence without any additional treatment for 2 years after the endoscopic procedure.
This case presents a male patient with rectal MALT lymphoma who was successfully treated with endoscopic submucosal dissection without adjuvant therapy. MALT lymphoma has a good prognosis among gastrointestinal non-Hodgkin's lymphomas. The exact pathological mechanism of nongastric MALT lymphomas has yet to be elucidated. Rectal MALT lymphoma is a rare type of extranodal non-Hodgkin B-cell lymphoma, and its pathogenesis and therapeutic strategies will require more attention in daily clinical practice. Magnifying endoscopy is of great diagnostic value, and EUS can be used as an effective method to detect the depth of infiltration. ESD is a mature endoscopic therapy. Patients with Stage I disease limited to the gastrointestinal tract can be treated successfully with ESD monotherapy. Our case not only reports the unusual presentation of a rare disease presenting as a large mass but also reports the successful use of ESD alone to treat rectal MALT lymphoma without recurrence. However, an increasing number of MALT lymphomas have been reported, and more research about standard management for this disease is needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Iwamuro M, Japan; Kawabata H, Japan; Pattarajierapan S, Thailand S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Tevlin R, Larkin JO, Hyland JM, O'Connell PR, Winter DC. Primary colorectal lymphoma - A single centre experience. Surgeon. 2015;13:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52:1410-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 3. | Wöhrer S, Kiesewetter B, Fischbach J, Müllauer L, Troch M, Lukas J, Mayerhoefer ME, Raderer M. Retrospective comparison of the effectiveness of various treatment modalities of extragastric MALT lymphoma: a single-center analysis. Ann Hematol. 2014;93:1287-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Fischbach W. Colorectal MALT lymphoma: a rare clinical entity. Z Gastroenterol. 2018;56:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Dionigi G, Annoni M, Rovera F, Boni L, Villa F, Castano P, Bianchi V, Dionigi R. Primary colorectal lymphomas: review of the literature. Surg Oncol. 2007;16 Suppl 1:S169-S171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Gay ND, Chen A, Okada CY. Colorectal Lymphoma: A Review. Clin Colon Rectal Surg. 2018;31:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Jeon MK, So H, Huh J, Hwang HS, Hwang SW, Park SH, Yang DH, Choi KD, Ye BD, Myung SJ, Yang SK, Byeon JS. Endoscopic features and clinical outcomes of colorectal mucosa-associated lymphoid tissue lymphoma. Gastrointest Endosc. 2018;87:529-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Violeta Filip P, Cuciureanu D, Sorina Diaconu L, Maria Vladareanu A, Silvia Pop C. MALT lymphoma: epidemiology, clinical diagnosis and treatment. J Med Life. 2018;11:187-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Kiesewetter B, Raderer M. Immunomodulatory treatment for mucosa-associated lymphoid tissue lymphoma (MALT lymphoma). Hematol Oncol. 2020;38:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Adachi K, Ohtsuka H, Kozai Y. Primary Rectal Mucosa-Associated Lymphoid Tissue Lymphoma. Clin Gastroenterol Hepatol. 2016;14:e52-e53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Raderer M, Kiesewetter B, Ferreri AJ. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA Cancer J Clin. 2016;66:153-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 12. | Salar A. Gastric MALT lymphoma and Helicobacter pylori. Med Clin (Barc). 2019;152:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Zucca E, Bertoni F. The spectrum of MALT lymphoma at different sites: biological and therapeutic relevance. Blood. 2016;127:2082-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 14. | Yachida T, Matsuda T, Sakamoto T, Nakajima T, Kakugawa Y, Maeshima AM, Taniguchi H, Kushima R, Tobinai K, Kobara H, Masugata H, Masaki T, Saito Y. Endoscopic features of colorectal lymphoma according to histological type. JGH Open. 2022;6:257-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Schwartz BL, Lowe RC. Successful Endoscopic Resection of Mucosa-Associated Lymphoid Tissue Lymphoma of the Colon. ACG Case Rep J. 2019;6:e00228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Hangge PT, Calderon E, Habermann EB, Glasgow AE, Mishra N. Primary Colorectal Lymphoma: Institutional Experience and Review of a National Database. Dis Colon Rectum. 2019;62:1167-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Kanneganti K, Balar B. Mucosa-associated lymphoid tissue lymphoma presenting as massive gastrointestinal bleeding: a case report. Case Rep Gastroenterol. 2008;2:296-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Matsuo S, Mizuta Y, Hayashi T, Susumu S, Tsutsumi R, Azuma T, Yamaguchi S. Mucosa-associated lymphoid tissue lymphoma of the transverse colon: a case report. World J Gastroenterol. 2006;12:5573-5576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Foukas PG, Bisig B, de Leval L. Recent advances upper gastrointestinal lymphomas: molecular updates and diagnostic implications. Histopathology. 2021;78:187-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Juárez-Salcedo LM, Sokol L, Chavez JC, Dalia S. Primary Gastric Lymphoma, Epidemiology, Clinical Diagnosis, and Treatment. Cancer Control. 2018;25:1073274818778256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 21. | Nakamura S, Matsumoto T. Gastrointestinal lymphoma: recent advances in diagnosis and treatment. Digestion. 2013;87:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Nonaka K, Nishimura M, Kita H. Role of narrow band imaging in endoscopic submucosal dissection. World J Gastrointest Endosc. 2012;4:387-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | He X, Xu P, Wang X, Jiang S, Gong D, Wu N. The association of gene rearrangement and lymphoma diagnosis: A prospective observational study. Medicine (Baltimore). 2020;99:e20733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Wündisch T, Thiede C, Morgner A, Dempfle A, Günther A, Liu H, Ye H, Du MQ, Kim TD, Bayerdörffer E, Stolte M, Neubauer A. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol. 2005;23:8018-8024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 218] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 25. | Niino D, Yamamoto K, Tsuruta O, Maeda T, Yakushijin Y, Aoki R, Kimura Y, Hashikawa K, Kiyasu J, Takeuchi M, Sugita Y, Ohshima K. Regression of rectal mucosa-associated lymphoid tissue (MALT) lymphoma after antibiotic treatments. Pathol Int. 2010;60:438-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Kelley SR. Mucosa-associated lymphoid tissue (MALT) variant of primary rectal lymphoma: a review of the English literature. Int J Colorectal Dis. 2017;32:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Won JH, Kim SM, Kim JW, Park JH, Kim JY. Clinical features, treatment and outcomes of colorectal mucosa-associated lymphoid tissue (MALT) lymphoma: literature reviews published in English between 1993 and 2017. Cancer Manag Res. 2019;11:8577-8587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Grünberger B, Wöhrer S, Streubel B, Formanek M, Petkov V, Puespoek A, Haefner M, Hejna M, Jaeger U, Chott A, Raderer M. Antibiotic treatment is not effective in patients infected with Helicobacter pylori suffering from extragastric MALT lymphoma. J Clin Oncol. 2006;24:1370-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Kiesewetter B, Simonitsch-Klupp I, Mayerhoefer ME, Dolak W, Lukas J, Raderer M. First Line Systemic Treatment for MALT Lymphoma-Do We Still Need Chemotherapy? Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Kobayashi T, Takahashi N, Hagiwara Y, Tamaru J, Kayano H, Jin-nai I, Bessho M, Niitsu N. Successful radiotherapy in a patient with primary rectal mucosa-associated lymphoid tissue lymphoma without the API2-MALT1 fusion gene: a case report and review of the literature. Leuk Res. 2008;32:173-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Han J, Zhu Z, Zhang C, Xie HP. Successful Endoscopic Resection of Primary Rectal Mucosa-Associated Lymphoid Tissue Lymphoma by Endoscopic Submucosal Dissection: A Case Report. Front Med (Lausanne). 2021;8:715256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Ishikawa E, Nakamura M, Satou A, Shimada K, Nakamura S. Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma in the Gastrointestinal Tract in the Modern Era. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 33. | Thieblemont C, Bastion Y, Berger F, Rieux C, Salles G, Dumontet C, Felman P, Coiffier B. Mucosa-associated lymphoid tissue gastrointestinal and nongastrointestinal lymphoma behavior: analysis of 108 patients. J Clin Oncol. 1997;15:1624-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 266] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 34. | Shah RM, Kuo V, Schwartz A. Endoscopic mucosal resection and cure for rectal mucosa-associated lymphoid tissue lymphoma. Proc (Bayl Univ Med Cent). 2020;34:305-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Park CH, Yang DH, Kim JW, Kim JH, Min YW, Lee SH, Bae JH, Chung H, Choi KD, Park JC, Lee H, Kwak MS, Kim B, Lee HJ, Lee HS, Choi M, Park DA, Lee JY, Byeon JS, Park CG, Cho JY, Lee ST, Chun HJ. Clinical Practice Guideline for Endoscopic Resection of Early Gastrointestinal Cancer. Clin Endosc. 2020;53:142-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 36. | Tannoury J, Amiot A, Lemonnier F, Dupuis J, Gagnière C, Belhadj K, Bras FL, Sobhani I, Haioun C, Copie-Bergman C, Lévy M. Colonic mucosa-associated lymphoid tissue lymphoma: a case series. Leuk Lymphoma. 2020;61:582-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Ahlawat S, Kanber Y, Charabaty-Pishvaian A, Ozdemirli M, Cohen P, Benjamin S, Haddad N. Primary mucosa-associated lymphoid tissue (MALT) lymphoma occurring in the rectum: a case report and review of the literature. South Med J. 2006;99:1378-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |