Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7553
Peer-review started: February 11, 2022
First decision: March 23, 2022
Revised: April 7, 2022
Accepted: June 4, 2022
Article in press: June 4, 2022
Published online: July 26, 2022
Processing time: 149 Days and 12 Hours
Urticaria is one of the most common causes of emergency room visits. It is defined as an acute inflammatory dermatosis, characterized by localized degranulation of mast cells, with consequent dermal microvascular and formation of edematous and pruritic plaques called hives. Urticaria affects the skin and tissues of the superficial mucosa. Sometimes it is accompanied by angioedema, which is characterized by deeper edema of the dermis and subcutaneous cellular tissue known as the urticarial-angioedema syndrome. About 15%-25% of the general population has suffered at least one type of urticaria at some point during their lifetime and hyperpermeability estimated at 7.6%-16% and has experienced acute urticaria that is usually self-limited and spontaneously resolves without requiring medical attention.
We present the case of a young male patient who was referred to our department with a clinical picture of 4 mo of pruritus associated with hives of variable sizes, irregular borders, with interlesional confluence, that were non-painful, without involvement of the palms and soles of the feet but with a tendency to progression in a generalized manner. He had multiple emergency room visits and poor response to antihistamines and systemic corticosteroids. Imaging studies demonstrated nodules in the lower lingula segment, at the level of the greater fissure and in the anterior contour of the left anterior basal segment associated with parahiliar adenopathies in the absence of findings suggestive of infectious or autoimmune etiology. Segmental lobectomy was performed by thoracoscopy with resection of a lung nodule in the lingula and biopsy of the para-aortic mediastinal ganglion. The histopathological report showed the presence of poorly differentiated invasive adenocarcinoma with a solid morphological and acinar pattern with immunohistochemical description of lung tissue that expresses strong positive and diffuse reaction for thyroid transcription factor 1 (TTF-1) with negativity to P40 for a histopathological diagnosis of malignant epithelial neoplasia with expression of infiltrating adenocarcinoma. Spontaneous chronic urticaria is considered possibly secondary to lung adenocarcinoma.
Chronic spontaneous urticaria is considered a paraneoplastic dermatosis with a controversial association in the literature. In the presented case, a young patient presented with chronic refractory urticaria and after an exhaustive clinical work-up was found to have a diagnosis of poorly differentiated lung adenocarcinoma with high expression of TTF-1. According to the Curth criteria, the urticaria presented by the patient is related to the oncological diagnosis. In addition, the high expression of TTF-1 documented in this case could be acting as an autoantigen that would cause chronic spontaneous urticaria. Further research evaluating a causal relationship between the TFF-1 protein and urticaria in lung cancer is needed.
Core Tip: This case of urticaria associated with adenocarcinoma highlights the histopathological report where the thyroid transcription factor 1 (TTF-1) was highly expressed by the lung tissue and its relationship with lung cancer. TTF-1 regulates the transcription of specific genes in the thyroid, lung, and diencephalon. This transcription factor is also known as a thyroid-specific enhancer-binding protein and is used in pathology as a marker to determine whether a tumor arises from the lung or thyroid.
- Citation: Jiménez LF, Castellón EA, Marenco JD, Mejía JM, Rojas CA, Jiménez FT, Coronell L, Osorio-Llanes E, Mendoza-Torres E. Chronic urticaria associated with lung adenocarcinoma — a paraneoplastic manifestation: A case report and literature review . World J Clin Cases 2022; 10(21): 7553-7564
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7553.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7553
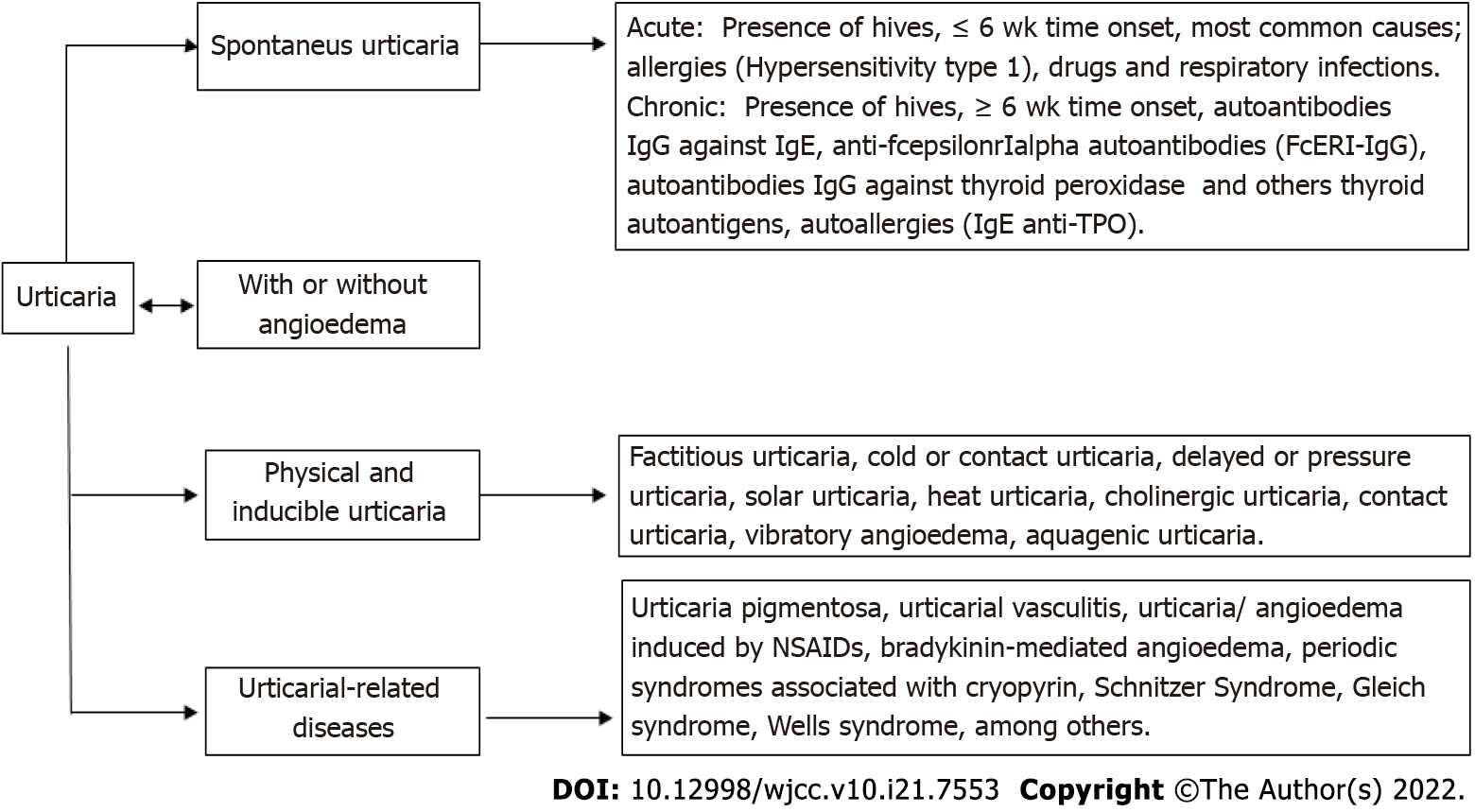
Urticaria is characterized by transient itchy wheals, angioedema, or both. It is a debilitating disease that places a substantial burden on patients and society. Urticaria is classified as acute (lasting < 6 wk) or chronic (persisting for > 6 wk)[1]. Urticaria represents a group of diseases including spontaneous urticaria and inducible urticaria (formerly called physical); the remaining group comprises angioedema and urticarial vasculitis[2].
The dermatological lesion (hives) has three typical characteristics: Central edema of variable size, surrounded by reflex erythema; Itching associated with a burning sensation of a transitory nature due that the skin returns to its normal appearance, generally in a period ranging from 30 min to 24 h[3]. Angioedema is defined by: Sudden and marked edema of the deep dermis and adipose tissue; Greater frequency of pain than itching, sometimes concomitant; Frequent involvement of the mucous membranes; and resolution of the condition at approximately 72 h[4]. The 40%-50% of cases of urticaria are associated with angioedema; 40% may present with urticaria without angioedema, and the remaining 10%-20% may present with angioedema without urticaria[5].
Urticaria is generally classified as acute or chronic, depending on the duration of symptoms and the presence or absence of triggering stimuli[6]. Acute urticaria is defined as the presence of hives, accompanied or not by angioedema, which resolve in a period of < 6 wk. Chronic urticaria is defined as the presence of hives, accompanied or not by angioedema, for a period > 6 wk, continuously or intermittently[7]. It has been estimated that the prevalence of chronic urticaria ranges between 0.5% and 5%, being more common in adults, with a maximum age of onset between 20 years and 40 years, with a high prevalence in females[8].
Chronic urticaria (CU) is divided into two types: chronic spontaneous urticaria (CSU) and chronic inducible urticaria (CindU); the first refers to the presence of wheals with or without angioedema greater than 6 wk without known external determinants. However, approximately 45% of these patients present with IgG isotype-specific autoantibodies that recognize immunoglobulin E (IgE) in the mast cell membrane. These autoantibodies can directly recognize the alpha chain of high-affinity receptor for IgE (FcεRIα) with consequent release of histamine, tryptase, cytokines, among other pro-inflammatory mediators[9]; CSU is also associated with anti-thyroid antibodies in approximately 10%-42.5% of cases[3]. In recent years, the concept of “auto-allergies” has been coined as a possible pathophysiological mechanism underlying mast cell activation and degranulation events[10] and IgE-type autoantibodies against thyroid peroxidase (anti-TPO IgE) have been identified between 10% and 61% of patients with CSU[11-13]. These autoantibodies have also been found in a variety of dermatological disorders related to mast cell activation such as atopic dermatitis and bullous pemphigoid[14,15].
Figure 1 illustrates the classification of urticaria according to the World Allergy Organization. CindU is produced by physical or environmental stimuli and represents between 20% and 30% of all chronic urticaria[16]. The association between CSU (without vasculitis and angioedema) and malignancy is not completely clear. Urticaria is not one of the truly specific paraneoplastic dermatoses suggestive of cancer; however, its appearance and association with other cancers such as lymphoma, leukemia, and ovarian carcinoma has been controversially documented[17]. The current scientific evidence about CSU as a clinical manifestation associated with lung adenocarcinoma is also very limited with few studies or case reports available. The following is a case report of a patient who was presented with refractory CSU as a possible paraneoplastic syndrome associated with poorly differentiated lung adenocarcinoma.
A 25-year-old male patient who presented with 4 mo insidious skin rash, located in the proximal and anterior regions of the right thigh that was intermittent, sometimes migratory, with intensification during the evenings and overnight, with little improvement to treatment with second-generation antihistamines and topical antipruritic drugs.
The clinical picture was later associated with the appearance of wheal-type dermatological lesions of variable sizes, irregular borders, which progressed in a generalized manner, prompting him to consult emergency services on multiple occasions. The patient was treated with systemic, topical oral corticosteroids, and antihistamines with partial improvement, followed by exacerbation of symptoms, so he was hospitalized.
The patient denied immunopathological history and allergies to medications or foods. The patient does not have past drug history.
The patient had no personal or family history of lung cancer or urticaria.
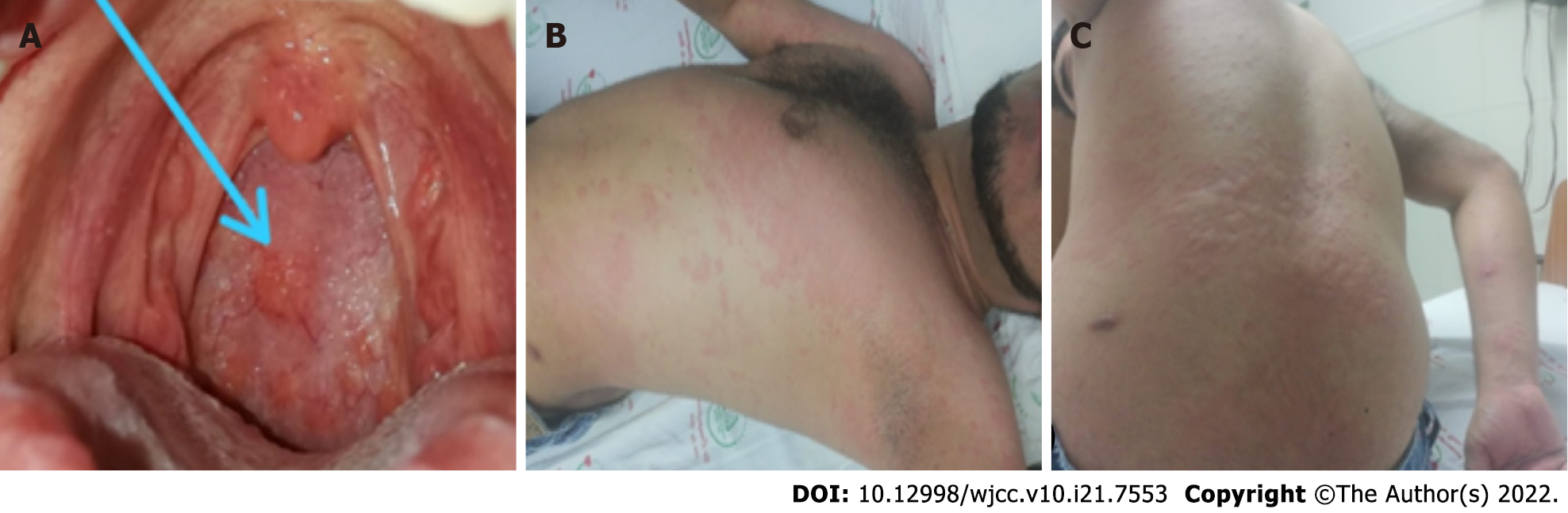
On physical examination, the patient presented; blood pressure (BP) 120/80 mmHg, heart rate 72 bpm, respiratory rate 17 bpm, body temperature 36.3 °C, oxygen saturation 98%, fraction of inspired oxygen (FiO2) 21%, weight 75 kg, height 1.7 m, and body mass index (BMI) 25.9 kg/m2. Nodular lesions of approximately 0.5 cm were observed. The patient also presented generalized erythematous wheal-like skin lesions with raised serpiginous edges with interlesional confluence, non-tender to palpation and with a tendency to pale by acupressure, without involvement of the palms and soles (Figure 2). In the oropharynx, we found nodular lesions of approximately 0.5 cm in diameter (Figure 2A) that were erythematous, wheal-like lesions with raised and serpiginous edges. Highly pruritic lesions with intralesional confluence were found on the anterior trunk and upper extremities (Figure 2B) and lesions with similar characteristics were also found on the posterior trunk (Figure 2C).
Laboratory tests were performed with the following reports; hemoglobin level 14.9 g/dL, hematocrit 47.8%, mean corpuscular volume 69.9 fL, mean corpuscular hemoglobin 21.8 pg, leukocyte count 22460/mm³, neutrophils 94.2%, lymphocytes 2.5%, monocytes 2.2%, eosinophils 0%, basophils 0.2%, platelets 366000/mm³ and peripheral blood smear without alterations in the white and erythroid series or in platelets. Serum chemistries demonstrated blood urea nitrogen (BUN) 20.6 mg/dL, creatinine 1.1 mg/dL, Na 139 mmol/L, Cl 97.5 mmol/L, K 4.47 mmol/L, aspartate transaminase (AST) 20.3, alanine transaminase (ALT) 29.2, allergic-specific IgE (RAST for allergens) negative seafood and latex, total IgE 62.7 IU/mL (V.N: 0 - 200), and Ra test < 30 (less than 30). Study of infectious and autoimmune diseases showed HVB negative, HVC negative, HIV negative, and VDRL not reactive. antinuclear antibodies (ANA) IgG 10.92 (less than 20), anti-RO 2.67 (less than 20), anti-LA 2.4 (less than 20), anti-SM 2.23 (less than 20), anti-RNP 2.18 (less than 20), C-anca, P-anca negative, C3 120 mg/dL, C4 15.2 mg/dL (Tables 1-3). Tumor marker levels are presented in Table 2.
| Parameter | Result | Reference range |
| Blood count | ||
| Leukocytes | 22460 | 5000-10000/mm3 |
| Neutrophils | 94.2% (21160) | 45%-75% |
| Lymphocytes | 2.5% (570) | 30%-40% |
| Monocytes | 2.2% (490) | 0%-8% |
| Eosinophils | 0% (0) | 0%-5% |
| Basophils | 0.2% (40) | 0%-1% |
| Red blood cells | 6.8 million/mm3 | 3.7-5.1 million/mm3 |
| Hemoglobin | 14.9 g/dL | 12-14 g/dL |
| Hematocrit | 47.8% | 35%-46% |
| Mean corpuscular volume | 69.9 ft | 80-100 ft |
| Mean corpuscular hemoglobin | 21.8 | 27-33 pg |
| Platelets | 366000 | 150000-450000/mm3 |
| Peripheral blood smear | ||
| Red blood cells series | Normal number, morphology preserved | - |
| Platelets | Normal number, morphology preserved | - |
| White blood cell series | Normal size, shape, granulations and lobulations | - |
| Parameter | Result | Reference range |
| Urine test | ||
| Color | Yellow | - |
| Appearance | Slightly turbid | - |
| Density | 1020 | 1010-1020 |
| pH | 6 | 4.5-7.5 |
| Nitrites | Negative | - |
| Leukocyte esterase | Negative | - |
| Protein | Negative | < 30 mg/dL |
| Glucose | Negative | 0-15 mg/dL |
| Bacteria | + | - |
| Ketonic bodies | Negative | - |
| Leukocytes | 1-3 | 1-10 by camp |
| Red blood cells | 2-4 | 1-5 by camp |
| Renal function | ||
| Blood urea nitrogen | 20.1 mg/dL | 9-25 mg/dL |
| Creatinine | 1.1 mg/dL | 0.7-1.3 mg/dL |
| Urea | 44.08 mg/dL | 10-45 mg/dL |
| Other biomarkers in blood | ||
| Troponin | 0.01 mg/dL | < 0.01 mg/dL |
| Creatine phosphokinase | 38 mg/dL | 28-174 mg/dL |
| Uric acid | 4.2 mg/dL | 3.5-7.2 mg/dL |
| Tumor markers | ||
| Alpha fetoprotein | 0.50 | 54.8 U/L |
| Carcinoembryonic Antigen | 1.02 | 0-3 U/L |
| CA 19-9 | 31 | 0-37 U/L |
| Parameter | Result |
| Antibodies | |
| Antinuclear antibodies IgG | 10.92 |
| Anti-RD | 2.67 |
| Anti-LA | 2.4 |
| Anti-SM | 2.23 |
| Anti-RNP | 2.18 |
| C-ANCA P-ANCA | Negative |
| IgE | 62.7 |
| RA test | < 30 |
| C3 | 120 mg/dL (reference range: 90-180 mg/dL) |
| C4 | 215.2 mg/dL (reference range: 10-40 mg/dL) |
| Infectious diseases test | |
| Hepatitis B virus test | Negative |
| Hepatitis C virus test | Negative |
| Human Immunodeficiency virus test | Negative |
| Venereal disease research laboratory test | Negative |
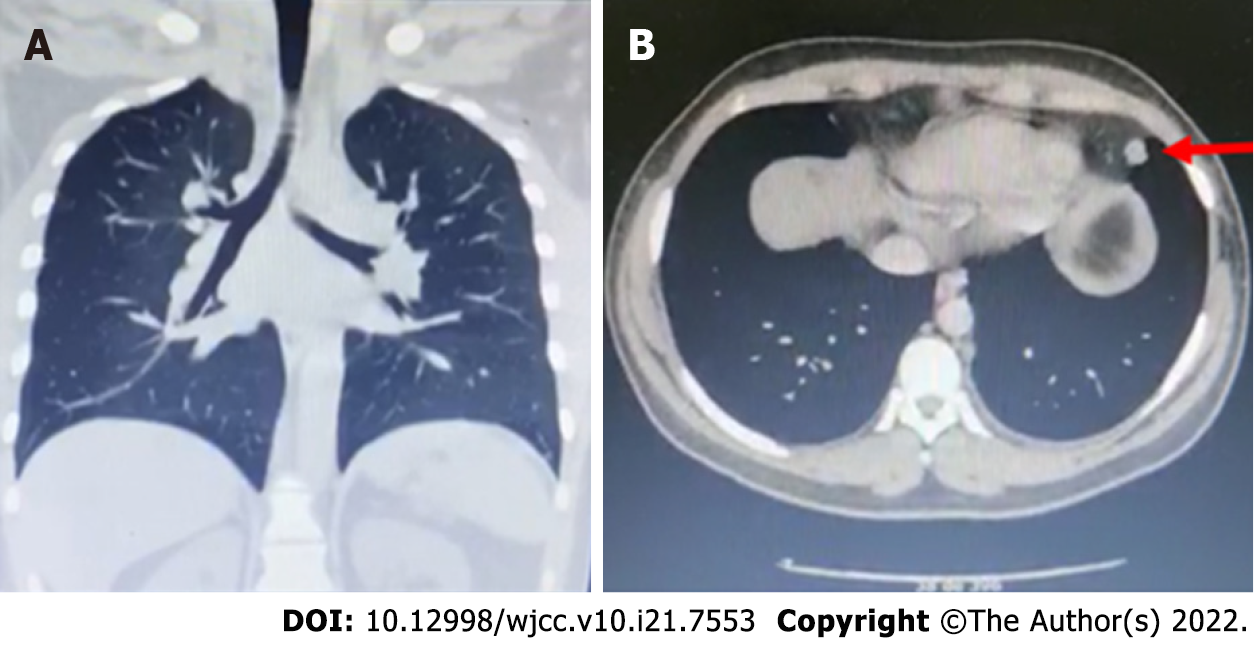
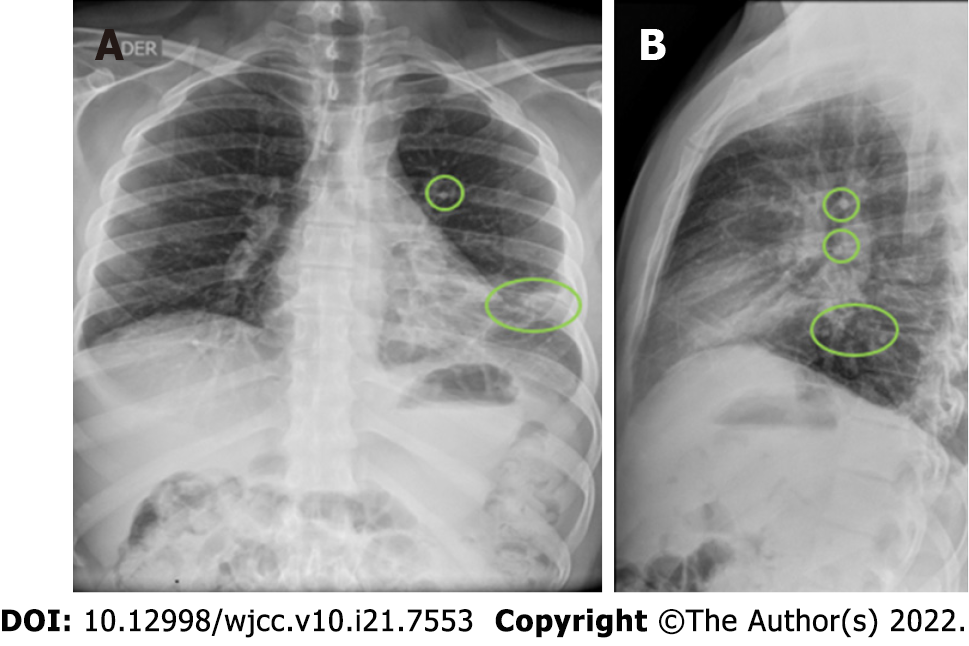
A total abdominal ultrasound showed results within normal limits; chest X-ray and chest computed tomography (CT) (Figure 3) were also performed. Chest X-ray showed bilateral perihilar interstitial opacities without identifying areas of pulmonary consolidation, basal subsegmental atelectasis, and free costophrenic angles. Chest CT with contrast showed several small nodules at the level of the lower lingula segment with the two largest in their medial aspect measuring 7.9 mm and 13.6 mm x 10.8 mm (Figure 4). In addition, nodules adhered to the greater fissure in the anterior contour of the left anterior basal segment with adenopathy of 16 mm x 11 mm present at the retrocaval paratracheal level and subcarinal lymphadenopathy of approximate 46 mm x 48 mm (Figure 4).
The diagnosis of CSU associate with lung carcinoma is clinical. Therefore, the identification of the underlying cause may pose a challenge for the treating physician. Common diagnostic algorithms are mainly focused on identifying autoimmunity, infections and thyroid disease as the most common causes while malignant neoplasms are not commonly part of the differential diagnosis[18].
Based on the description and chest imaging findings, thoracic surgery performed a thoracoscopic segmental lobectomy with resection of the lingular pulmonary nodule and biopsy of the para-aortic mediastinal ganglion. The histopathological report showed the presence of poorly differentiated invasive adenocarcinoma with a solid morphological pattern (80%) and acinar (20%) with lung tissue expressing a strong positive and diffuse reaction for the thyroid transcription factor 1 (TTF-1) marker with negativity to P40. The resulting histopathological diagnosis was malignant epithelial neoplasia with immunohistochemical expression of infiltrating adenocarcinoma. The first step in the management of these patients is the use of tumor pathology to confirm the presence of metastatic cancer followed by determination of subtype and confirmation of tissue of origin by immunohistochemistry.
The patient was initially treated with a carboplatin/paclitaxel chemotherapy regimen followed by alectinib 600 mg by mouth every 12 h. In addition, multiple therapeutic thoracenteses and conformational technical external radiotherapy at the level of the thoracolumbar spine were required.
The patient progressed with polyostotic bone metastases in the lumbar, dorsal, and cervical vertebral bodies, for which he was assessed by the neurosurgery service however they determined there was no indication for surgical management, so he was treated with radiotherapy. The patient currently denies new episodes of urticaria after the start of chemotherapy, nor has he required the use of antihistamines.
In a study by Chen et al[19], 12720 patients with chronic urticaria and treatment with long-term antihistamines and without a history of malignant tumors, autoimmune diseases, atopy or allergic diseases were included. The aim of that study was to investigate the relative risk of cancer among patients with chronic urticaria in the Taiwanese population. Seven hundred and four cancers were found among patients with chronic urticaria. An increased risk of cancer was observed (standardized incidence ratio, 2.2; 95%CI: 2.0-2.3), especially hematological malignancy (4.1; 3.1-5.4). The relative risk of cancer varied by age and was higher in patients between 20-years-old and 39-years-old compared to the general population. Most cancer cases were detected within the first year of diagnosis. The risk of non-Hodgkin lymphoma was higher (standardized incidence ratio, 4.4; 95%CI: 3.0-6.1) among hematologic cancers.
Larenas-Linnemann et al[20] similarly described 26 cases in which CSU appears to be caused by a malignant neoplasm (mainly carcinoma or hematological neoplasia). CSU preceded the diagnosis of neoplasia by some months (2-8 mo in 80% of the cases) and was resolved after chemotherapy and/or surgical resection of the malignant neoplasm[20]. In 3 patients, the outbreak of urticaria alerted the treating physician to the recurrence of the neoplasm.
Paraneoplastic syndromes are disorders caused by cancer but not as a direct result of invasion of the affected organ or tissue, nor are they related to its size, direct spread or metastasis[21]. These can be seen in approximately 7%-15% of cancer patients and can be classified according to the involvement of different systems. Among the main groups most frequently identified are endocrine, neurological, hematological, and musculocutaneous, among others[22].
Paraneoplastic dermatoses are associated with an internal malignant neoplasm and are not caused directly by the tumor itself or its metastases[23]. The criteria to identify paraneoplastic dermatoses were reported by Curth in 1976, which remain in use today[24]. Curth's Criteria for Paraneoplastic Dermatoses are commonly used in clinical practice to aide in diagnosis (Table 4). The major criteria include simultaneous or very close onset of the neoplasia and the parallel evolution of the two conditions. Minor criteria include uniformity (a specific malignancy is constantly associated with a specific dermatosis), statistical significance (there is a statistically significant association between malignancy and dermatosis based in case-control studies) and rarity (rarity in the type of skin pathology).
| Criteria | Main characteristic | Main findings |
| Majorcriteria | Concurrent appearance | Dermatosis and malignancy occur simultaneously. |
| Parallel evolution | If the malignancy is treated successfully or recurs, the dermatosis follows a similar course. | |
| Minor criteria | Uniformity | A specific malignancy is constantly associated with a specific dermatosis. |
| Statistical significance | There is a statistically significant association between malignancy and dermatosis based on case-control studies. | |
| Rarity | Rarity in the type of skin pathology. Very frequent processes are eliminated because their high prevalence can cause them to be merely coincidental. |
Paraneoplastic dermatoses (PND) can be classified according to the anatomy involved, etiology, or pathophysiologic mechanism. They can also be classified as hereditary or acquired and according to the probability of association with a malignancy. This last classification allows grouping PND into: Real, optional and controversial (Table 5)[25,26].
| Type of PND | Characteristics |
| Real dermatoses | Basex paraneoplastic acrokeratosis, migratory necrolytic erythema, gyratum repens erythema, paraneoplastic pemphigus, florid cutaneous papillomatosis, palmar fasciitis and arthritis, acquired lacunar hypertrichosis. |
| Facultative dermatoses | Acquired ichthyosis, vasculitis, erythroderma, dermatomyositis, filiform hyperkeratosis, Sweet's syndrome, Pyoderma gangrenosum, pruritus, superficial migratory phlebitis. |
| Controversial dermatoses | Centrifugal annular erythema, Cutaneous vasculitis, necrobiotic xanthogranuloma, primary amyloidosis, scleroderma, porphyria cutanea tardis, bullous pemphigoid, linear IgA dermatitis, Raynaud's phenomenon, urticaria |
According to recent reports, the relationship between urticaria and malignant conditions is still under discussion. Despite this, a retrospective cohort study showed urticaria was linked with and increased risk of non-Hodgkin lymphoma[24,27]. Likewise, urticaria can be associated with malignant neoplasms such as lymphoma, leukemia and ovarian carcinoma[28]. Association of urticaria with colon adenocarcinoma has also been described, as evidenced in a case report corresponding to a 42-year-old patient with CU in whom high-grade infiltrating colon adenocarcinoma (stage C1 according to the Duke classification) was detected. CU was completely resolved after tumor resection surgery[29].
In Stockholm, an in-hospital study showed that the association between CU and cancer was not statistically significant. Malignant neoplasms detected 5 years after the onset of urticaria were rectum, colon, stomach, malignant melanoma, skin, glioblastoma, and lymphoma[29]. In South Korea, an epidemiological study was carried out in patients with CU, in which an association between this manifestation and malignant neoplasms, such as hematological tumors, stomach, thyroid, liver and prostate cancer, was found[30]. However, it is unknown whether the coexistence of these manifestations share a pathophysiological mechanism or is coincidental, so further research is required to clarify this possible relationship[31]. Therefore, it is worth noting that the majority of studies that associate CU and malignancy have been performed with low quality evidence[32].
The primary site of origin is commonly unknown in adenocarcinomas are the main neoplasms of unknown primary site and CU as a preneoplastic symptom is a rather unusual finding. Currently, the pathophysiological mechanisms that explains the association between adenocarcinoma and CU are still unknown. As in other neoplasms, resolution of CU symptoms occurs after tumor treatment. In two case reports and retrospective reviews, urticaria was associated with adenocarcinomas between 11% and 18%, respectively[31]. Five cases of pulmonary neoplasms associated with chronic urticaria have been reported, of which 3 cases (60%) corresponded to adenocarcinomas, 1 case (20%) to small cell carcinoma and 1 case (20%) to large cell carcinoma. In addition, a case of chronic pruritus associated adenocarcinoma without dermal lesions was also reported[33].
Considering the high prevalence of thyroid disease in patients with CSU and the role of the thyroid transcription factor 1 (TTF-1) as an immunohistochemical biomarker for diagnosis of adenocarcinoma-like neoplasms, the authors of this case thought that high expression of TTF-1 could be acting as an autoantigen triggering CSU[34]. There were no abnormalities in the requested tumor markers in the patient. After an extensive literature search in different databases, no relationship was found between elevation of tumor markers and the appearance of urticaria.
The TTF-1-positive cells found in the lung are type II pneumocytes and club cells. In the thyroid, follicular and parafollicular cells are also positive for TTF-1. For lung cancers, adenocarcinomas are usually positive, while squamous carcinomas and large cell carcinomas are rarely positive. Small cell carcinomas (of any primary site) are normally positive[35,36]. However, others have found that TTF-1 staining is often positive in lung adenocarcinomas, large cell carcinomas, small cell lung carcinomas, non-small cell neuroendocrine tumors, and small cell extrapulmonary carcinomas. It is also positive in thyroid cancer and is used to monitor metastases and recurrences[37]. Larenas-Linnemann et al[20], carried out an extensive review of the literature (26 cases in total), in which they argue that chronic urticaria can be caused by cancer and that it can resolve with its cure. Out of the 26 cases studied, 77% presented with chronic urticaria with poor response to treatment with antihistamines and required oral corticosteroids to control symptoms. Most of the patients (15/22) had presented with urticaria between 2 and 8 mo before the diagnosis of malignancy. The most frequent type of cancer was carcinoma (17 of 25, 68%), and only 24% were of hematological origin, including one seminoma and one astrocytoma. In 19 of 25 cases, the neoplasm was detected in an early and asymptomatic phase. The researchers report that early diagnosis of the neoplasm was due to the exhaustive diagnostic approach of the treating physicians in identification of the cause of the urticaria. After treatment with chemotherapy or tumor resection, the urticaria resolved in all patients[20].
Greiner et al[38] reported the case of a 56-year-old patient, with no history of atopy or allergic diseases, who presented with urticarial dermatological lesions associated with small cell lung carcinoma. The researchers reported that the urticaria was refractory to treatment with intravenous corticosteroids and second-generation antihistamines. The symptoms resolved completely with a higher dose of corticosteroids (methylprednisolone) and surgical intervention for the lung carcinoma. Following tumor removal, the symptoms of urticaria resolved significantly.
In another case report in which urticarial lesions resolved completely with antineoplastic treatment (surgical and/or pharmacological)[19]. Campanelli et al[28] reported a case of chronic urticaria as a paraneoplastic manifestation in a patient with colon adenocarcinoma with no family history of cancer and atopy. After surgical treatment, left hemicolectomy, plus adjuvant chemotherapy for 5 wk, the urticaria completely disappeared. Follow-up of the case 13 mo after completion of antineoplastic treatment showed complete resolution of cancer and urticaria. This clinical scenario is very similar to our reported case. The patient reported that the dermatological lesions (wheals and itching) resolved with the antineoplastic treatment received.
In a retrospective population-based cohort study from Taiwan, Chen et al[19] demonstrated that patients with chronic urticaria have a 2.2 (95%CI: 2.0-2.3) increased standardized incidence (SIR) of developing cancer, especially hematologic malignancy (SIR = 4.1; 3.1-5.4) and non-Hodgkin's lymphoma (SIR = 4.4, 95%CI: 3.0-6.1). Of a total of 12720 patients with chronic urticaria who were treated with antihistamines and did not have a history of malignant tumors, autoimmune diseases, atopy or allergic diseases; there were 704 cancers among the patients studied. Hematological neoplasms were the most commonly observed type of cancer[19]. Despite these findings, the 2017 review and update of the EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis, and treatment of urticaria[39], states that there is insufficient evidence of a Causal correlation of urticaria with neoplastic diseases to support routine screening for malignancy in diagnosing underlying causes of urticaria.
Based on this analysis, we could infer that the high expression of TTF-1 in lung carcinomas may lead to increased expression of TG, TPO and TSHR at the thyroid level. The findings in this case suggest that the high expression of TTF-1 documented could be acting as an autoantigen that would cause CSU. The proposed mechanism may include disruption of glandular immune tolerance leading to an autoimmune response characterized by the synthesis of antithyroid autoantibodies, which in turn degranulates mast cells and mast cells, resulting in the clinical appearance of wheals and itching. However, to date, there are multiple gaps in knowledge and little scientific evidence in the specialized literature. Therefore, more studies are necessary to elucidate the potential relationship between chronic urticaria and malignancy and TTF-1 expression. This case report provides new knowledge that enriches the available scientific literature and that serves as a foundation for the construction of future works that allow evaluation of a potential causal relationship between chronic urticaria and cancer.
The detailed and systematic bibliographic search in the various databases (Clinical Key, PubMed, Scopus) using the relationship between spontaneous chronic urticaria and TTF1 in concomitance with lung adenocarcinoma, did not yield information that establishes the relationship between these variables. Consequently, future research evaluating the possible causal relationship between TFF-1 protein and CSU in patients with lung adenocarcinoma is needed.
Chronic spontaneous urticaria is considered a paraneoplastic dermatosis with a controversial association in the literature. In the case described, a young patient presented with chronic refractory urticaria and after an exhaustive clinical work-up was found to have a diagnosis of poorly differentiated lung adenocarcinoma with high expression of TTF-1. According to the Curth criteria, the urticaria presented by the patient is related to the oncological diagnosis.
In addition, the high expression of TTF-1 documented in this case could be acting as an autoantigen that would cause CSU. Further research evaluating a causal relationship between TFF-1 protein and urticaria in lung cancer is needed.
We thank Dr. Aileen Chang for the English edition.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: Colombia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fazilat-Panah D, Iran; Tang H, China A-Editor: Liu X, China S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Leslie T. Urticaria and angioedema. Medicine. 2021;49:377-380. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | 3 Gonzalez-Diaz SN, Sanchez-Borges M, Rangel-Gonzalez DM, Guzman-Avilan RI, Canseco-Villarreal JI, Arias-Cruz A. Chronic urticaria and thyroid pathology. World Allergy Organ J. 2020;13:100101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Sussman G, Abuzakouk M, Bérard F, Canonica W, Oude Elberink H, Giménez-Arnau A, Grattan C, Hollis K, Hunter S, Knulst A, Lacour JP, Lynde C, Marsland A, McBride D, Maurer M, Nakonechna A, Ortiz de Frutos J, Reynolds M, Sweeney C, Tian H, Weller K, Wolin D, Balp MM. Angioedema in chronic spontaneous urticaria is underdiagnosed and has a substantial impact: Analyses from ASSURE-CSU. Allergy. 2018;73:1724-1734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Winters M. Clinical Practice Guideline Initial Evaluation and Management of Patients Presenting with Acute Urticaria or Angioedema. American Academy of Emergency Medicine (AAEM). [cited July 17, 2021]. Available from: https://www.aaem.org/resources/statements/position/clinical-practice-guideline-initial-evaluation-and-management-of-patients-presenting-with-acute-urticaria-or-angioedema. |
| 6. | Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, Bernstein JA, Bindslev-Jensen C, Brzoza Z, Buense Bedrikow R, Canonica GW, Church MK, Craig T, Danilycheva IV, Dressler C, Ensina LF, Giménez-Arnau A, Godse K, Gonçalo M, Grattan C, Hebert J, Hide M, Kaplan A, Kapp A, Katelaris CH, Kocatürk E, Kulthanan K, Larenas-Linnemann D, Leslie TA, Magerl M, Mathelier-Fusade P, Meshkova RY, Metz M, Nast A, Nettis E, Oude-Elberink H, Rosumeck S, Saini SS, Sánchez-Borges M, Schmid-Grendelmeier P, Staubach P, Sussman G, Toubi E, Vena GA, Vestergaard C, Wedi B, Werner RN, Zhao Z, Maurer M; Endorsed by the following societies: AAAAI, AAD, AAIITO, ACAAI, AEDV, APAAACI, ASBAI, ASCIA, BAD, BSACI, CDA, CMICA, CSACI, DDG, DDS, DGAKI, DSA, DST, EAACI, EIAS, EDF, EMBRN, ESCD, GA²LEN, IAACI, IADVL, JDA, NVvA, MSAI, ÖGDV, PSA, RAACI, SBD, SFD, SGAI, SGDV, SIAAIC, SIDeMaST, SPDV, TSD, UNBB, UNEV and WAO. The EAACI/GA²LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 832] [Article Influence: 118.9] [Reference Citation Analysis (1)] |
| 7. | Bernstein JA, Lang DM, Khan DA, Craig T, Dreyfus D, Hsieh F, Sheikh J, Weldon D, Zuraw B, Bernstein DI, Blessing-Moore J, Cox L, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph CR, Schuller DE, Spector SL, Tilles SA, Wallace D. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 428] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 8. | Powell RJ, Leech SC, Till S, Huber PA, Nasser SM, Clark AT; British Society for Allergy and Clinical Immunology. BSACI guideline for the management of chronic urticaria and angioedema. Clin Exp Allergy. 2015;45:547-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Bracken SJ, Abraham S, MacLeod AS. Autoimmune Theories of Chronic Spontaneous Urticaria. Front Immunol. 2019;10:627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 10. | Maurer M, Altrichter S, Schmetzer O, Scheffel J, Church MK, Metz M. Immunoglobulin E-Mediated Autoimmunity. Front Immunol. 2018;9:689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 11. | Altrichter S, Peter HJ, Pisarevskaja D, Metz M, Martus P, Maurer M. IgE mediated autoallergy against thyroid peroxidase--a novel pathomechanism of chronic spontaneous urticaria? PLoS One. 2011;6:e14794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 12. | Atta AM, Rodrigues MZ, Sousa CP, Medeiros Júnior M, Sousa-Atta ML. Autoantibody production in chronic idiopathic urticaria is not associated with Helicobacter pylori infection. Braz J Med Biol Res. 2004;37:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Concha LB, Chang CC, Szema AM, Dattwyler RJ, Carlson HE. IgE antithyroid antibodies in patients with Hashimoto's disease and chronic urticaria. Allergy Asthma Proc. 2004;25:293-296. [PubMed] |
| 14. | Hradetzky S, Werfel T, Rösner LM. Autoallergy in atopic dermatitis. Allergo J Int. 2015;24:16-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | van Beek N, Lüttmann N, Huebner F, Recke A, Karl I, Schulze FS, Zillikens D, Schmidt E. Correlation of Serum Levels of IgE Autoantibodies Against BP180 With Bullous Pemphigoid Disease Activity. JAMA Dermatol. 2017;153:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 16. | Amaya D, Sánchez A, Sánchez J. Urticaria inducible: serie de casos y revisión de la literatura. Biomédica. 2016;36:10-21. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Poole S, Fenske NA. Cutaneous markers of internal malignancy. II. Paraneoplastic dermatoses and environmental carcinogens. J Am Acad Dermatol. 1993;28:147-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Quirt J, Lima H, Waserman S. Urticaria: a multidisciplinary disease. Where are we now? Curr Derm Rep. 2015;4:8-14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Chen YJ, Wu CY, Shen JL, Chen TT, Chang YT. Cancer risk in patients with chronic urticaria: a population-based cohort study. Arch Dermatol. 2012;148:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Larenas-Linnemann D, Saini SS, Azamar-Jácome AA, Maurer M. Chronic urticaria can be caused by cancer and resolves with its cure. Allergy. 2018;73:1562-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Henry K. Paraneoplastic syndromes: Definitions, classification, pathophysiology and principles of treatment. Semin Diagn Pathol. 2019;36:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Zapata K, Ramírez A. Manifestaciones cutáneas de las neoplasias malignas. Rev Asoc Col Dermatol. 2009;17:109-120. |
| 23. | Rivera A, Hernández M. Dermatosis paraneoplásicas. Revisión de la bibliografía Med Int Mex. 2011;27:586-595. |
| 24. | Chapa J, Ocampo J. Dermatosis paraneoplásicas. Dermatología CMQ. 2015;13:220-226. |
| 25. | Doutre MS. Urticaria. EMC Dermatología. 2020;54:1-21. [DOI] [Full Text] |
| 26. | Monestier S, Richar MA. Paraneoplastic dermatoses. Dermatology. 2018;52:1-17. |
| 27. | Joshi S, Arikan P. Urticaria. Ferri¨s Clinical Advisor. 9 ed. Elsevier, 2021: 1426-1427e. |
| 28. | Campanelli A, Prins C, Saurat JH. Chronic urticaria revealing a colonic adenocarcinoma. J Am Acad Dermatol. 2005;52:1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Lindelöf B, Sigurgeirsson B, Wahlgren CF, Eklund G. Chronic urticaria and cancer: an epidemiological study of 1155 patients. Br J Dermatol. 1990;123:453-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Kim BR, Yang S, Choi JW, Choi CW, Youn SW. Epidemiology and comorbidities of patients with chronic urticaria in Korea: A nationwide population-based study. J Dermatol. 2018;45:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Nazari Z, Ghaffarr J, Ghaffari N. Chronic urticaria associated with malignancies: A review article. Chron Dis J. 2019;7:1-5. [DOI] [Full Text] |
| 32. | Yvin E, Delaunay J, Lozac'h P, Lavigne C, Martin L, Urbanski G. [Chronic superficial urticaria associated with solid cancers: Case report and literature review]. Ann Dermatol Venereol. 2019;146:377-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Urbach E. Endogenous allergy. Arch Derm Syphilol. 1942;45:697-722. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Guazzi S, Price M, De Felice M, Damante G, Mattei MG, Di Lauro R. Thyroid nuclear factor 1 (TTF-1) contains a homeodomain and displays a novel DNA binding specificity. EMBO J. 1990;9:3631-3639. [PubMed] |
| 35. | Weir BA, Woo MS, Getz G, Perner S, Ding L, Beroukhim R, Lin WM, Province MA, Kraja A, Johnson LA, Shah K, Sato M, Thomas RK, Barletta JA, Borecki IB, Broderick S, Chang AC, Chiang DY, Chirieac LR, Cho J, Fujii Y, Gazdar AF, Giordano T, Greulich H, Hanna M, Johnson BE, Kris MG, Lash A, Lin L, Lindeman N, Mardis ER, McPherson JD, Minna JD, Morgan MB, Nadel M, Orringer MB, Osborne JR, Ozenberger B, Ramos AH, Robinson J, Roth JA, Rusch V, Sasaki H, Shepherd F, Sougnez C, Spitz MR, Tsao MS, Twomey D, Verhaak RG, Weinstock GM, Wheeler DA, Winckler W, Yoshizawa A, Yu S, Zakowski MF, Zhang Q, Beer DG, Wistuba II, Watson MA, Garraway LA, Ladanyi M, Travis WD, Pao W, Rubin MA, Gabriel SB, Gibbs RA, Varmus HE, Wilson RK, Lander ES, Meyerson M. Characterizing the cancer genome in lung adenocarcinoma. Nature. 2007;450:893-898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 887] [Cited by in RCA: 878] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 36. | Kwei KA, Kim YH, Girard L, Kao J, Pacyna-Gengelbach M, Salari K, Lee J, Choi YL, Sato M, Wang P, Hernandez-Boussard T, Gazdar AF, Petersen I, Minna JD, Pollack JR. Genomic profiling identifies TITF1 as a lineage-specific oncogene amplified in lung cancer. Oncogene. 2008;27:3635-3640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 37. | Espinoza CR, Schmitt TL, Loos U. Thyroid transcription factor 1 and Pax8 synergistically activate the promoter of the human thyroglobulin gene. J Mol Endocrinol. 2001;27:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Greiner D, Schöfer H, Boehncke WH. Urticaria associated with a small cell carcinoma of the lung. Cutis. 2002;69:49-50. [PubMed] |
| 39. | Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, Church MK, Ensina LF, Giménez-Arnau A, Godse K, Gonçalo M, Grattan C, Hebert J, Hide M, Kaplan A, Kapp A, Abdul Latiff AH, Mathelier-Fusade P, Metz M, Nast A, Saini SS, Sánchez-Borges M, Schmid-Grendelmeier P, Simons FE, Staubach P, Sussman G, Toubi E, Vena GA, Wedi B, Zhu XJ, Maurer M; European Academy of Allergy and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69:868-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 694] [Article Influence: 63.1] [Reference Citation Analysis (0)] |