Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7386
Peer-review started: January 4, 2022
First decision: February 21, 2022
Revised: March 4, 2022
Accepted: May 22, 2022
Article in press: May 22, 2022
Published online: July 26, 2022
Processing time: 188 Days and 1.2 Hours
Research suggests that approximately 6% of adult patients admitted to hospitals in the United States present with sepsis and there has been a minimal change in the incidence of this condition in the last decade. Furthermore, patients with cancer generally have a higher incidence of sepsis due to immunosuppression caused by cancer or its treatment.
To assess if cancer increases the mortality rates in sepsis patients by pooling evidence from contemporary studies.
PubMed, Embase, and Google Scholar databases were searched from January 1, 2001 to December 15, 2021 for studies comparing outcomes of sepsis patients based on the presence of active cancer. Mortality data were pooled using a random-effects model, with the odds ratio (OR) and 95% confidence interval (CI) calculated. Meta-regression was conducted to assess the influence of confounders on mortality rates.
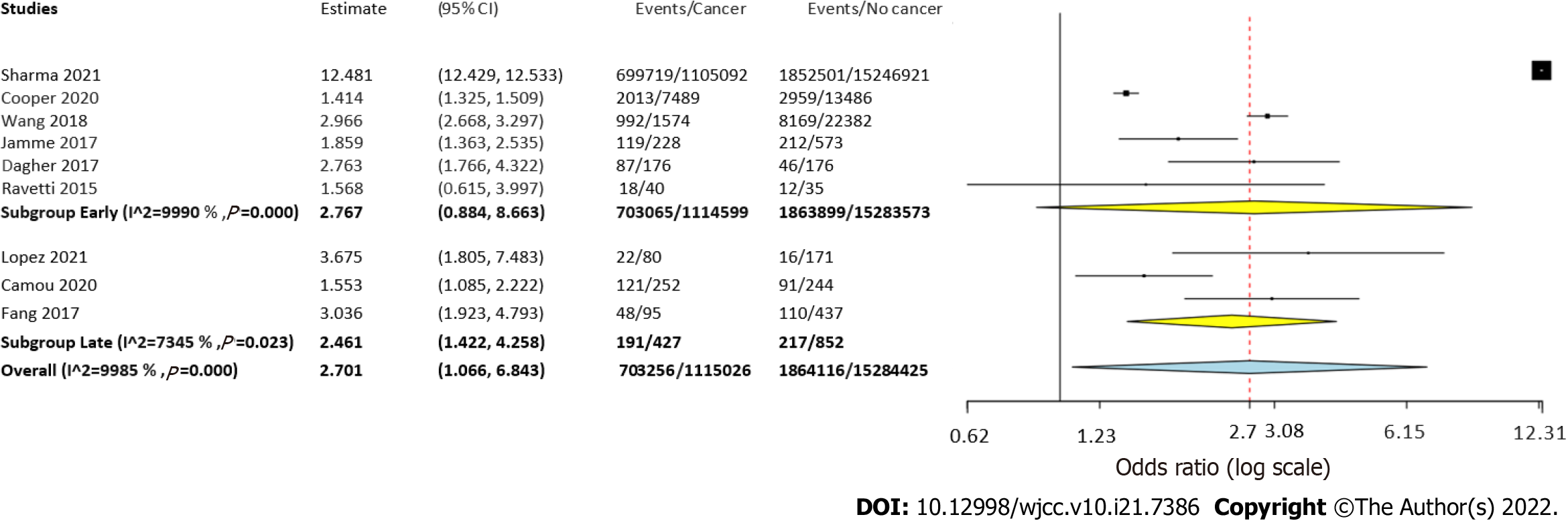
Nine studies were included. The meta-analysis demonstrated a non-significant tendency towards increased risk of early mortality (OR = 2.77, 95%CI: 0.88-8.66, I2 = 99%) and a statistically significantly increased risk of late mortality amongst sepsis patients with cancer as compared to non-cancer sepsis patients (OR = 2.46, 95%CI: 1.42-4.25, I2 = 99%). Overall, cancer was found to significantly increase the risk of mortality in sepsis patients (OR = 2.7, 95%CI: 1.07-6.84, I2 = 99%). Meta-analysis indicated a statistically significantly increased risk of mortality in patients with solid tumors as well as hematological malignancies. Meta-regression indicated that an increase in the prevalence of comorbid pulmonary and renal diseases increased the risk of mortality in cancer patients with sepsis. Mortality rates increased with an increase in the percentage of patients with urinary tract infections while an inverse relationship was seen for infections of cutaneous origin.
Contemporary evidence indicates that the presence of any cancer in sepsis patients significantly increases the risk of mortality. Scarce data suggest that mortality is equally increased for both solid and hematological cancers. Current evidence is limited by high heterogeneity and there is a need for further studies taking into account several confounding variables to present better evidence.
Core Tip: Therapeutic advances in the past two decades have resulted in several advances in the management of cancer as well as sepsis patients. However, it is unclear if active cancer results in worse clinical outcomes in sepsis patients. We pooled the data from nine recent studies to demonstrate that cancer results in a 2.7 times increased risk of mortality in sepsis patients. The outcomes are similar for both solid tumors and hematological cancers. There is a need for further research taking into account several confounding variables to present better evidence.
- Citation: Xiang MJ, Chen GL. Impact of cancer on mortality rates in patients with sepsis: A meta-analysis and meta-regression of current studies. World J Clin Cases 2022; 10(21): 7386-7396
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7386.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7386
Sepsis is a sudden-onset life-threatening organ dysfunction that occurs due to a dysregulated immune response to any infection[1]. The difference between sepsis and septic shock is that the latter is a sub-set of sepsis wherein circulatory and cellular–metabolic abnormalities are intense enough to significantly increase patient mortality[2]. Indeed, sepsis has a global health burden that is associated with high healthcare costs. Research suggests that approximately 6% of adult patients admitted to hospitals in the United States present with sepsis and there has been a minimal change in the incidence of this condition in the last decade[3]. Over the past few years, there has been intense research to discern novel therapies in the management of sepsis[4]; however, the condition is still associated with high rates of morbidity and mortality[5]. A meta-analysis of data from high-income countries indicates that intensive care unit (ICU) mortality with sepsis is approximately 37.3% while hospital mortality and 1-mo mortality range from 39% to 36.8%, respectively[6].
Similar to sepsis, cancer is another leading cause of mortality worldwide. Global data suggest that cancer-related mortality has increased by 25.4% from 2007 to 2017[7]. In comparison with patients without cancer, patients with malignancies have an increased risk of sepsis. Taccone et al[8] in a study on 3147 patients admitted to European ICUs have shown that the prevalence of sepsis in patients with hematological malignancies and solid tumors was 71% and 41.5%, respectively, in comparison to 35.9% in patients without cancer. Possible reasons for such high sepsis rates could be the immunosuppression caused by cancer or its treatment[9]. However, despite the overall increase in cancer-related global mortality, temporal data suggest that the survival of cancer patients with sepsis has increased over time. Zuber et al[10] in a 10-year study on the French population have demonstrated a 25.4% decrease in mortality of cancer patients due to sepsis from 1997 to 2008. In another study, Pène et al[11] compared data of cancer patients with septic shock from two periods, 1998-2001 and 2002-2005. The authors noted that improvement in therapeutic options for sepsis significantly improved survival by 20% between these periods. Considering these data, it would be pertinent to understand if cancer as comorbidity still impacts survival in patients with sepsis. While several recent studies have attempted to answer this clinical question[9,12,13], to the best of our knowledge, no review has attempted to systematically analyze the current evidence. Hence, the purpose of our study was to assess if cancer increases the mortality rates in sepsis patients by pooling evidence from contemporary studies.
The protocol of our review was registered on PROSPERO with registration No. CRD42021291886. We followed the reporting guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (PRISMA) for the current review[14].
A systematic and comprehensive search was undertaken with the help of a medical librarian to explore the electronic databases of PubMed, Embase, and Google Scholar. We also searched “Reference Citation Analysis” for any additional studies. Two authors of the review were involved in the database search which was carried out independently. The time limit of the search was from January 1, 2001 to December 15, 2021. This was done to synthesize only current evidence and exclude older studies. The search terms “cancer”, “malignancy”, “sepsis”, and “septic shock” were used for all databases. Details are presented in Supplementary Table 1. Following the database search, we deduplicated the results. All the remaining studies were analyzed by their titles and abstracts. Articles relevant to the subject of our review were identified and their full texts were extracted. These articles were then examined by two reviewers independently for final inclusion in the review. Any discrepancies in study selection were resolved by consensus. Finally, we also searched the reference list of included studies to look for any other possible inclusions.
The inclusion criteria of the review were as follows: (1) All types of cohort (prospective and retrospective), cross-sectional, and case-control studies conducted on adult patients with sepsis. We did not predefine sepsis and any definition used by the study was acceptable; (2) Studies were to compare outcomes of patients with cancer vs those without cancer; and (3) Outcomes of interest was mortality.
The exclusion criteria were: (1) Studies conducted on patients treated before 2001; (2) Studies on cancer survivors and not on patients with active cancer; (3) Studies not reporting separate data for sepsis patients; (4) Non-English language studies; and (5) Studies reporting duplicate data. Studies with complete overlap of data were excluded. However, studies with partial overlap were to be considered for inclusion.
Two authors independently extracted the following data: Author details, publication year, study type, study location, the database used, the definition of sepsis, sample size, demographic details, comorbidities, the origin of infection, type of cancer, lactate levels, sequential organ failure assessment (SOFA) score, use of invasive ventilation, and follow-up.
The methodological quality of studies was assessed using the Newcastle-Ottawa scale (NOS)[15]. It was conducted by two authors independent of each other. Any disagreements were solved by a discussion. Studies were assessed for selection of study population, comparability, and outcomes, with each domain being awarded a maximum of four, two, and three points, respectively. The maximum score which can be awarded was nine.
Meta-analysis was performed using “Review Manager” [RevMan, version 5.3; Nordic Cochrane Centre (Cochrane Collaboration), Copenhagen, Denmark; 2014]. Both crude and multivariable-adjusted data on mortality were to be extracted from individual studies. However, the majority of the studies reported only crude mortality data, and hence a meta-analysis of adjusted data could not be carried out. Mortality data were pooled using odds ratios (OR) with 95% confidence interval (CI). The meta-analysis was conducted using a random-effects model. Heterogeneity was assessed using the I2 statistic. I2 values of 25%-50% represented low, values of 50%-75% medium, and more than 75% substantial heterogeneity. A sensitivity analysis was carried out to assess the contribution of each study to the pooled estimate by removing one study at a time and recalculating the pooled effect estimates for the remaining studies. Subgroup analyses were carried out based on the follow-up period and type of cancer. Mortality data up to 28 d were grouped as early mortality while 90-180 d of follow-up data were grouped as late mortality. To assess for inter-study heterogeneity, we conducted a random-effects univariate meta-regression analysis using Open MetaAnalyst software[16]. The covariates included in the meta-regression were: Age, male gender, comorbidities of hypertension, diabetes mellitus, pulmonary disease, renal disease, and cardiac disease, and origin of infection (pulmonary, abdominal, urinary tract, or cutaneous).
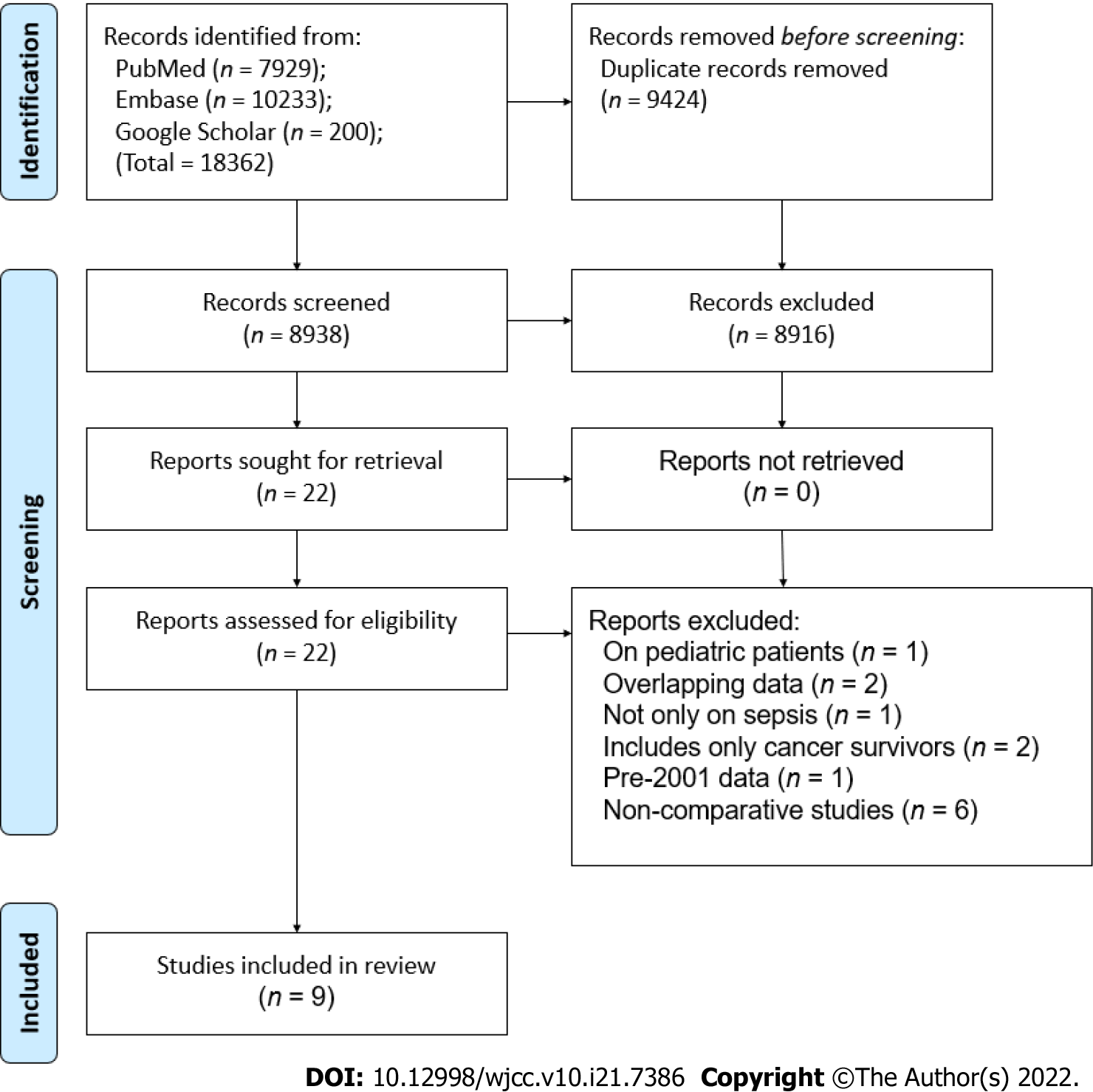
The PRISMA flow chart of the study is presented in Figure 1. A total of 8938 unique articles were found after the literature search, of which 8916 were excluded after the title and abstract screening and 22 were selected for full-text analysis. Thirteen studies did not meet the inclusion criteria and were excluded while the remaining nine were selected for the review[9,12,17-23].
Details of the included studies are presented in Table 1. The studies were published between 2015 to 2021, reporting data from different countries around the world. One study[22] was a matched case-control study, one was a prospective cohort[23], while the remaining were retrospective cohorts in nature. The study period ranged from 2001 to 2019. One recent study from the United States had a very large sample size, with 1105092 cancer and 15246921 non-cancer patients. The sample size of the remaining studies ranged from 40 to 7489 patients in the cancer group and 35 to 22382 in the non-cancer group. The mean/median age of the study population was > 60 years across studies. Data on lactate levels, SOFA scores, and use of invasive ventilation were not universally reported by the included studies. Four studies used the recent sepsis-3 consensus definition to classify patients with sepsis. Except for one study[13] which included patients only with solid tumors, the remaining studies included all types of cancer patients. The NOS score of the studies ranged from 6 to 8.
| Ref. | Location | Database | Study period | Sample size | Mean/median age (yr) | Male gender (%) | Lactate levels (mmol/L) | SOFA score | Invasive ventilation (%) | Diagnosis of sepsis | Types of cancer | NOS score | ||||||
| Cancer | Non-cancer | Cancer | Non-cancer | Cancer | Non-cancer | Cancer | Non-cancer | Cancer | Non-cancer | |||||||||
| Sharma et al[12], 2021 | United States | National inpatient sample | 2008-2017 | ST: 3120798; HM: 793014 | 15246921 | ST: 70.1; HM: 65.7 | 64.5 | ST: 52.8; HM: 57.8 | 48.8 | NR | NR | NR | NR | ST: 15.8; HM: 17.9 | 19 | ICD codes | All types | 6 |
| López et al[17], 2021 | Chile | Clínica Alemana de Santiago | 2017-2019 | 80 | 171 | 67.7 | 63.4 | 63.8 | 53.8 | 2.9 ± 2 | 2.9 ± 3.3 | 7.1 ± 3.5 | 6.7 ± 3.4 | NR | NR | Sepsis-3 consensus definition | All types | 7 |
| Cooper et al[18], 2020 | United States | Brigham and Women’s Hospital | 2003-2014 | ST: 4623; HM: 2866 | 13486 | ST: 64; HM: 58 | 62 | ST: 54.5; HM: 58.6 | 54.7 | NR | NR | NR | NR | ST: 33.9; HM: 27.2 | 47.2 | CDC Adult Sepsis Event criteria | All types | 6 |
| Camou et al[19], 2020 | France | CHU Bordeaux | 2012-2016 | ST: 133; HM: 119 | 244 | ST: 65; HM: 63 | 68 | ST: 61; HM: 59 | 55.7 | ST: 3.9 (2.1-6.8); HM: 3 (1.6-4.8) | 3.1 (1.8-8.4) | ST: 8 (7-11); HM: 10 (8-11) | 9 (7-13) | ST: 36; HM: 31 | 52.4 | Sepsis-3 consensus definition | All types | 7 |
| Wang et al[20], 2018 | Israel | Medical Information Mart for Intensive Care III | 2001-2012 | 1574 | 22382 | NR | NR | 57.7 | 53.5 | NR | NR | 5 (3-8) | 5 (3-8) | NR | NR | ICD codes | All types | 6 |
| Fang et al[21], 2017 | Taiwan | Kaohsiung Chang Gung Memorial Hospital | 2013-2016 | 95 | 437 | 62.2 | 67.4 | 64.2 | 57.9 | 2.3 ± 2 | 1.8 ± 1.6 | 9.4 ± 3.9 | 9.5 ± 3.5 | NR | NR | Sepsis-3 consensus definition | All types | 7 |
| Abou Dagher et al[22], 2017 | Lebanon | Beirut Medical Center | 2010-2015 | 176 | 176 | 65.4 | 74.7 | 63.6 | 51.7 | NR | NR | NR | NR | NR | NR | Surviving Sepsis Campaign guidelines | All types | 8 |
| Ravetti et al[23], 2015 | Brazil | Mater Dei Hospital | 2012-2014 | 40 | 35 | 65.5 | 68.7 | 55 | 57.1 | NR | NR | 6.2 ± 2.7 | 7.4 ± 2.9 | NR | NR | 1992 Sepsis consensus definition | All types | 6 |
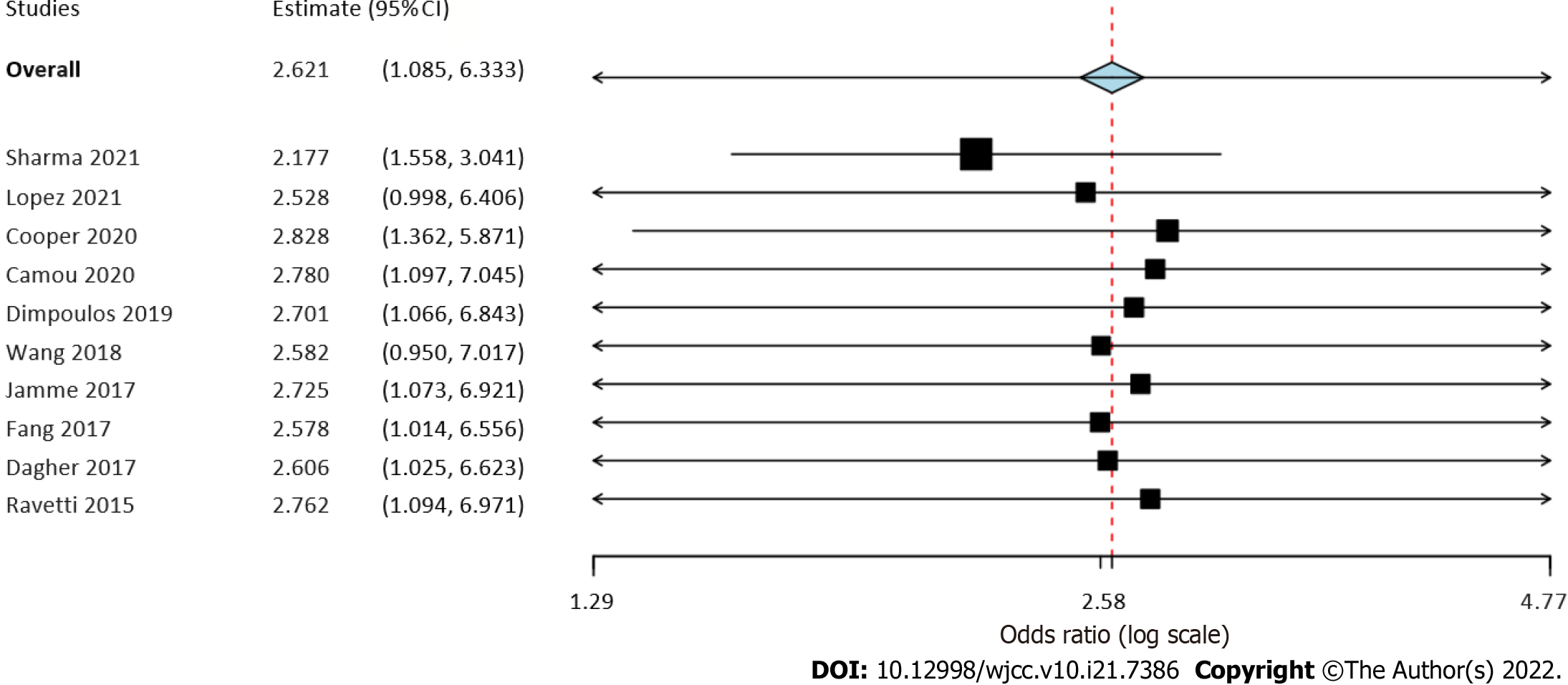
Six studies reported data on early mortality between cancer and non-cancer patients with sepsis. The meta-analysis demonstrated a non-significant tendency towards increased risk of early mortality amongst cancer patients with sepsis as compared to those without cancer (OR = 2.77, 95%CI: 0.88-8.66, I2 = 99%) (Figure 2). On the other hand, we noted that cancer patients had a statistically significantly increased risk of late mortality as compared to non-cancer sepsis patients (OR = 2.46, 95%CI: 1.42-4.25, I2 = 99%) (Figure 2). Overall, combining data from all nine studies, cancer was found to significantly increase the risk of mortality in sepsis patients (OR = 2.7, 95%CI: 1.07-6.84, I2 = 99%) (Figure 2). On sensitivity analysis, the results consistently demonstrated an increased risk of mortality with cancer on the exclusion of any study. However, the results were non-significant but still indicative of an increased risk of mortality on the exclusion of the studies of Wang et al[20] and López et al[17] (Figure 3).
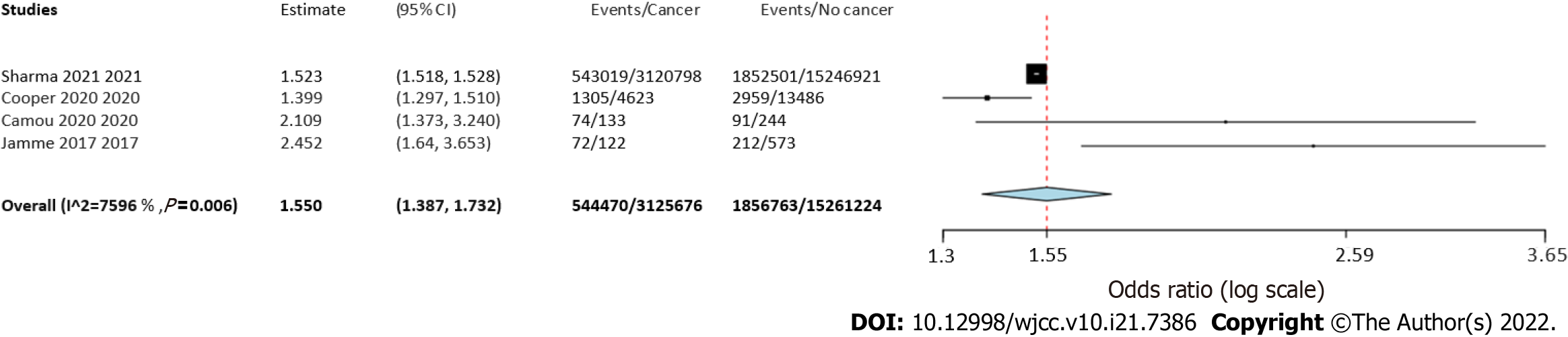
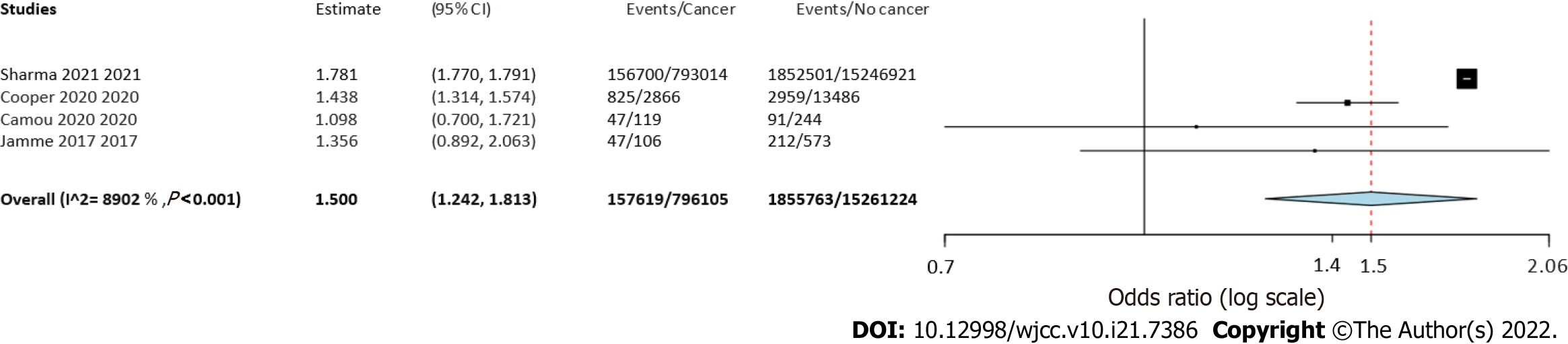
Four studies reported separate data on solid tumors and hematological malignancies. Meta-analysis indicated a statistically significantly increased risk of mortality in patients with solid tumors (OR = 1.55 95%CI: 1.39-1.73, I2 = 76%) (Figure 4) as well as hematological malignancies (OR = 1.5 95%CI: 1.24-1.81, I2 = 89%) (Figure 5).
A total of 12 covariates were selected in the meta-regression analysis based on the reporting of data by the included studies. Details of meta-regression analysis are presented in Table 2. Scatter plots are presented as Supplementary Figures 1-12. Meta-regression indicated that two comorbidities, namely, pulmonary disease and renal disease, significantly influenced the risk of mortality. An increase in the prevalence of comorbid pulmonary and renal diseases increased the risk of mortality in cancer patients with sepsis. Amongst the source of infection covariates used in the analysis, we noted that infections of the urinary tract and cutaneous origin significantly influenced the mortality rates. Mortality rates increased with an increase in the percentage of patients with urinary tract infections while an inverse relationship was seen for infections of cutaneous origin.
| Covariate | Coefficient | SE | 95%CI | P value | Scatter plot |
| Mean age | 0.001 | < 0.001 | -0.001 to 0.003 | 0.35 | Supplementary Figure 1 |
| Male gender | -0.011 | 0.033 | -0.075 to 0.054 | 0.73 | Supplementary Figure 2 |
| Hypertension | 0.024 | 0.013 | -0.001 to 0.049 | 0.06 | Supplementary Figure 3 |
| Diabetes Mellitus | 0.022 | 0.023 | -0.024 to 0.068 | 0.35 | Supplementary Figure 4 |
| Pulmonary disease | 0.035 | 0.017 | 0.002 to 0.068 | 0.03 | Supplementary Figure 5 |
| Renal disease | 0.048 | 0.017 | 0.015 to 0.080 | < 0.01 | Supplementary Figure 6 |
| Cardiac disease | 0.035 | 0.020 | -0.005 to 0.075 | 0.08 | Supplementary Figure 7 |
| Bacteremia | -0.004 | 0.004 | -0.012 to 0.004 | 0.35 | Supplementary Figure 8 |
| Pulmonary origin | -0.008 | 0.010 | -0.028 to 0.011 | 0.39 | Supplementary Figure 9 |
| Abdominal origin | -0.002 | 0.005 | -0.012 to 0.009 | 0.76 | Supplementary Figure 10 |
| Urinary tract origin | 0.013 | 0.005 | 0.003 to 0.022 | 0.01 | Supplementary Figure 11 |
| Cutaneous origin | -0.062 | 0.007 | -0.076 to -0.048 | < 0.01 | Supplementary Figure 12 |
Cancer has been an important cause of sepsis-related hospitalizations for decades. Data from the United States suggest that in the 1990s, approximately 12% of all hospital admissions for sepsis were due to cancer[24]. Furthermore, cancer-related sepsis was associated with a significantly increased risk of mortality as compared to sepsis without any comorbid malignancies[25,26]. However, much has changed in the past two decades with several advances in the management of cancer as well as sepsis patients. Personalized cancer treatment is now possible with cytogenetic evaluations[27]. Progress in hematopoietic stem cell transplant has made the procedure safer and more successful[28]. Technological strides and pharmaceutical research have reduced the adverse events associated with radiotherapy and chemotherapy[29,30]. Chimeric antigen receptor therapy and oncolytic virus therapy are rapidly establishing their place in the field of cancer treatment[31,32]. In this context, the clinical question which arises is: Does active cancer still result in worse clinical outcomes in sepsis patients? In an attempt to answer this clinical query, we designed the current systematic review to include only contemporary data. This was achieved by two important steps. First, we restricted the search limits to 2001. Second, we included only those studies wherein the study period was after 2001.
In our meta-analysis of nine studies, we noted that active cancer was associated with a 2.7 times increased risk of mortality as compared to sepsis patients without underlying cancer. In the subgroup analysis based on follow-up duration, the results were statistically significant for late mortality, but not for early mortality. However, considering the wide 95%CI of early mortality (0.88 to 8.66) with the lower end very close to 1, the results still indicate a tendency of increased risk of early mortality amongst cancer patients. The credibility of the results was further confirmed on sensitivity analysis wherein there was a consistent increased risk of mortality in cancer patients. For the two studies[17,20], wherein the results were non-significant, the lower end of 95%CI was 0.99 and 0.95 and thereby indicative of a tendency for worse outcomes amongst cancer patients. Increased mortality in cancer patients with sepsis could be attributed to the immunocompromised status due to cancer therapy or the disease itself. For example, chemotherapy-induced neutropenia is a common immune defect seen in patients with malignancies. All-cause neutropenia has been shown to increase the risk of mortality amongst cancer patients[33]. Immunotherapy and corticosteroids used to manage cancer can also inhibit the immune system[12]. Lu et al[34] have shown that the use of corticosteroids increases the 30-d mortality risk in metastatic cancer patients with sepsis. Furthermore, animal studies have shown that tumor development can inhibit T cell activation due to viral or bacterial infection and reduce the response of antigen-presenting cells[35]. The results of our review are supported by other studies demonstrating the role of immunosuppression in clinical outcomes of sepsis patients. Tolsma et al[33] have shown that any immunocompromised status is independently associated with an increased risk of mortality in sepsis patients. Another recent study by Lindell et al[36] has shown that prior malignancies, hemophagocytic lymphohistiocytosis, congenital immunodeficiency, and hematopoietic cell transplant significantly increase the risk of early mortality in children with severe sepsis or septic shock. Another reason for worse outcomes in cancer patients could be related to selection bias as the majority of the studies were not case-matched and retrospective in nature. It is plausible that aggressive therapy may not be offered to cancer patients due to the perceived risk of high mortality.
An important limitation of the included studies and our review is that we could not assess the impact of specific cancers on sepsis-related mortality due to wanting of data. At best, a sub-group analysis differentiating hematological and solid malignancies was conducted, which indicated an increased risk of mortality with either cancer. The ORs for both hematological and solid malignancies were similar, indicating a 1.5 times increased risk of mortality. However, individual included studies have reported variation in the risk of sepsis-related mortality between solid tumors and hematological malignancies. Camou et al[19] have reported higher mortality rates with solid tumors while Sharma et al[12] have reported higher rates with hematological malignancies. Contrastingly but consistent with our results, Bou Chebl et al[37] in a recent study have noted no difference in sepsis-related mortality rates between the two cancer types even with similar rates of intravenous fluid administration, vasopressor use, steroid use, or intubation in the two subgroups. Considering the scarce data available in the literature, further studies are needed to differentiate sepsis characteristics and outcomes amongst patients with solid tumors and hematological malignancies.
The results of our review need to be interpreted with caution on account of the high heterogeneity of the meta-analysis. Since the heterogeneity persisted even after subgroup analyses, we performed a meta-regression using 12 confounding variables based on the availability of data from the included studies. We noted that comorbid pulmonary and renal disease were associated with higher mortality rates in cancer patients. Indeed, a healthy pulmonary system is essential for survival in the case of critically ill patients. Several studies have shown that amongst solid cancers, lung cancer is associated with the highest sepsis-related mortality[12,24]. Second, we also noted that infections of urinary tract origin were associated with higher mortality rates while the reverse was true for infections of cutaneous origin. Nevertheless, it is important to note that our results were derived from a small cohort of studies and should be interpreted with caution. A recent review by Motzkus and Luckmann[38] assessing the relationship between the origin of infection and sepsis-related mortality could not conclusively establish a link between the two. The authors noted that misclassification of infection and disease states are serious possibilities that prohibit strong conclusions.
There are other limitations to our review which need to be mentioned. Foremost, only a limited number of studies were available for inclusion in the review, and the majority of the studies were retrospective in nature. The inherent bias of such studies is well recognized. Second, every study in our review included a heterogeneous population of patients with differences in patient demographics, comorbidities, cancer type, cancer therapy, the origin of infection, sepsis therapy, etc. Since homogenous populations were not included in individual studies, there was bound to be high heterogeneity in our meta-analysis. Third, varied definitions of sepsis were used in the included studies. It is plausible that such differences could have influenced outcomes. Lastly, the majority of the studies reported only crude mortality data. It is known that several confounders can influence mortality rates after sepsis and a pooled analysis of adjusted data would have provided better evidence.
Despite these limitations, our study is the first to pool evidence on the impact of cancer on outcomes of patients with sepsis. Only current studies were included in our review to provide recent evidence. All of the included studies were published recently, which is indicative of the clinical relevance of the topic. A detailed meta-regression was conducted to assess the influence of different confounders on the pooled effect size.
Contemporary evidence indicates that the presence of any cancer in sepsis patients significantly increases the risk of mortality. Scarce data suggest that mortality is equally increased for both solid and hematological cancers. Current evidence is limited by high heterogeneity and there is a need for further studies taking into account several confounding variables to present better evidence.
Research suggests that approximately 6% of adult patients admitted to hospitals in the United States present with sepsis and there has been a minimal change in the incidence of this condition in the last decade. Furthermore, patients with cancer generally have a higher incidence of sepsis due to immunosuppression caused by cancer or its treatment.
Despite the high incidence of cancer and sepsis in the global population, there has been limited research on the impact of cancer on outcomes of patients with sepsis. It would be pertinent to understand if cancer as a comorbidity impacts survival in patients with sepsis so that appropriate measures could be taken to reduce the incidence of adverse outcomes.
The purpose of our study was to assess if cancer increases the mortality rates in sepsis patients by pooling evidence from contemporary studies.
PubMed, Embase, and Google Scholar databases were searched from January 1, 2001 to December 15, 2021 for studies comparing outcomes of sepsis patients based on the presence of active cancer. Mortality data was pooled using odds ratio (OR) and 95% confidence intervals (CI) in a random-effects model. Meta-regression was conducted to assess the influence of confounders on mortality rates.
Nine studies were included. Meta-analysis demonstrated a non-significant tendency towards increased risk of early mortality (OR = 2.77, 95%CI: 0.88-8.66, I2 = 99%) and a statistically significantly increased risk of late mortality amongst cancer patients as compared to non-cancer sepsis patients (OR = 2.46, 95%CI: 1.42-4.25, I2 = 99%). Overall, cancer was found to significantly increase the risk of mortality in sepsis patients (OR = 2.7, 95%CI: 1.07-6.84, I2 = 99%). Meta-analysis indicated a statistically significantly increased risk of mortality in patients with solid tumors as well as hematological malignancies. Meta-regression indicated that an increase in the prevalence of comorbid pulmonary and renal diseases increased the risk of mortality in cancer patients with sepsis. Mortality rates increased with an increase in the percentage of patients with urinary tract infections while an inverse relationship was seen for infections of cutaneous origin.
Contemporary evidence indicates that the presence of any cancer in sepsis patients significantly increases the risk of mortality. Scarce data suggest that mortality is equally increased for both solid and hematological cancers.
Cancer patients with sepsis should be considered as a high-risk group for mortality. These patients should receive intensive therapy and highly-monitored treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghimire R, Nepal; Mikulic D, Croatia S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Qi WW
| 1. | Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 1009] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 2. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17094] [Article Influence: 1899.3] [Reference Citation Analysis (2)] |
| 3. | Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, Jernigan JA, Martin GS, Septimus E, Warren DK, Karcz A, Chan C, Menchaca JT, Wang R, Gruber S, Klompas M; CDC Prevention Epicenter Program. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009-2014. JAMA. 2017;318:1241-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 1284] [Article Influence: 160.5] [Reference Citation Analysis (0)] |
| 4. | Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med. 2014;20:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 541] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 5. | Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K; International Forum of Acute Care Trialists. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1639] [Cited by in RCA: 2304] [Article Influence: 256.0] [Reference Citation Analysis (0)] |
| 6. | Vincent JL, Jones G, David S, Olariu E, Cadwell KK. Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit Care. 2019;23:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 279] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 7. | GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736-1788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5338] [Cited by in RCA: 4902] [Article Influence: 700.3] [Reference Citation Analysis (1)] |
| 8. | Taccone FS, Artigas AA, Sprung CL, Moreno R, Sakr Y, Vincent JL. Characteristics and outcomes of cancer patients in European ICUs. Crit Care. 2009;13:R15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 281] [Article Influence: 17.6] [Reference Citation Analysis (1)] |
| 9. | Gudiol C, Albasanz-Puig A, Cuervo G, Carratalà J. Understanding and Managing Sepsis in Patients With Cancer in the Era of Antimicrobial Resistance. Front Med (Lausanne). 2021;8:636547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 10. | Zuber B, Tran TC, Aegerter P, Grimaldi D, Charpentier J, Guidet B, Mira JP, Pène F; CUB-Réa Network. Impact of case volume on survival of septic shock in patients with malignancies. Crit Care Med. 2012;40:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Pène F, Percheron S, Lemiale V, Viallon V, Claessens YE, Marqué S, Charpentier J, Angus DC, Cariou A, Chiche JD, Mira JP. Temporal changes in management and outcome of septic shock in patients with malignancies in the intensive care unit. Crit Care Med. 2008;36:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Sharma A, Nguyen P, Taha M, Soubani AO. Sepsis Hospitalizations With Versus Without Cancer: Epidemiology, Outcomes, and Trends in Nationwide Analysis From 2008 to 2017. Am J Clin Oncol. 2021;44:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Dimopoulos G, Rovina N, Patrani M, Antoniadou E, Konstantonis D, Vryza K, Vlachogianni G, Kyprianou M, Routsi C, Giamarellos-Bourboulis EJ; Hellenic Sepsis Study Group. Past history of stage I/II solid tumor malignancy impacts considerably on sepsis mortality: a propensity score matching analysis from the hellenic sepsis study group. BMC Infect Dis. 2019;19:831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 4555] [Article Influence: 1138.8] [Reference Citation Analysis (0)] |
| 15. | Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [cited 1 June 2021]. In: Ottawa Hospital Research Institute [Internet]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 16. | Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 517] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 17. | López R, Pérez-Araos R, Baus F, Moscoso C, Salazar Á, Graf J, Montes JM, Samtani S. Outcomes of Sepsis and Septic Shock in Cancer Patients: Focus on Lactate. Front Med (Lausanne). 2021;8:603275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Cooper AJ, Keller SP, Chan C, Glotzbecker BE, Klompas M, Baron RM, Rhee C. Improvements in Sepsis-associated Mortality in Hospitalized Patients with Cancer vs Those without Cancer. A 12-Year Analysis Using Clinical Data. Ann Am Thorac Soc. 2020;17:466-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Camou F, Didier M, Leguay T, Milpied N, Daste A, Ravaud A, Mourissoux G, Guisset O, Issa N. Long-term prognosis of septic shock in cancer patients. Support Care Cancer. 2020;28:1325-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Wang YG, Zhou JC, Wu KS. High 28-day mortality in critically ill patients with sepsis and concomitant active cancer. J Int Med Res. 2018;46:5030-5039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Fang WF, Chen YM, Lin CY, Huang KT, Kao HC, Fang YT, Huang CH, Chang YT, Wang YH, Wang CC, Lin MC. Immune profiles and clinical outcomes between sepsis patients with or without active cancer requiring admission to intensive care units. PLoS One. 2017;12:e0179749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Abou Dagher G, El Khuri C, Chehadeh AA, Chami A, Bachir R, Zebian D, Bou Chebl R. Are patients with cancer with sepsis and bacteraemia at a higher risk of mortality? BMJ Open. 2017;7:e013502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Ravetti CG, Moura AD, Vieira ÉL, Pedroso ÊR, Teixeira AL. sTREM-1 predicts intensive care unit and 28-day mortality in cancer patients with severe sepsis and septic shock. J Crit Care. 2015;30:440.e7-440.13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Williams MD, Braun LA, Cooper LM, Johnston J, Weiss RV, Qualy RL, Linde-Zwirble W. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit Care. 2004;8:R291-R298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 253] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4208] [Cited by in RCA: 4297] [Article Influence: 195.3] [Reference Citation Analysis (0)] |
| 26. | Van de Louw A, Cohrs A, Leslie D. Incidence of sepsis and associated mortality within the first year after cancer diagnosis in middle aged adults: A US population based study. PLoS One. 2020;15:e0243449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Rack KA, van den Berg E, Haferlach C, Beverloo HB, Costa D, Espinet B, Foot N, Jeffries S, Martin K, O'Connor S, Schoumans J, Talley P, Telford N, Stioui S, Zemanova Z, Hastings RJ. European recommendations and quality assurance for cytogenomic analysis of haematological neoplasms. Leukemia. 2019;33:1851-1867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 28. | Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood. 2016;127:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 415] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 29. | Nakamura K, Sasaki T, Ohga S, Yoshitake T, Terashima K, Asai K, Matsumoto K, Shioyama Y, Honda H. Recent advances in radiation oncology: intensity-modulated radiotherapy, a clinical perspective. Int J Clin Oncol. 2014;19:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Khodadadi M, Alijani S, Montazeri M, Esmaeilizadeh N, Sadeghi-Soureh S, Pilehvar-Soltanahmadi Y. Recent advances in electrospun nanofiber-mediated drug delivery strategies for localized cancer chemotherapy. J Biomed Mater Res A. 2020;108:1444-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Rafei H, Daher M, Rezvani K. Chimeric antigen receptor (CAR) natural killer (NK)-cell therapy: leveraging the power of innate immunity. Br J Haematol. 2021;193:216-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 32. | Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016;107:1373-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 506] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 33. | Tolsma V, Schwebel C, Azoulay E, Darmon M, Souweine B, Vesin A, Goldgran-Toledano D, Lugosi M, Jamali S, Cheval C, Adrie C, Kallel H, Descorps-Declere A, Garrouste-Orgeas M, Bouadma L, Timsit JF. Sepsis severe or septic shock: outcome according to immune status and immunodeficiency profile. Chest. 2014;146:1205-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 34. | Lu X, Wang X, Gao Y, Yu S, Zhao L, Zhang Z, Zhu H, Li Y. Efficacy and safety of corticosteroids for septic shock in immunocompromised patients: A cohort study from MIMIC. Am J Emerg Med. 2021;42:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Allen BM, Hiam KJ, Burnett CE, Venida A, DeBarge R, Tenvooren I, Marquez DM, Cho NW, Carmi Y, Spitzer MH. Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nat Med. 2020;26:1125-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 228] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 36. | Lindell RB, Nishisaki A, Weiss SL, Traynor DM, Fitzgerald JC. Risk of Mortality in Immunocompromised Children With Severe Sepsis and Septic Shock. Crit Care Med. 2020;48:1026-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 37. | Bou Chebl R, Safa R, Sabra M, Chami A, Berbari I, Jamali S, Makki M, Tamim H, Abou Dagher G. Sepsis in patients with haematological vs solid cancer: a retrospective cohort study. BMJ Open. 2021;11:e038349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Motzkus CA, Luckmann R. Does Infection Site Matter? J Intensive Care Med. 2017;32:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |