Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7356
Peer-review started: March 1, 2022
First decision: April 8, 2022
Revised: April 25, 2022
Accepted: June 14, 2022
Article in press: June 14, 2022
Published online: July 26, 2022
Processing time: 132 Days and 5.6 Hours
Peripheral nerve sheath tumors (PNSTs), a rare group of neoplasms in the orbit, comprise only 4% of all orbital tumors. At present, there are very few studies detailing the features of these tumors identified using imaging technology.
To compare the differences in location, morphology, magnetic resonance imaging (MRI) signal intensity/computed tomography (CT) value, and enhancement degree of tumors of different pathological PNSTs types.
Clinical, pathological, CT, and MRI data were analyzed retrospectively in 34 patients with periorbital sheath tumors diagnosed using histopathology from January 2013 to August 2021.
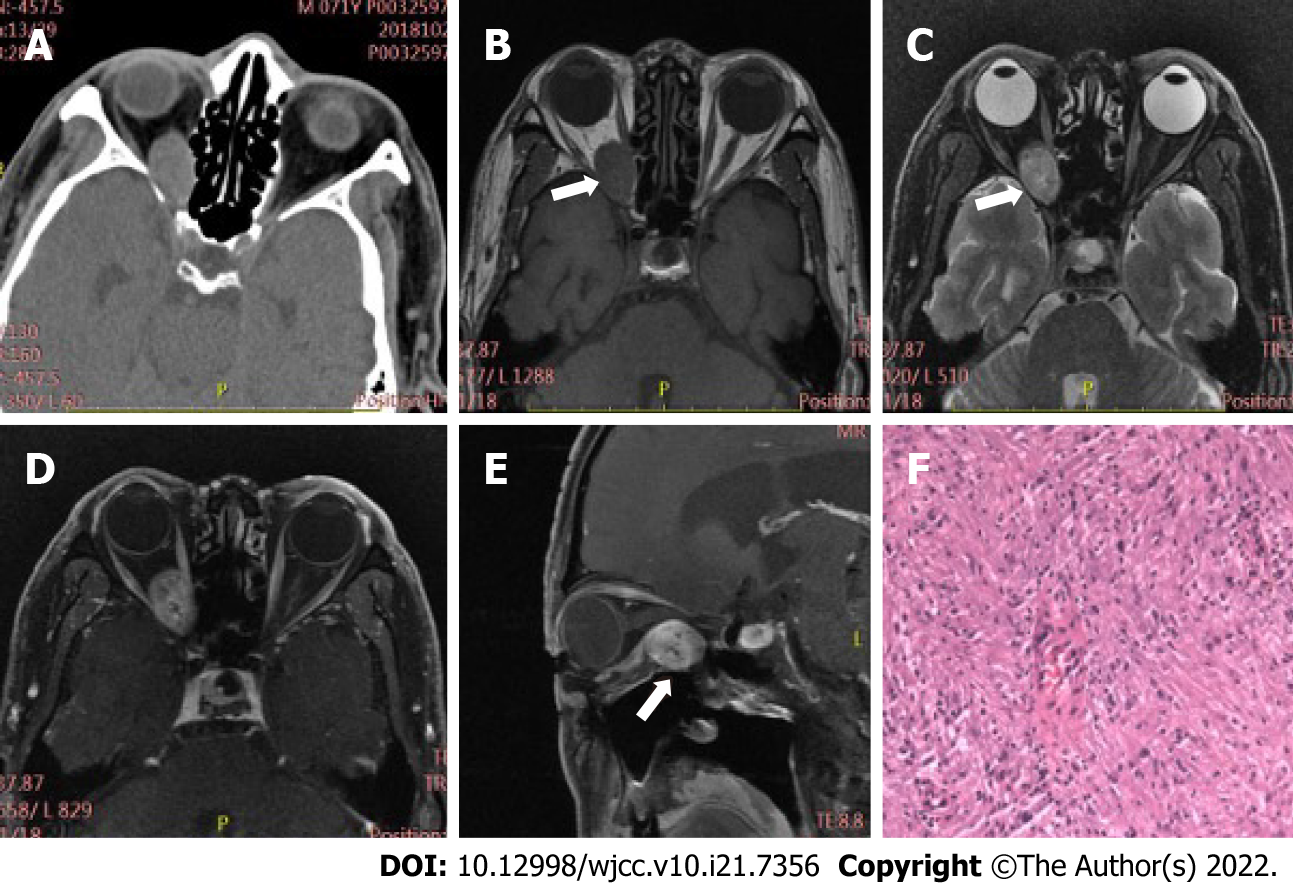
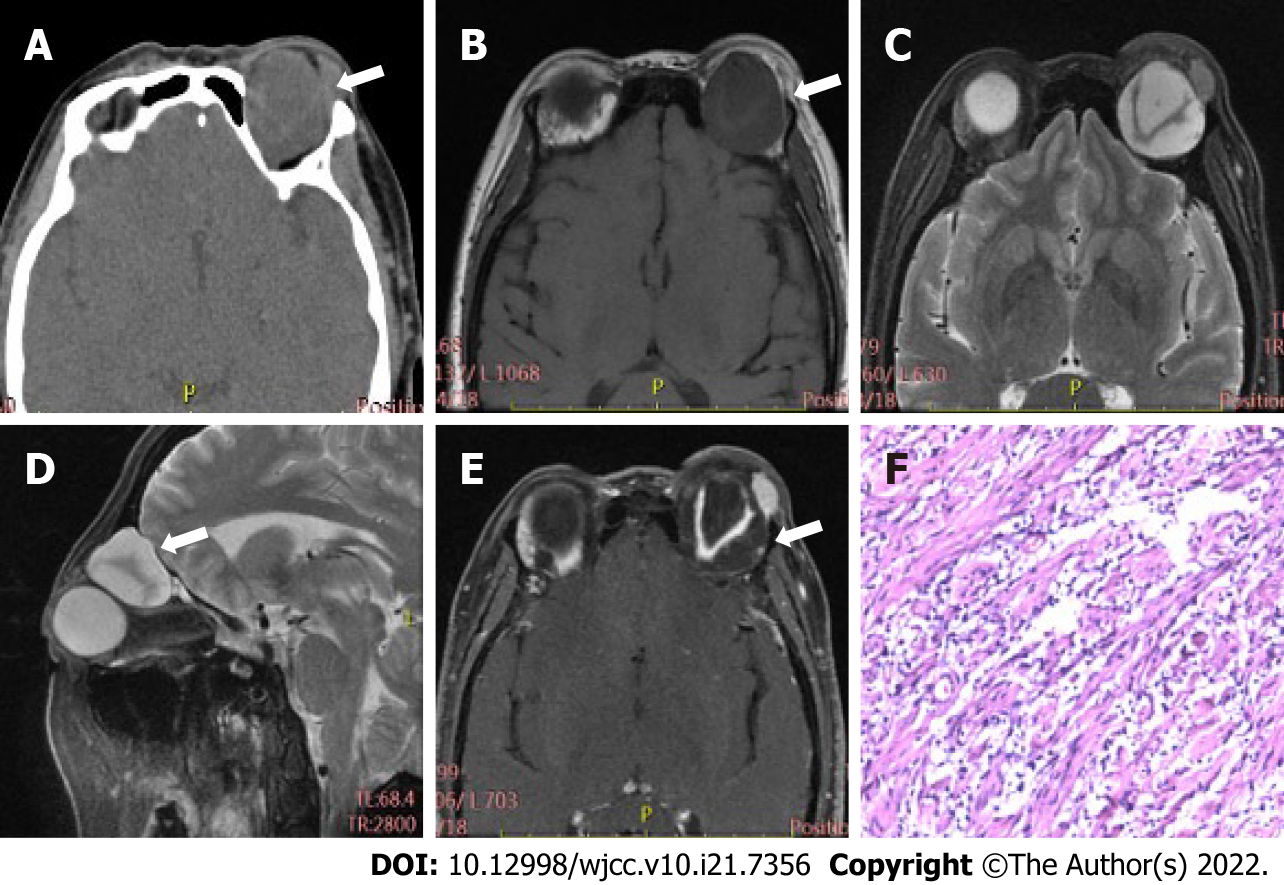
Among 34 cases of orbital peripheral nerve sheath tumors, 21 were schwannomas, 12 were neurofibromas, and 1 was a plexiform neurofibroma. Common clinical symptoms presented by patients with these types of tumors include eyelid swelling, exophthalmos, and limited eye movement. Schwannomas mostly occur in the intramuscular space with small tumor volume and rare bone involvement. Neurofibromas develop in the extrapyramidal space with larger tumor volume and more bone involvement. Radiologically, schwannomas and neurofibromas are characterized by regular morphology and uneven density and signal. One case of plexiform neurofibroma showed tortuous and diffuse growth along the nerve, with a worm-like appearance on imaging.
Different pathological types of orbital peripheral nerve sheath tumors have unique imaging characteristics. Comprehensive consideration of the patient's clinical and imaging manifestations is of great value in the diagnosis of orbital peripheral nerve sheath tumors.
Core Tip: We analyzed clinical, pathological, computed tomography (CT), and magnetic resonance imaging (MRI) data retrospectively in 34 patients with periorbital sheath tumors diagnosed using histopathology during more than 7 years. The differences in location, morphology, MRI signal intensity/CT value, and enhancement degree of tumors of different pathological types were compared. Radiologically, schwannomas and neurofibromas are characterized by regular morphology and uneven density and signal. One case of plexiform neurofibroma showed tortuous and diffuse growth along the nerve, with a worm-like appearance on imaging.
- Citation: Dai M, Wang T, Wang JM, Fang LP, Zhao Y, Thakur A, Wang D. Imaging characteristics of orbital peripheral nerve sheath tumors: Analysis of 34 cases. World J Clin Cases 2022; 10(21): 7356-7364
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7356.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7356
In 1768, Akenside first published a scientific description of a patient with multiple tumors involving the peripheral nerves[1]. Over ten decades later, von Recklinghausen demonstrated that neurofibromas belong to a single nerve[2]. In 1910, neurinoma was histologically independent of neurofibromas and was later named schwannoma after confirming that the tumor originated in Schwann cells[3]. An in-depth study subdivided and reclassified peripheral nerve tumors that develop from the neural crest and neuroectoderm as peripheral nerve sheath tumors (PNSTs). According to the 2020 World Health Organization classification of soft tissue tumors, PNSTs are divided into categories, including neu
The infraorbital nerve tissue is rich, including the optic nerve entering the middle cranial fossa through the optic nerve canal, oculomotor nerve, trochlear nerve, abductor nerve, the first branch of the trigeminal nerve entering the orbit through the supraorbital fissure (eye meridian), the supraorbital nerve from the supraorbital fissure to the forehead, and the infraorbital nerve entering the orbit through the infraorbital fissure[6]. Except for the optic nerve, all infraorbital nerves are peripheral nerves. Orbital PNSTs have a low incidence rate, accounting for only approximately 4% of all orbital tumors. They are thought to originate from sensory nerves and are frequently located in the superior and medial orbital compartments. However, PNSTs rarely invade the orbit and ocular adnexa[7]. Orbital PNSTs are often characterized by variable tumor locations, nonspecific clinical symptoms, occasional diagnostic dilemmas, and challenging treatment plans[8]. Although it may be difficult to differentiate orbital masses based on imaging data, computed tomography (CT) and magnetic resonance imaging (MRI) features can still provide clues for diagnosis[9]. For example, localized tumors on CT often demonstrate smooth margins, round, ovoid, homogenous density, or lobulation[10].
In this study, we summarized the imaging, pathological, and clinical characteristics of 34 patients in detail, which provides valuable references and ideas for the differential diagnosis of this disease.
We recruited 34 histologically-diagnosed cases of PNSTs admitted to the Department of Ophthalmology, Xi’an People’s Hospital (Xi’an Fourth Hospital), Shaanxi, China, from January 2013 to August 2021. Specifically, 21 cases were schwannomas, 12 cases were neurofibromas, and 1 case was plexiform neurofibroma. Three groups were defined according to tumor type. None of these patients had undergone any kinds of therapy or had any other malignancy. All patients underwent imaging examinations: 28 patients underwent CT scan, MRI-plain scan, and enhanced scans; two patients received both MRI-plain scan and enhanced scan; 2 patients underwent MRI-plain scan only; and 2 patients received CT scan only.
Our study was approved by the Institutional Committee for Research Involving Human Subjects of the Xi’an People’s Hospital (Xi’an Fourth Hospital). Informed consent forms listing relevant information needed to be collected were signed and obtained from the participants. Demographic and personal data, including gender, age, and clinical symptoms, were collected from outpatient medical records. We retrospectively analyzed the clinical and imaging features of these patients.
CT scanning using a 16-row spiral CT machine (Siemens, SomAToM Emotion, Germany). The scanning parameters were as follows: plain scan slice thickness, 2.0 mm; slice increment, 2.0 mm; and reconstruction slice thickness, 0.75 mm. CT scans of four patients were performed on a 64-slice CT machine (GE Revolution EVO, United States). The detector width was 20.0 mm, and the slice thickness, pitch, and reconstruction slice thickness were 2.5 mm, 0.969 mm, and 0.75 mm, respectively. Sagittal and coronal reconstructions were performed.
MRI scans of all patients were performed using a 3.0 magnetic resonance scanner (GE, Signa HDxt, United States) and a skull 8-channel phased array coil. Plain orbital MRI scan sequences included T1-weighted imaging (T1WI) axial repetition time (TR)/echo time (TE; TR/TE: 400 ms/10 ms), T2-weighted imaging (T2WI)/fat saturation (FS) axial (TR/TE: 2800 ms/70 ms), T2WI/FS oblique sagittal (TR/TE: 1500 ms/70 ms) and T2WI/Short TI Inversion Recovery (STIR) coronal (TR/TE: 6600 ms/40 ms). The orbital MR enhancement sequence included the T1WI/FS axial position, oblique sagittal position (TR/TE: 500 ms/10 ms) and coronal position (TR/TE: 450 ms/10 ms). The slice thickness was 2 mm, and the slice increment was 1 mm. Meglumine gadopentetate (Grant No. j20171008, Bayer), a contrast agent, was rapidly injected at a dosage of 0.2 mL/kg.
Two senior imaging physicians used uniform criteria to evaluate the images. If the results were inconsistent, a consistent diagnosis was reached through consultation. Physicians evaluated the following characteristics: tumor location (internal and external space of the muscle cone), shape (regular or irregular), size, CT value, MRI signal intensity (taking cerebral gray matter as reference), signal uniformity (uniform or non-uniform), enhancement degree (mild, moderate, obvious, non-enhancement), and enhancement pattern (uniform or non-uniform).
Data were collected using Microsoft Excel, and statistical analyses were performed using IBM SPSS Statistics for Windows version 26.0. The count data are expressed as cases and percentages (%), and a chi-square test was performed to compare the variables among the three groups. We adopted a continuity-adjusted formula for the chi-square test if the value of the expected cases in one cell was greater than or equal to 1 but less than 5. Fisher’s exact test was used if a cell had few expected cases (i.e., < 1) in the table. We considered a 2-tailed P value < 0.05 as statistically significant. Since there was only one case of plexiform fibroma, we fused the plexiform case to the neurofibroma group to compare the patients' general characteristics.
In this study, we recruited 34 patients with PNSTs, including 21 with schwannoma, 12 with neurofibroma, and 1 with plexiform neurofibroma. Their ages ranged from 18 to 77 years old. Table 1 presents the demographic and clinical characteristics of the study population. The mean age of the schwannoma patients (47.33 ± 3.602 years) was not significantly different from that of the patients with neurofibroma (41.77 ± 4.595 years). There were 17 male and 17 female patients, of whom the male-to-female ratio of schwannoma was approximately 4:3, and that of neurofibroma was 5:8. The most common clinical symptoms of the three tumors were eyelid swelling, exophthalmos, and limited eye movement, and the difference in prevalence based on tumor type was not statistically significant (all P > 0.05).
| Schwannoma (n = 21) (%) | Neurofibroma + plexiform neurofibroma (n =13) (%) | P value | |
| Gender | 0.290 | ||
| Male | 12 (57.1) | 5 (38.5) | |
| Female | 9 (42.9) | 8 (61.5) | |
| Age (mean ± SD, yr) | 47.33 ± 3.602 | 41.77 ± 4.595 | 0.347 |
| Clinical symptoms | |||
| Exophthalmos | 14 (66.7) | 9 (69.2) | 1.000 |
| Limited eye movement | 11 (52.4) | 7 (53.8) | 0.934 |
| Eyelid swelling | 14 (66.7) | 7 (53.8) | 0.455 |
| Decreased vision | 4 (19.0) | 1 (7.7) | 0.682 |
| Dizzy | 6 (28.6) | 2 (15.4) | 0.642 |
| With NF-I | 0 (0.0) | 4 (30.8) | 0.015a |
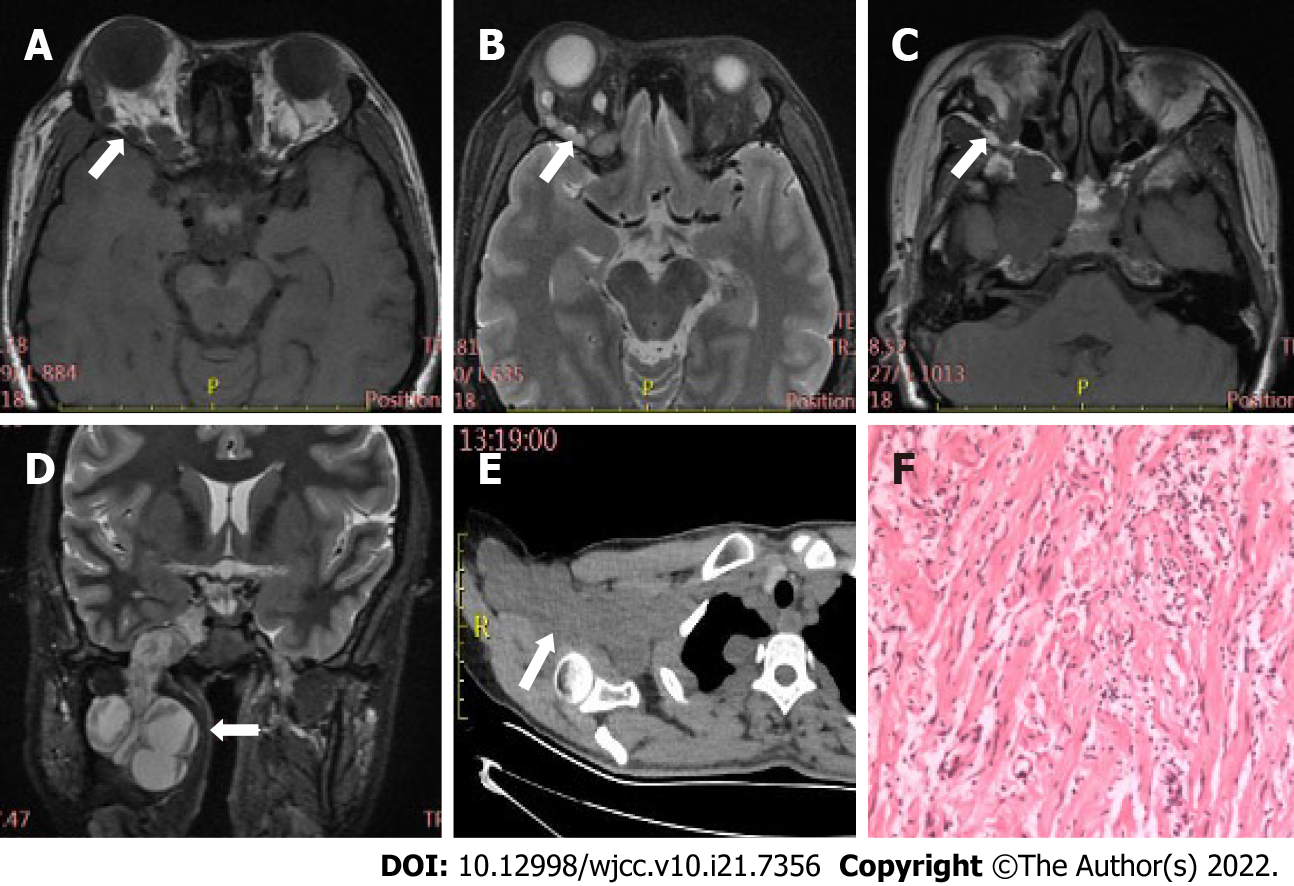
Table 2 displays differences in imaging findings between the group of schwannoma patients and the neurofibroma group. Generally, statistically significant differences in tumor location and bone involvement were observed between two groups, while other radiological features, such as tumor morphology, density, and signal uniformity, were similar. Concretely speaking, the imaging manifestations of the two tumors had regular morphology, and uneven density and signal. Schwannomas mostly occur in the intramuscular space and have relatively small volume, thus having uncommon bone involvement, as shown in Figure 1. However, neurofibroma, often having large tumor volume and adjacent bone compression, mostly occurs in the extrapyramidal space (Figure 2). As for the case of plexiform neurofibroma, MRI examination showed that the tumor grew diffusely and tortuously along the nerve; multiple slightly long T1 and slightly long T2 signal nodules were found in the right orbit. Further, small flake and strip T2 signal shadows were seen in the T2WI lesions, with a diameter of about 4 to 5 mm. Through the infraorbital hole and supraorbital fissure, the tumor grew into the intracranial, deep cervical, and subclavian spaces (Figure 3).
| Imaging features | Schwannoma (n = 21) (%) | Neurofibroma (n = 12) (%) | Chi-square value | P value |
| Location (Intramuscular space) | 11.933 | 0.000a | ||
| Yes | 19 (90.5) | 3 (25.0) | ||
| No | 2 (9.5) | 9 (75.0) | ||
| Location (extrapyramidal space) | 11.933 | 0.000a | ||
| Yes | 2 (9.5) | 9 (75.0) | ||
| No | 19 (90.5) | 3 (25.0) | ||
| Regular morphology | 0.002 | 0.968 | ||
| Yes | 16 (76.2) | 10 (83.3) | ||
| No | 5 (23.8) | 2 (16.7) | ||
| Bone involvement | 5.085 | 0.024a | ||
| Yes | 3 (14.3) | 7 (58.3) | ||
| No | 18 (85.7) | 5 (41.7) | ||
| Homogeneous density/signal intensity | 0.000 | 1.000 | ||
| Yes | 6 (28.6) | 3 (25.0) | ||
| No | 15 (71.4) | 9 (75.0) |
Although multiple studies have described the clinical or histological features of orbital PNST, the literature still lacks a systematic imaging description of these tumors[11,12]. We performed a comprehensive radiological review of several PNSTs from the orbit in the context of the current classification framework.
PNSTs often occur in the neck and head but are infrequent in the orbit. Based on five large studies of biopsy-proven orbital tumors, Sweeney et al[10] reported the occurrence probability of PNSTs in different orbital tumors: schwannoma 0.7%-2.3%, neurofibroma 0.4%-3.0%, malignant PNST 0%-0.2%. Unlike malignant PNSTs, which have a peak incidence in the seventh decade, benign orbital PNSTs are considered tumors of adulthood, with the exception of plexiform neurofibromas, of which approximately 50% are diagnosed in early childhood[13,14]. In our study, the mean age of patients with schwannoma and neurofibroma was 47.33 ± 3.602 years and 42.17 ± 4.977 years, respectively. The patient with plexiform neurofibromas was 37 years old; only one case was included in the study because of the low prevalence of this type of PNST. Generally, the age of onset in these patients is consistent with the epidemiological characteristics of this disease.
The molecular etiology of PNSTs remains unclear[15]. Previous studies have demonstrated that loss of the tumor suppressor gene NF-1 (17q 11.2) is related to 28% of neurofibromas and almost all plexiform neurofibromas, but not neurilemmomas[16]. In our study, none of the 21 schwannoma cases were accompanied by NF-1, although three neurofibroma cases (25.0%) and only one plexiform neurofibroma case had NF-1.
Schwannomas arise from myelin-producing Schwann cells and grow principally via cell hyperplasia, often showing a predominance of spindle-shaped Schwann cells[17]. Pathologically, a schwannoma is a smooth/unsmooth tan or yellow mass with occasional hemorrhage, calcification, or atypical cystic changes[18]. Based on cell morphology, schwannomas are divided into two types: Antoni A and B patterns. The Antoni A pattern often displays well-differentiated spindle cells with palisade nuclei, whereas the Antoni B pattern is characterized by bipolar and multipolar cells suspended in a loose myxoid matrix[19,20]. Imaging findings of schwannomas correlate with the biphasic pattern observed histopathologically. Most tumors dominated by Antoni A are solid, whereas those dominated by Antoni B are cystic and more vascular, which explains the diversity of imaging manifestations of neurilemmomas. MRI showed a slightly high signal in the Antoni A pattern and a significantly high signal in the Antoni B pattern on T2WI. On the contrast-enhanced scan, the enhanced signal of the Antoni B pattern was significantly stronger than that of the Antoni A pattern. Owing to cystic changes and hemorrhage, schwannomas may have mixed signals. In a study by Young et al[21], the imaging characteristics of 13 patients with histologically proven schwannomas of the orbital frontal nerve were analyzed. Data showed extraconal location of all front nerve schwannomas, 10 of 13 patients had bone remodeling on CT, and 1 had calcification on pre-contrast CT. On pre-contrast CT, most lesions were heterogeneously isodense to hypodense. On post-contrast CT, all patients showed heterogeneous mild to moderate contrast enhancement. On T1-weighted MRI, most were heterogeneously iso- to hypointense, whereas on T2-weighted MRI, all were heterogeneously iso- to hyperintense, with portions of hypointensity within the tumor[21]. In comparison with our results, their data support an obviously high rate of bone involvement and a low rate of homogeneous density/signal intensity, which may be attributed to the single orbital nerve type.
Neurofibromas originate from non-myelinated, neoplastic Schwann cells, often presenting as smooth, solitary, and sometimes gelatinous masses with proliferation of collagen fibers and pathological mucinous matrix[22]. Because of mucoid degeneration, orbital neurofibromas often appear as uneven low-density masses on CT. On MRI, a characteristic target sign can be seen, the center of which is the involved thickened nerve bundle or dense collagen and fibrous tissue, showing equal or slightly longer T1 and shorter T2 signals, and the periphery is a mucus matrix with a hypersignal[23]. The tumor can extend to the intracranial region along the supraorbital fissure, and the surrounding orbital wall may have compressed changes if the tumor is large. Plexiform neurofibromas can be divided into superficial, tissue replacement, and invasive types, based on tumor location, range, and growth mode[24]. Superficial neurofibromas are limited to the skin and subcutaneous tissue without involution of the muscular layer, whereas the invasive type often shows diffuse invasive growth, involving more than three layers of skin structure[25]. The tissue replacement type is mostly nodular and grows along the nerve plexus with a clear boundary[26]. MRI scans of this type revealed tortuous and diffuse tumors along the nerve, multiple beaded, with a worm-like appearance, similar to case 3 in our study.
Schwannomas, neurofibromas, and plexiform neurofibromas are benign tumors with favorable prognoses[27]. Complete excision of these benign tumors with full effort to maintain capsular integrity is the mainstay treatment. However, for a patient whose documented serial imaging suggests a solitary schwannoma or neurofibroma affecting the apex, or where resection is not feasible, orbital decompression alone may be beneficial[10]. In our study, complete gross resection was performed for 33 patients based on imaging findings, and partial resection was performed for plexiform neurofibroma. The final diagnosis was clarified based on the pathology report of the postoperative specimens. These patients were followed-up for one-nine years, and there was no tumor recurrence.
The limitation of our study is the relatively small sample size, with only one case of plexiform neurofibroma. We believe that our results will encourage further studies using larger sample sizes and will help elucidate the imaging characteristics of all types of orbital PNSTs.
PNSTs are rare neoplasms of the orbits. Different tumor types have distinct imaging characteristics. MR multi-direction imaging displays individual structures of the tumors, while CT can enable users to better observe the details of the involvement of the adjacent orbital bone. Since one imaging modality cannot see all the imaging variables, it is recommended to use plain and enhanced MRI scans as the main examination method, and CT scanning as an important supplementary means. A full under
Peripheral nerve sheath tumors (PNSTs) is a rare group of neoplasms in the orbit. Although computed tomography (CT) and magnetic resonance imaging (MRI) features could provide clues for the diagnosis of PNSTs, there are very few studies detailing the features of these tumors identified using imaging technology at present.
The comprehensive characteristics of 34 patients with PNSTs were collected, and we found that imaging played as an important role in the diagnosis of this rare tumor.
This study was designed to compare the clinical, pathological, CT, and MRI data in 34 patients with periorbital sheath tumors, including 21 schwannomas, 12 neurofibromas, and 1 plexiform neurofibroma.
All data were analyzed retrospectively in 34 patients with periorbital sheath tumors diagnosed using histopathology from January 2013 to August 2021.
Schwannomas mostly occur in the intramuscular space with small tumor volume and rare bone involvement. Neurofibromas develop in the extrapyramidal space with larger tumor volume and more bone involvement. One case of plexiform neurofibroma showed tortuous and diffuse growth along the nerve, with a worm-like appearance on imaging.
Imaging manifestations have great value in the diagnosis of orbital peripheral nerve sheath tumors.
Further studies will elucidate the imaging characteristics of all types of orbital PNSTs using larger sample sizes, and focus on the value of imaging in the surgery of orbital PNSTs.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neuroimaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yap RVC, Philippines; Zakaria MN, Malaysia S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Leung KCP, Ko TCS. Orbital hybrid peripheral nerve sheath tumors. Taiwan J Ophthalmol. 2020;10:181-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Anderson JL, Gutmann DH. Neurofibromatosis type 1. Handb Clin Neurol. 2015;132:75-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Jessen KR, Mirsky R, Lloyd AC. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb Perspect Biol. 2015;7:a020487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 565] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 4. | Murphey MD, Kransdorf MJ. Staging and Classification of Primary Musculoskeletal Bone and Soft-Tissue Tumors According to the 2020 WHO Update, From the AJR Special Series on Cancer Staging. AJR Am J Roentgenol. 2021;217:1038-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Abdel Razek AAK, Gamaleldin OA, Elsebaie NA. Peripheral Nerve Sheath Tumors of Head and Neck: Imaging-Based Review of World Health Organization Classification. J Comput Assist Tomogr. 2020;44:928-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Apaydin N, Kendir S, Karahan ST. The Anatomical Relationships of the Ocular Motor Nerves with an Emphasis on Surgical Anatomy of the Orbit. Anat Rec (Hoboken). 2019;302:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Ghassibi MP, Ulloa-Padilla JP, Dubovy SR. Neural Tumors of the Orbit -- What Is New? Asia Pac J Ophthalmol (Phila). 2017;6:273-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Zhang ML, Suarez MJ, Bosley TM, Rodriguez FJ. Clinicopathological features of peripheral nerve sheath tumors involving the eye and ocular adnexa. Hum Pathol. 2017;63:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Kapur R, Mafee MF, Lamba R, Edward DP. Orbital schwannoma and neurofibroma: role of imaging. Neuroimaging Clin N Am. 2005;15:159-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Sweeney AR, Gupta D, Keene CD, Cimino PJ, Chambers CB, Chang SH, Hanna E. Orbital peripheral nerve sheath tumors. Surv Ophthalmol. 2017;62:43-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Tailor TD, Gupta D, Dalley RW, Keene CD, Anzai Y. Orbital neoplasms in adults: clinical, radiologic, and pathologic review. Radiographics. 2013;33:1739-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 12. | Yong KL, Beckman TJ, Cranstoun M, Sullivan TJ. Orbital Schwannoma-Management and Clinical Outcomes. Ophthalmic Plast Reconstr Surg. 2020;36:590-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Friedrich RE, Scheuer HT, Zustin J, Luebke AM, Hagel C, Scheuer HA. Microdont Developing Outside the Alveolar Process and Within Oral Diffuse and Plexiform Neurofibroma in Neurofibromatosis Type 1. Anticancer Res. 2021;41:2083-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Pointdujour-Lim R, Lally SE, Shields JA, Eagle RC Jr, Shields CL. Orbital Schwannoma: Radiographic and Histopathologic Correlation in 15 Cases. Ophthalmic Plast Reconstr Surg. 2018;34:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Tripathy SR, Mishra SS, Deo RC, Mohanta I, Das D, Satapathy MC. Trochlear Nerve Neurofibroma in a Clinically NF-1-Negative Patient; A Case Report and Review of Literature. World Neurosurg. 2016;89:732.e13-732.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Rose GE, Wright JE. Isolated peripheral nerve sheath tumours of the orbit. Eye (Lond). 1991;5 ( Pt 6):668-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Hilton DA, Hanemann CO. Schwannomas and their pathogenesis. Brain Pathol. 2014;24:205-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 18. | Koeller KK, Shih RY. Intradural Extramedullary Spinal Neoplasms: Radiologic-Pathologic Correlation. Radiographics. 2019;39:468-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 19. | Miettinen MM, Antonescu CR, Fletcher CDM, Kim A, Lazar AJ, Quezado MM, Reilly KM, Stemmer-Rachamimov A, Stewart DR, Viskochil D, Widemann B, Perry A. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol. 2017;67:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 266] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 20. | D'Almeida Costa F, Dias TM, Lombardo KA, Raghunathan A, Giannini C, Kenyon L, Saad AG, Gokden M, Burger PC, Montgomery EA, Rodriguez FJ. Intracranial cellular schwannomas: a clinicopathological study of 20 cases. Histopathology. 2020;76:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Young SM, Kim YD, Jeon GS, Woo KI. Orbital Frontal Nerve Schwannoma-Distinctive Radiological Features. Am J Ophthalmol. 2018;186:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Poon JC, Ogilvie T, Dixon E. Neurofibroma of the porta hepatis. J Hepatobiliary Pancreat Surg. 2008;15:327-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Pavlus JD, Carter BW, Tolley MD, Keung ES, Khorashadi L, Lichtenberger JP 3rd. Imaging of Thoracic Neurogenic Tumors. AJR Am J Roentgenol. 2016;207:552-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Gourd E. New therapy for children with plexiform neurofibromas. Lancet Oncol. 2020;21:e238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Dombi E, Baldwin A, Marcus LJ, Fisher MJ, Weiss B, Kim A, Whitcomb P, Martin S, Aschbacher-Smith LE, Rizvi TA, Wu J, Ershler R, Wolters P, Therrien J, Glod J, Belasco JB, Schorry E, Brofferio A, Starosta AJ, Gillespie A, Doyle AL, Ratner N, Widemann BC. Activity of Selumetinib in Neurofibromatosis Type 1-Related Plexiform Neurofibromas. N Engl J Med. 2016;375:2550-2560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 478] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 26. | Delgado J, Jaramillo D, Ho-Fung V, Fisher MJ, Anupindi SA. MRI features of plexiform neurofibromas involving the liver and pancreas in children with neurofibromatosis type 1. Clin Radiol. 2014;69:e280-e284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Kim KS, Jung JW, Yoon KC, Kwon YJ, Hwang JH, Lee SY. Schwannoma of the Orbit. Arch Craniofac Surg. 2015;16:67-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |