Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7571
Peer-review started: February 11, 2022
First decision: March 23, 2022
Revised: April 2, 2022
Accepted: May 28, 2022
Article in press: May 28, 2022
Published online: July 26, 2022
Processing time: 149 Days and 17.2 Hours
Clear cell sarcoma (CCS) is a rare and highly malignant soft tissue tumor, usually occurring in the deep soft tissues of the distal tendons and aponeurosis of the extremities, especially the feet and knees. CCS originating in the head and neck is extremely rare. The clinical manifestations of CCS in the head and neck are not typical, and the imaging manifestations have certain characteristics, but the diagnosis still depends on pathological examination and genetic testing.
A 33-year-old male patient had paroxysmal headache for more than 4 years, accompanied by nausea and vomiting, which could be relieved after rest. Com
CCS should be considered in the differential diagnosis of soft tissue tumors of the head and neck, and their diagnosis depends on pathological examination and genetic testing.
Core Tip: Clear cell sarcoma (CCS) is a rare and highly malignant soft tissue tumor, usually occurring in the deep soft tissues of the distal tendons and aponeurosis of the extremities. CCS originating in the head and neck is extremely rare and usually presents as a painless growth mass. In this case, the lesion was located in the head and neck and presented as episodic headache. The lesion was perforated and involved the medulla oblongata. Differential diagnosis of malignant soft tissue tumors of the head and neck should consider CCS based on pathological and genetic tests.
- Citation: Liu CC, Huang WP, Gao JB. Primary clear cell sarcoma of soft tissue in the posterior cervical spine invading the medulla oblongata: A case report. World J Clin Cases 2022; 10(21): 7571-7576
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7571.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7571
Clear cell sarcoma (CCS) is a rare soft tissue tumor that is highly malignant, which often originates from deep soft tissue at the distal end of the tendon and aponeurosis of the extremities, rarely occurring in the head and neck[1]. The most common clinical manifestation is a painless mass. Computed tomography (CT) is helpful to find bone changes, and magnetic resonance imaging (MRI) can help determine the lesion scope. Although imaging findings have certain characteristics and can play an auxiliary role, the diagnosis still requires pathological and genetic testing. This paper describes the radiographic findings of a head and neck CCS in a young male patient.
A 33-year-old man was hospitalized with episodic headache for more than 4 years.
Four years ago, the patient had a sudden headache without obvious inducement, presenting dull pain radiating to the left shoulder, lasting for about 10 h each time, accompanied by nausea and vomiting, which could be relieved after rest, without any symptoms such as disturbance of consciousness, unstable walking, and numbness of limbs.
The patient was in good health prior to the present illness.
The patient had no previous or family history of similar illnesses.
The patient's neurological examination was normal and other physical examination showed no abnormalities.
Tumor marker examination showed that cancer antigen 125 was 336.20 U/mL (normal range ≤ 35 U/mL), and carbohydrate antigen 19-9 was 13414.00 U/mL (normal range ≤ 37 U/mL).
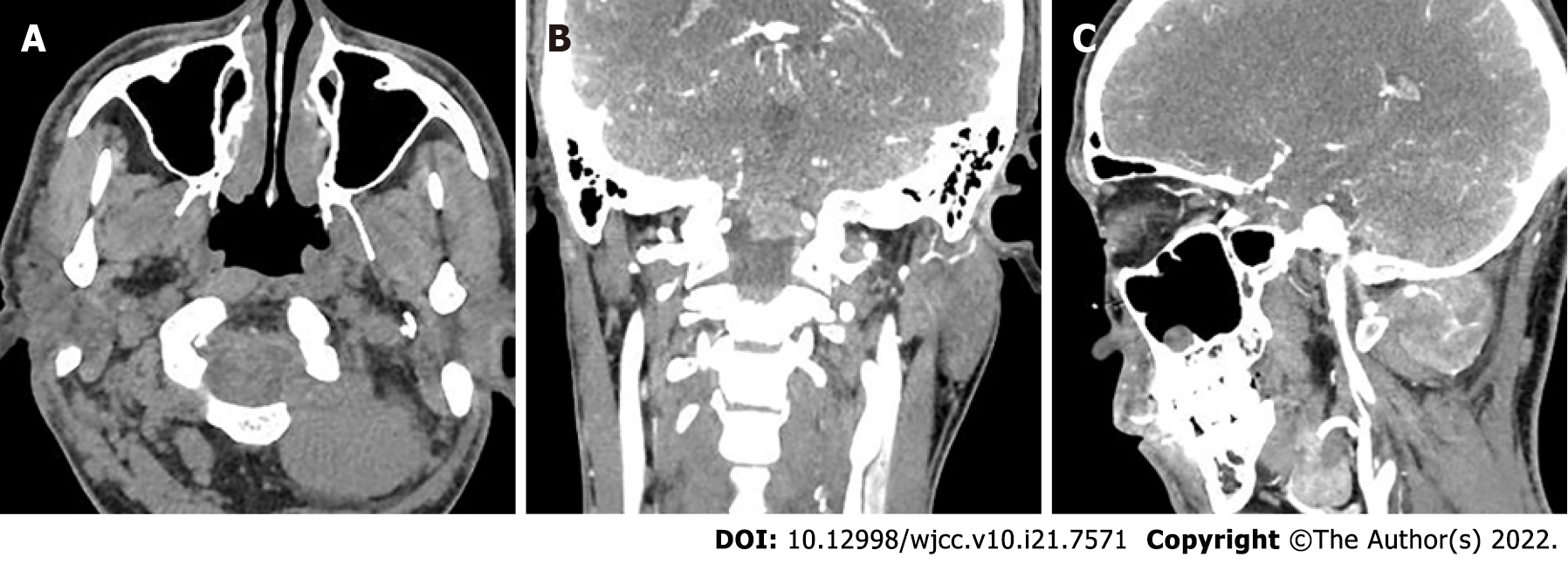
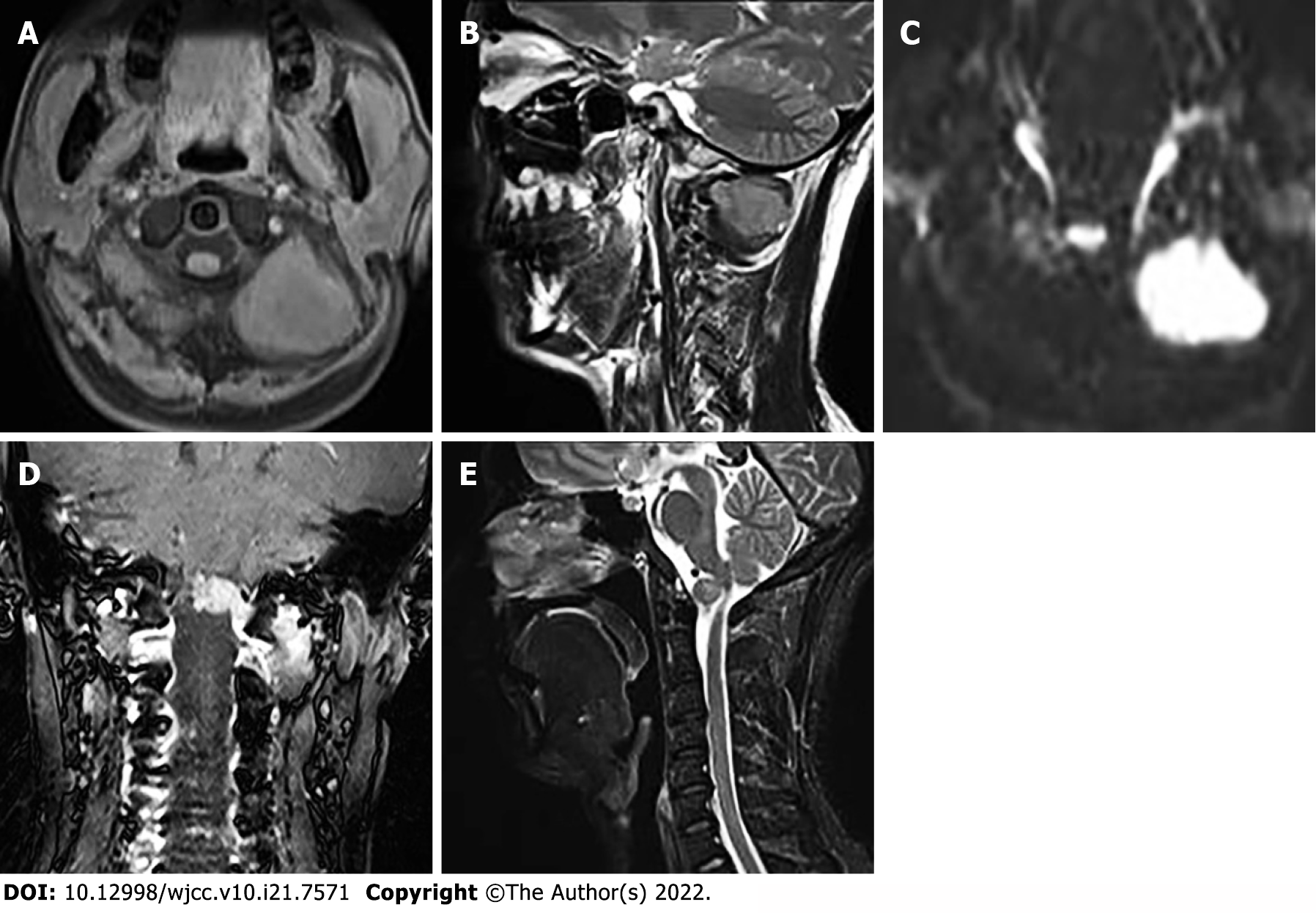
Head and neck computed tomography angiography showed a massive soft tissue mass at the left paraspinal level of 1-2, with a clear boundary (Figure 1A). The mass penetrated inward through the foramen magnum into the spinal canal of the cranio-cervical junction to the front of the cervical spinal cord (Figure 1B). The artery supplying blood to the tumor was tortuous (Figure 1C). On a contrast-enhanced scan, the lesion size was 4.0 cm × 4.7 cm × 3.4 cm (anterior and posterior diameter × left and right diameter × upper and lower diameter), showing uneven and obvious enhancement. There was a curved blood supplying artery in the lesion, locally surrounding the left vertebral artery with adjacent bone invasion. MRI examination showed that compared with adjacent muscles, the lesion showed isosignal on T1-weighted images (T1WI) (Figure 2A), slightly higher signal on T2-weighted images (T2WI) (Figure 2B), high signal on TIRM fat inhibitory sequence, and significantly higher signal on diffusion weighted imaging (DWI) (Figure 2C). The enhancement showed uneven and obvious enhancement, and the lesion invaded the anterior medulla oblongata through the left atlantoaxial foramina (Figure 2D) with a clear boundary. The protrusion of the mass locally compressed cervical spinal cord on T2WI (Figure 2E).
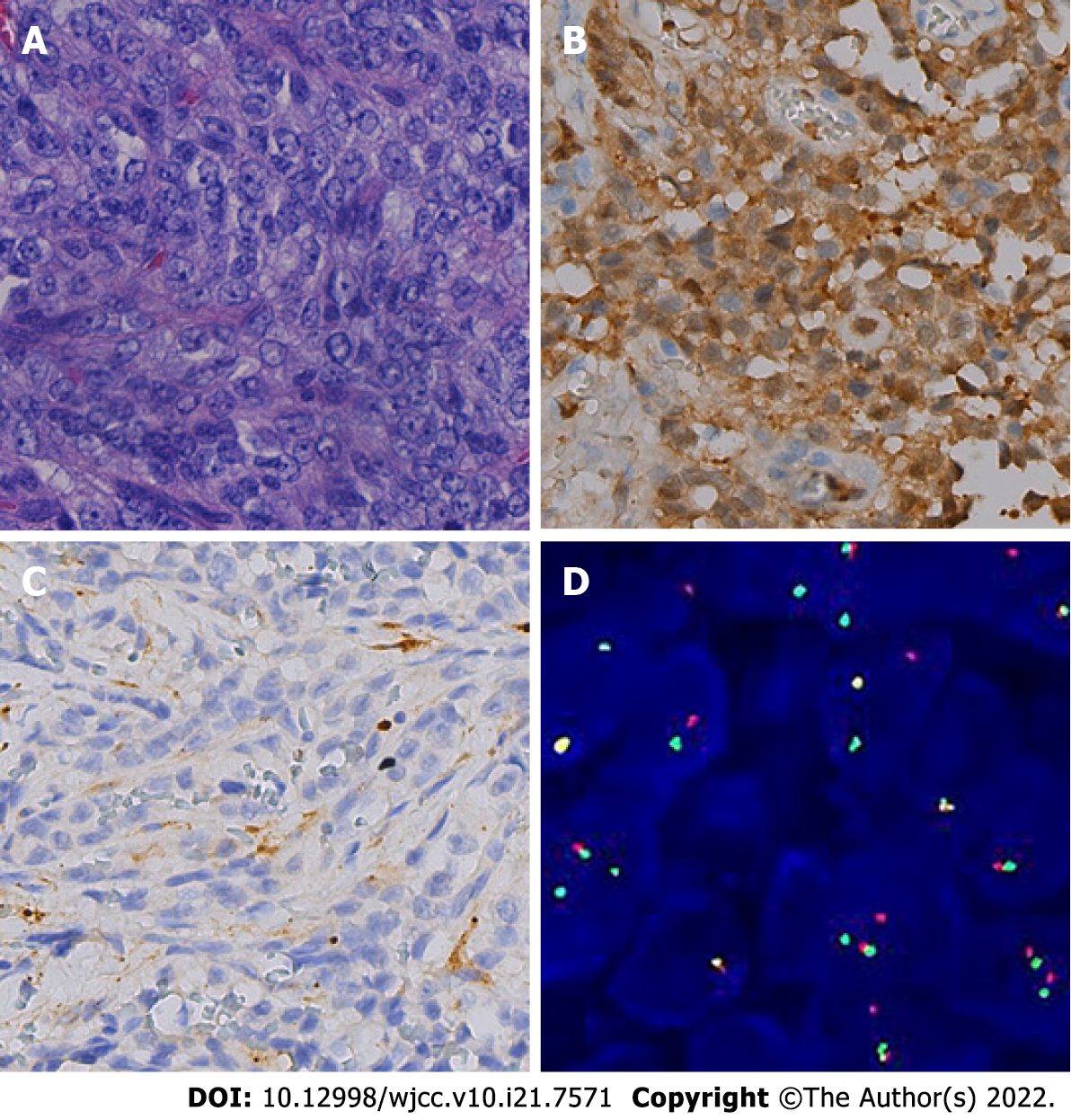
Finally, postoperative histopathology showed ovoid tumor cells separated by fibrous tissue into nests or bundles, with atypical mitosis (Figure 3A). Immunohistochemistry staining revealed S-100 protein (+), CD56 (+), synaptophysin (+), epithelial membrane antigen (±), Ki-67 (40%+), SMARCB1 protein (+), SRY-related high-mobility group box 10 (+), human melanin black-45 (HMB45; +), and melanocyte marker (Melan A; +) (Figure 3B and C). Fluorescence in situ hybridization showed 54% of the positive cells in 100 tumor cells, which was in line with EWSR1 gene break (Figure 3D).
Based on pathology and genetic examination, the patient was diagnosed as having primary CCS of soft tissue of the head and neck.
After admission, the patient underwent lesion resection of the foramen magnum by his attending physician, Guang-Yi Jiang. During surgery, the tumor was seen to be gray-red and tough, with abundant blood supply and adjacent bone erosion. Due to large tumor size, the tumor tissue was partially excised. The bone flap was reduced and connected and fixed with a titanium plate. An external drainage tube was placed subcutaneously, and the muscle group was sutured. The scalp was sutured layer by layer and then pressed and bandaged. Postoperative pathological examination was carried out. The patient was safely returned to the ward after surgery.
The patient showed no neurological abnormalities after surgery. After 2 mo, the patient received radiotherapy followed by antirotinib targeted therapy. After 6 mo, the patient was lost to follow-up by telephone.
CCS is a rare and highly invasive soft tissue tumor, accounting for about 1% of soft tissue sarcomas[2]. The average age of onset of this disease is 35 years old[3], and the most common site is deep soft tissue at the distal tendons and aponeurosis of the extremities, especially the feet and knees. CCS originating in the head and neck is extremely rare. More than half of patients with CCS present with a slow-growing, painless mass that is difficult to diagnose early. In this case, the patient had chronic headache, which was related to the compression of cervical spinal cord by the larger tumor size. At the same time, cancer antigen 125 and carbohydrate antigen 19-9 were elevated in the patient. Studies have shown that cancer antigen 125 may be a useful marker for the diagnosis of epithelioid sarcoma, and elevated carbohydrate antigen 19-9 is often indicative of tumors of epithelial origin[4]. However, pathologically, the origin of CCS tissue is unclear, and there are no reports about cancer antigen 125 and carbohydrate antigen 19-9 in CCS. Microscopically, there are uniformly arranged nests of dense transparent cells and scattered multinucleated giant cells separated by fibrous tissue septum, which is usually contiguous with tendon or aponeurosis. Immunohistochemistry is usually positive for S100, HMB45, Melan A, and vimentin. The morphological and immunohistochemical characteristics overlap with those of malignant melanoma, but there are differences in genetic changes between the two. CCS mainly involves periodic balanced translocation of EWSR1 and ATF1/CREB1 genes[5]. Risk factors for CCS include ionizing radiation, exposure to chemical substances (vinyl chloride, arsenic), and chronic tissue irritation (lymphedema and foreign body implantation). However, the patient in this case was previously healthy and had no related risk factors or family genetic history. The treatment for CCS is mainly surgical treatment, but it is difficult to achieve extensive resection due to its invasive characteristics. CCS is prone to local recurrence and distant metastasis after surgery, with a poor prognosis. Currently, the effect of radiotherapy and chemotherapy on the lesions has not been clarified[6]. The reported 5-year and 10-year survival rates of CCS are 60%-70% and 40%-50%, respectively[7].
The imaging findings of CCS in the head and neck are rarely reported. CT examination of this case showed uniform soft tissue density without calcification and necrosis. There was also large compression of the spinal cord, strong invasive growth within the spinal canal, adjacent bone dissolve bony destruction, no hardening, and no periosteal reaction. Tortuous vessels can be seen in the lesion, locally surrounding the left vertebral artery. The signal was uniform on MRI plain scan, equal-intensity on T1WI, slightly higher intensity on T2WI, and significantly higher intensity on DWI. The lesion was perforated and invaded into the anterior medulla oblongata through the left atlantoaxial foramina. The enhanced scan showed uneven and obvious enhancement, which was consistent with the characteristics of dense tumor cells and abundant blood vessels in pathological tissue. The areas with low enhancement may be related to the slow clearance of the contrast agent caused by fibrous tissue diaphragm.
CCS in the head and neck should be differentiated from neurofibroma, malignant schwannoma, and hemangioma. Neurofibromas are usually solid, with single or multiple cystic changes in the center. There is a hyperactive ring on T2WI and nerve travel from the tumor. On CT, malignant schwanoma is usually an inhomogeneous mass with unclear boundary, accompanied by bone destruction and facial nerve canal enlargement. On MRI, it shows isosignal on T1WI and hypersignal with linear septal hyposignal on T2WI, with cystic lesions and moderate enhancement. Hemangiomas occur in the head and neck of infants and are more common in women. Calcified phlebolith can be seen in the tumor. The density on plain CT scan is similar to that of muscle. The signal intensity on T2WI is uneven and there is significant enhancement.
As shown in this case, the clinical manifestations of CCS in the head and neck are not typical, and the imaging manifestations have certain characteristics, which are helpful for the diagnosis of the disease, though a clear diagnosis still requires pathological examination and genetic testing.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Johan MP, Japan; Lim SC, South Korea A-Editor: Liu X, China S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Hocar O, Le Cesne A, Berissi S, Terrier P, Bonvalot S, Vanel D, Auperin A, Le Pechoux C, Bui B, Coindre JM, Robert C. Clear cell sarcoma (malignant melanoma) of soft parts: a clinicopathologic study of 52 cases. Dermatol Res Pract. 2012;2012:984096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Dim DC, Cooley LD, Miranda RN. Clear cell sarcoma of tendons and aponeuroses: a review. Arch Pathol Lab Med. 2007;131:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Wang J, Thway K. Clear cell sarcoma-like tumor of the gastrointestinal tract: an evolving entity. Arch Pathol Lab Med. 2015;139:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Kato H, Hatori M, Kokubun S, Watanabe M, Smith RA, Hotta T, Ogose A, Morita T, Murakami T, Aiba S. CA125 expression in epithelioid sarcoma. Jpn J Clin Oncol. 2004;34:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Coindre JM, Hostein I, Terrier P, Bouvier-Labit C, Collin F, Michels JJ, Trassard M, Marques B, Ranchere D, Guillou L. Diagnosis of clear cell sarcoma by real-time reverse transcriptase-polymerase chain reaction analysis of paraffin embedded tissues: clinicopathologic and molecular analysis of 44 patients from the French sarcoma group. Cancer. 2006;107:1055-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Ibrahim RM, Steenstrup Jensen S, Juel J. Clear cell sarcoma-A review. J Orthop. 2018;15:963-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Cornillie J, van Cann T, Wozniak A, Hompes D, Schöffski P. Biology and management of clear cell sarcoma: state of the art and future perspectives. Expert Rev Anticancer Ther. 2016;16:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |