Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7565
Peer-review started: February 11, 2022
First decision: March 23, 2022
Revised: April 2, 2022
Accepted: May 27, 2022
Article in press: May 27, 2022
Published online: July 26, 2022
Processing time: 149 Days and 23.3 Hours
Giant cell-rich osteosarcoma (GCRO) is a rare histological variant of osteosarcoma. Spinal GCROs are extremely rare, with challenging diagnosis and management. Herein, we present a case of spinal GCRO at T2, which was not diagnosed in initial biopsy but after T2 corpectomy. We detailed the clinical course, management strategy, and outcome after a 4-year follow-up.
A 17-year-old female patient presented with back pain followed by ascending paresthesia. Spinal computed tomography (CT) and magnetic resonance imaging (MRI) revealed a collapsed T2 vertebra with an enhancing osteolytic mass. CT-guided biopsy showed inconclusive morphology. Pathology from T2 corpectomy revealed GCRO. The patient subsequently received neoadjuvant chemotherapy followed by salvage operation of T2 costotransversectomy with grossly-total resection adjuvant chemoradiation. Upon treatment completion, she had complete GCRO remission. The 4-year follow-up spinal MRI showed no tumor recurrence.
Spinal GCRO poses unique challenges in obtaining sufficient tissue diagnosis and complete surgical removal. However, long-term local control of spinal GCRO is possible following complete resection and adjuvant chemoradiation
Core Tip: Giant cell-rich osteosarcoma (GCRO) is a rare variant of conventional osteosarcoma that is easily misdiagnosed as giant cell tumors. Spinal GCRO poses unique challenges in obtaining a sufficient tissue diagnosis and complete surgical removal. We report a case of spinal GCRO, which was not diagnosed in initial computed tomography-guided biopsy but after T2 corpectomy. Given the relationship between the extent of resection and prognosis, a second salvage operation was performed and gross total resection was achieved. Thus, long-term local control is achievable following complete resection of salvage surgery and adjuvant chemoradiation even in spinal GCRO with previous subtotal resection.
- Citation: Tseng CS, Wong CE, Huang CC, Hsu HH, Lee JS, Lee PH. Spinal giant cell-rich osteosarcoma-diagnostic dilemma and treatment strategy: A case report. World J Clin Cases 2022; 10(21): 7565-7570
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7565.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7565
Osteosarcoma is the most common primary malignant bone tumor, with high-grade histopathological features and poor prognosis, mostly arising from long bones[1]. Osteosarcomas that arise from the spine are rare, accounting for 0.85%–3.0% of all osteosarcomas and 3.6%–14.5% of primary spinal tumors[2]. The sacrum is the most commonly involved location, followed by the lumbar and thoracic spine[2]. Giant cell tumors (GCTs) are typically benign with a low incidence of malignant transformation (< 1%) but can aggressively behave locally[3]. The typical radiographic features consist of a well-defined osteolytic lesion with a non-sclerotic margin and subchondral bone extension[4].
Giant cell-rich osteosarcoma (GCRO) is a rare histological variant of osteosarcoma that was first reported by Bathurst et al[5] in 1986. Based on the World Health Organization classification of soft tissue and bone in 2020, GCRO is categorized as conventional osteosarcomas, which belonged to the group of osteosarcoma not otherwise specified[6]. It accounts for approximately 3% of osteosarcomas and mostly occurs in the long bones[5]. Spinal GCROs are extremely rare, with challenging diagnosis and management compared with long-bone GCRO[7].
Herein, we report a case of spinal GCRO at T2 vertebrae, which was not diagnosed in the initial computed tomography (CT)-guided biopsy but after T2 corpectomy. We detailed the clinical course, management strategy, and outcome after a 4-year follow-up.
A 17-year-old female patient presented with back pain and leg numbness for 4 mo.
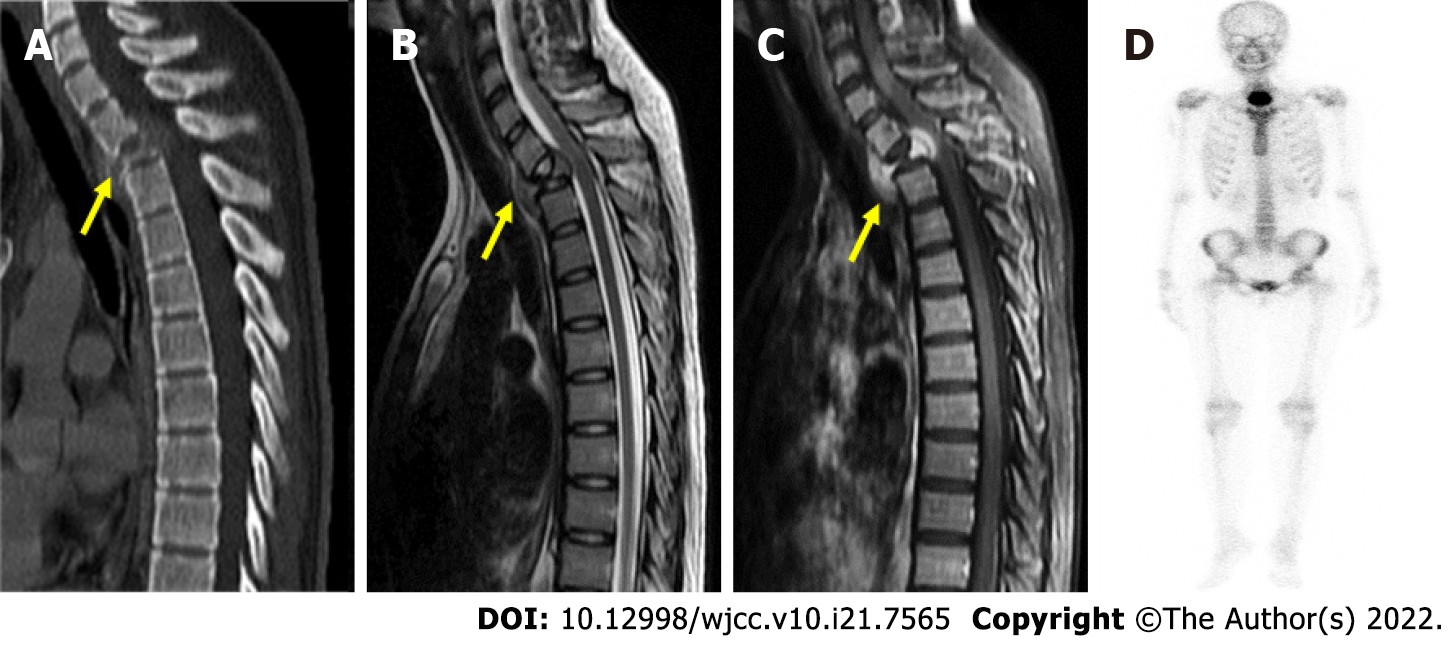
The patient had an unremarkable history and complained of insidious onset of intermittent back pain followed by bilateral ascending paresthesia for 4 mo. Neurological examination revealed paresthesia below the T5 level, 5/5 muscle strength in all four limbs, and bilaterally normal reflexes. Spinal CT and magnetic resonance imaging (MRI) revealed a collapsed T2 vertebra with an enhancing osteolytic mass that extended into the epidural and paraspinal region (Figure 1A-C). Bone scan showed an active bone lesion at the upper thoracic spine (Figure 1D).
Past illness history was not contributory.
Personal and family history was not contributory.
Neurological examination revealed paresthesia below the T5 level, 5/5 muscle strength in four limbs, and bilaterally normal reflexes.
Routine laboratory tests were unremarkable.
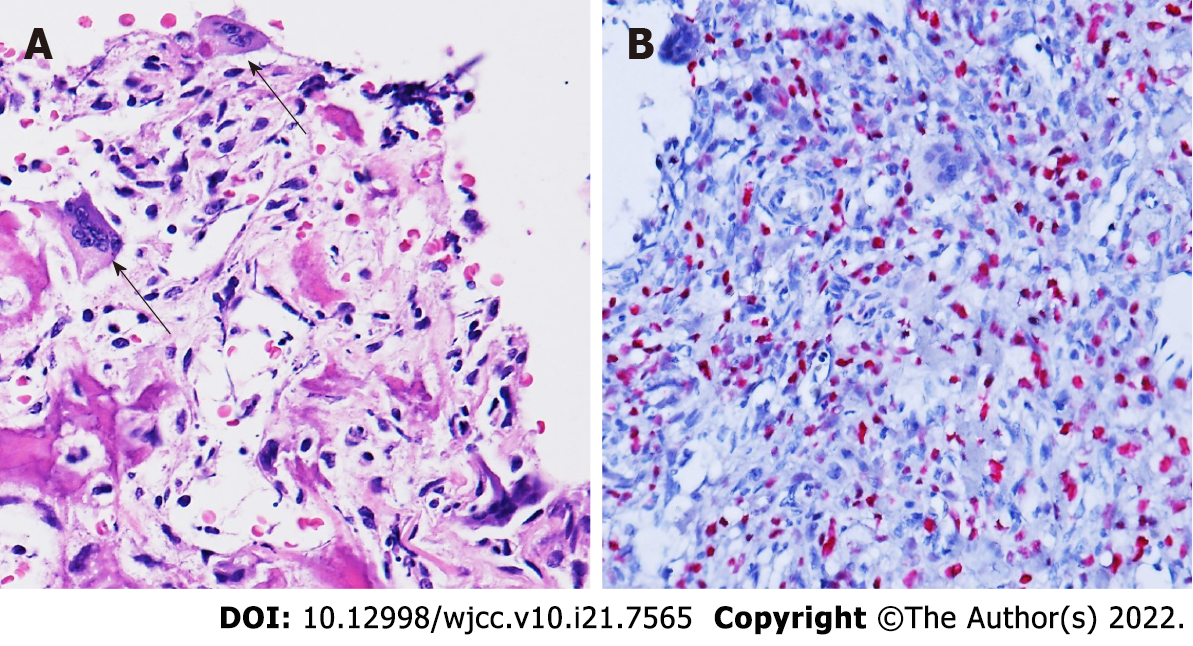
Pathological examination following T2 corpectomy reported GCRO of the spine (Figure 2).
The patient first received CT-guided biopsy, which showed inconclusive morphology; thus, surgical resection for tissue diagnosis was offered. The patient underwent anterior T2 corpectomy with tumor excision and reconstruction with a right iliac bone graft. Intraoperatively, the T2 vertebra was replaced by hypervascular soft tissues, and the pathology showed a hypercellular tumor composed of atypical mononuclear oval to plump spindle cells with osteoid formation, permeative infiltration to the preex
Considering the presence of residual tumor, the patient subsequently received an 8-wk course of neoadjuvant chemotherapy with cisplatin and doxorubicin, salvage operation of T2 costotransversectomy with gross total removal of the residual tumor, adjuvant radiation with 5000 cGy to the T1-T3 region, and a 30-wk course of adjuvant chemotherapy.
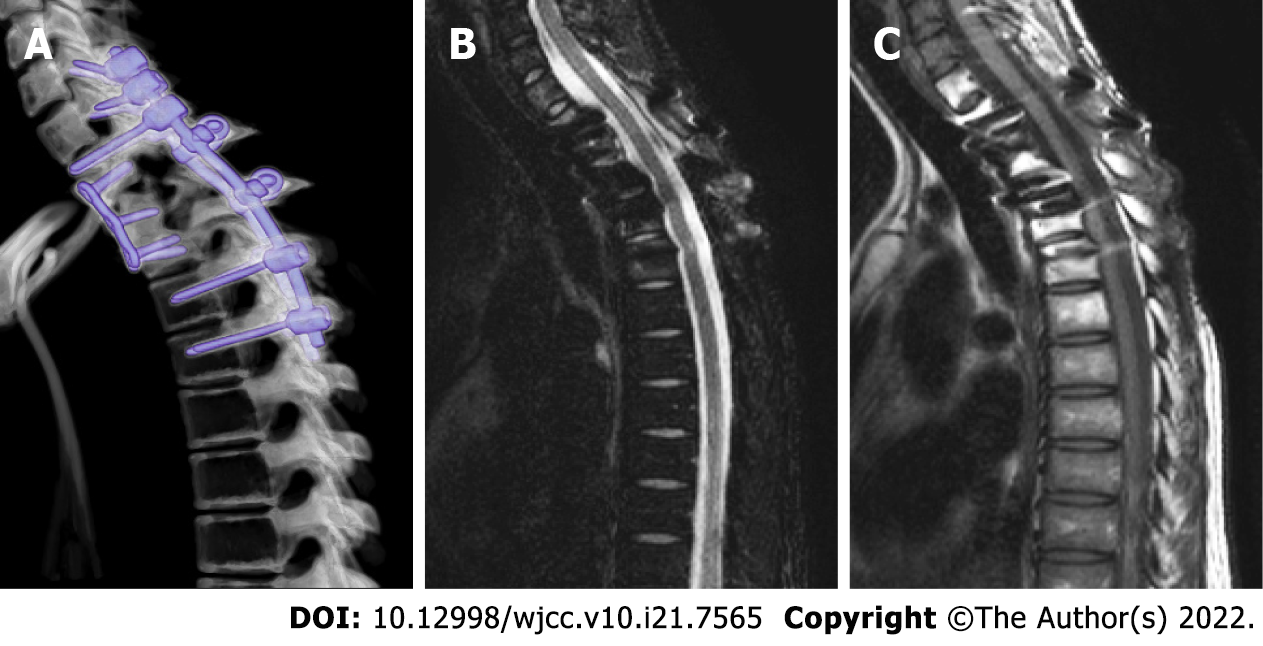
Upon treatment completion, she had complete neurologic recovery and GCRO remission. A 4-year follow-up spinal MRI showed no tumor recurrence (Figure 3).
GCRO is easily misdiagnosed as GCT due to its similarities in radiographic and histopathology features[8]. Radiographically, the radiographic features of GCRO and GCT are similar in the literature. However, most GCRO has little periosteal reactions, whereas a periosteal reaction was seen in 10%-30% of GCTs. Besides, the periosteal reaction identified in GCTs usually present in long bones, and the spinal location of the present case may further hinder the identification of these radiographic findings[9]. Histologically, both GCT and GCRO consist of diffuse infiltration of an abnormally increased number of giant cells[10]. The abundant giant cells in GCROs could thereby almost swamp the sarcoma cells, making GCRO easily misdiagnosed as GCT in pathology[11]. Moreover, the giant cells in GCRO and GCT both share a common H3F3A mutation, osteoclast-like activity, and a tendency to cause bone resorption[12]. Therefore, radiolucency in X-ray images and osteolytic lesions in CT is the commonly shared radiographic feature between GCT and GCRO[8,10,11]. The key diagnostic feature to distinguish CGRO from GCT is still the presence of eosinophilic and irregularly shaped osteoids, which are usually surrounded by a rim of osteoblasts. Furthermore, the key sarcomatous features of GCRO composed of atypical mononuclear oval to plump spindle cells with anaplasia and nuclear pleomorphism, invasive permeative infiltration, and formation of irregularly contoured eosinophilic osteoid, which are unlikely be present in GCT[8,10,13]. Moreover, recent studies have shown that MDM2 and CDK4 are amplified in low-grade osteosarcoma, which can potentially help distinguish GCT from GCRO. It has also been reported that a high Ki67 proliferative index > 20% in GCRO is useful in differentiating it from GCT[13-15]. Therefore, a generous amount of tissue sampling to prevent any sampling bias in surgical pathology is of paramount importance to make the correct diagnosis between GCRO and CGT[16].
Herein, the spinal location of the GCRO impacted its correct diagnosis in several aspects. First, the spinal location of the tumor might hinder a generous amount of surgical tissue sampling due to its proximity to the neurovascular structure. Moreover, decompression and/or separation surgery with stabilization followed by radiation is an accepted alternative treatment strategy, especially in patients with suspected spinal metastases and those unable to tolerate major operations[17]. Thus, an insufficient amount of tissue sampling may pose a higher risk of sampling bias in surgical pathology and misdiagnosis[16,18]. In this case, we selected anterior approach T2 corpectomy to allow a wide corpectomy with circumferential decompression and maximize the amount of tissue sampling since the initial pathology obtained from a CT-guided biopsy was inconclusive.
The survival rate of GCROs is similar to that of high-grade osteosarcoma[13] ranging from 60% to 70% at 5 years and decreases to approximately 20%–30% in patients with metastatic disease[5,19]; long-term local control is achievable with complete resection. The spinal location of the GCRO could impact its prognosis since complete resection with clear surgical margins is associated with better survival[10]. Complete spinal GCRO resection could be more technically challenging or require more aggressive approaches given the proximity of the neural, vascular, and visceral structures to the spinal column compared to the long-bone counterparts[20]. Therefore, the survival rate of primary osteosarcoma in pediatric spine is 18% at 5 years and 7% with distant metastasis[21]. However, gross total tumor resection should be attempted since it directly affects the prognosis[2,7,8]. A recent meta-analysis study also revealed the beneficial outcome after salvage surgery for residual primary spinal osteosarcoma[22]. In the present case, given the relationship between the extent of resection and prognosis, a second salvage operation following neoadjuvant chemotherapy was performed and grossly-total resection was achieved. The present case showed that long-term local control is achievable following complete resection of salvage surgery and adjuvant chemoradiation even in spinal GCRO with previous subtotal resection.
With limited knowledge, we propose in addition to regular physical and neurological exams, a whole-body bone scan 6-mo after salvage surgery is suggested to exclude any distant metastasis. Spinal CT is indicated to evaluation the degree of bony fusion on 3-6 mo postoperatively. MRI scans every 6-mo in the first 2 years and yearly in the subsequent years are mandatory to identify any recurrent tumor locally.
GCRO is a rare variant of conventional osteosarcoma that is easily misdiagnosed as GCT. Spinal GCRO poses unique challenges in obtaining a sufficient tissue diagnosis and complete surgical removal. However, long-term local control of spinal GCRO is possible following complete resection and adjuvant chemoradiation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Muthu S, India; Zhang L, China A-Editor: Liu X, China S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Brookes MJ, Chan CD, Baljer B, Wimalagunaratna S, Crowley TP, Ragbir M, Irwin A, Gamie Z, Beckingsale T, Ghosh KM, Rankin KS. Surgical Advances in Osteosarcoma. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Feng D, Yang X, Liu T, Xiao J, Wu Z, Huang Q, Ma J, Huang W, Zheng W, Cui Z, Xu H, Teng Y. Osteosarcoma of the spine: surgical treatment and outcomes. World J Surg Oncol. 2013;11:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | van der Heijden L, Dijkstra PD, van de Sande MA, Kroep JR, Nout RA, van Rijswijk CS, Bovée JV, Hogendoorn PC, Gelderblom H. The clinical approach toward giant cell tumor of bone. Oncologist. 2014;19:550-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 4. | Chakarun CJ, Forrester DM, Gottsegen CJ, Patel DB, White EA, Matcuk GR Jr. Giant cell tumor of bone: review, mimics, and new developments in treatment. Radiographics. 2013;33:197-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 5. | Bathurst N, Sanerkin N, Watt I. Osteoclast-rich osteosarcoma. Br J Radiol. 1986;59:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Choi JH, Ro JY. The 2020 WHO Classification of Tumors of Bone: An Updated Review. Adv Anat Pathol. 2021;28:119-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 188] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 7. | Mosquera-Salas L, Salazar-Falla N, Perez B, Sangiovanni S, Sua LF, Fernández-Trujillo L. Acute respiratory failure as initial manifestation of conventional osteosarcoma rich in giant cells: a case report. J Med Case Rep. 2020;14:228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Shinozaki T, Fukuda T, Watanabe H, Takagishi K. Giant Cell-Rich Osteosarcoma Simulating Giant Cell Tumor of Bone. KITAKANTO Med J. 2004;54:147-151. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Murphey MD, Nomikos GC, Flemming DJ, Gannon FH, Temple HT, Kransdorf MJ. From the archives of AFIP. Imaging of giant cell tumor and giant cell reparative granuloma of bone: radiologic-pathologic correlation. Radiographics. 2001;21:1283-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 294] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Mallick A, Shah N, Mahmud SA, Das SK. Giant cell-rich osteosarcoma - A rare case. J Oral Maxillofac Pathol. 2020;24:S67-S72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Lee JC, Huang HY. Soft Tissue Special Issue: Giant Cell-Rich Lesions of the Head and Neck Region. Head Neck Pathol. 2020;14:97-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Presneau N, Baumhoer D, Behjati S, Pillay N, Tarpey P, Campbell PJ, Jundt G, Hamoudi R, Wedge DC, Loo PV, Hassan AB, Khatri B, Ye H, Tirabosco R, Amary MF, Flanagan AM. Diagnostic value of H3F3A mutations in giant cell tumour of bone compared to osteoclast-rich mimics. J Pathol Clin Res. 2015;1:113-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Chow LT. Giant cell rich osteosarcoma revisited-diagnostic criteria and histopathologic patterns, Ki67, CDK4, and MDM2 expression, changes in response to bisphosphonate and denosumab treatment. Virchows Arch. 2016;468:741-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Hirose K, Okura M, Sato S, Murakami S, Ikeda JI, Noda Y, Fukuda Y, Morii E, Toyosawa S. Gnathic giant-cell-rich conventional osteosarcoma with MDM2 and CDK4 gene amplification. Histopathology. 2017;70:1171-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Santana LADM, Felix FA, de Arruda JAA, da Silva LP, Brito ÉAC, Takeshita WM, Trento CL. A rare case of a metastatic giant cell-rich osteosarcoma of the mandible: Update and differential diagnostic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131:e163-e169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Thunnissen FB, Ambergen AW, Koss M, Travis WD, O'Leary TJ, Ellis IO. Mitotic counting in surgical pathology: sampling bias, heterogeneity and statistical uncertainty. Histopathology. 2001;39:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Conti A, Acker G, Kluge A, Loebel F, Kreimeier A, Budach V, Vajkoczy P, Ghetti I, Germano' AF, Senger C. Decision Making in Patients With Metastatic Spine. The Role of Minimally Invasive Treatment Modalities. Front Oncol. 2019;9:915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Renshaw AA, Gould EW. Measuring errors in surgical pathology in real-life practice: defining what does and does not matter. Am J Clin Pathol. 2007;127:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Zhao X, Wu Q, Gong X, Liu J, Ma Y. Osteosarcoma: a review of current and future therapeutic approaches. Biomed Eng Online. 2021;20:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 172] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 20. | Katonis P, Datsis G, Karantanas A, Kampouroglou A, Lianoudakis S, Licoudis S, Papoutsopoulou E, Alpantaki K. Spinal osteosarcoma. Clin Med Insights Oncol. 2013;7:199-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Kim HJ, McLawhorn AS, Goldstein MJ, Boland PJ. Malignant osseous tumors of the pediatric spine. J Am Acad Orthop Surg. 2012;20:646-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Shankar GM, Clarke MJ, Ailon T, Rhines LD, Patel SR, Sahgal A, Laufer I, Chou D, Bilsky MH, Sciubba DM, Fehlings MG, Fisher CG, Gokaslan ZL, Shin JH. The role of revision surgery and adjuvant therapy following subtotal resection of osteosarcoma of the spine: a systematic review with meta-analysis. J Neurosurg Spine. 2017;27:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |