Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7302
Peer-review started: November 13, 2021
First decision: April 7, 2022
Revised: April 17, 2022
Accepted: May 26, 2022
Article in press: May 26, 2022
Published online: July 26, 2022
Processing time: 239 Days and 20.5 Hours
Delayed intracranial hemorrhage (DICH), a potential complication of ventriculoperitoneal (VP) shunts, has been associated with high mortality, but its risk factors are still unclear.
To investigate the risk factors of DICH after VP shunts.
We compared the demographic and clinical characteristics of DICH and non-DICH adult patients with VP shunts between January 2016 and December 2020.
The 159 adult VP shunt patients were divided into 2 groups according to the development of DICH: the DICH group (n = 26) and the non-DICH group (n = 133). No statistically significant difference was found in age, sex, laboratory examination characteristics or preoperative modified Rankin Scale (mRS) score between the DICH and non-DICH groups (P > 0.05); however, a history of an external ventricular drain (EVD) [P = 0.045; odds ratio (OR): 2.814; 95%CI: 1.024-7.730] and postoperative brain edema around the catheter (P < 0.01; OR: 8.397; 95%CI: 3.043-23.171) were associated with a high risk of DICH. A comparison of preoperative mRS scores between the DICH group and the non-DICH group showed no significant difference (P = 0.553), while a significant difference was found in the postoperative mRS scores at the 3-mo follow-up visit (P = 0.024).
A history of EVD and postoperative brain edema around the catheter are independent risk factors for DICH in VP shunt patients. DICH patients with a high mRS score are vulnerable to poor clinical outcomes.
Core Tip: A restrospective study of 109 patients after ventriculoperitoneal shunts indicates that a history of external ventricular drain and postoperative brain edema around the catheter are independent risk factors for delayed intracrainal hemorrhage (DICH). The DICH patients are vulnerable to poor clinical outcomes with a high modified Rankin Scale score.
- Citation: Chen JC, Duan SX, Xue ZB, Yang SY, Li Y, Lai RL, Tan DH. Risk factors for delayed intracranial hemorrhage secondary to ventriculoperitoneal shunt: A retrospective study. World J Clin Cases 2022; 10(21): 7302-7313
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7302.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7302
Ventriculoperitoneal (VP) shunting is a commonly performed surgical procedure for the treatment of hydrocephalus. Reports show that VP shunts are associated with various potential complications, such as infection, shunt obstruction and shunt malformation[1-4]. Delayed intracranial hemorrhage (DICH) refers to a subsequent cerebral hemorrhage that was not found in the first postoperative computed tomography (CT) scan of the VP shunt. Compared with other complications, DICH was regarded as a rare complication of VP shunts[5,6]. In 1985, Matsumura et al[7] provided a case report that was the first to describe DICH[7]. Since then, many case reports related to DICH[8] of the VP shunt have been published[9-13]. DICH, a severe complication with a high mortality (50%), has caused concern for neurosurgeons for the past few years. Recognizing the risk factors for DICH, could benefit neurosurgeons and improve treatment for patients. Several retrospective studies related to DICH were recently performed to explore the risk factors and prognosis related to DICH[14-18]. However, the risk factors for DICH have yet to be fully defined and more data are still needed. This retrospective study aims to include more patients and variables and explore the potential risk factors and mechanisms of DICH.
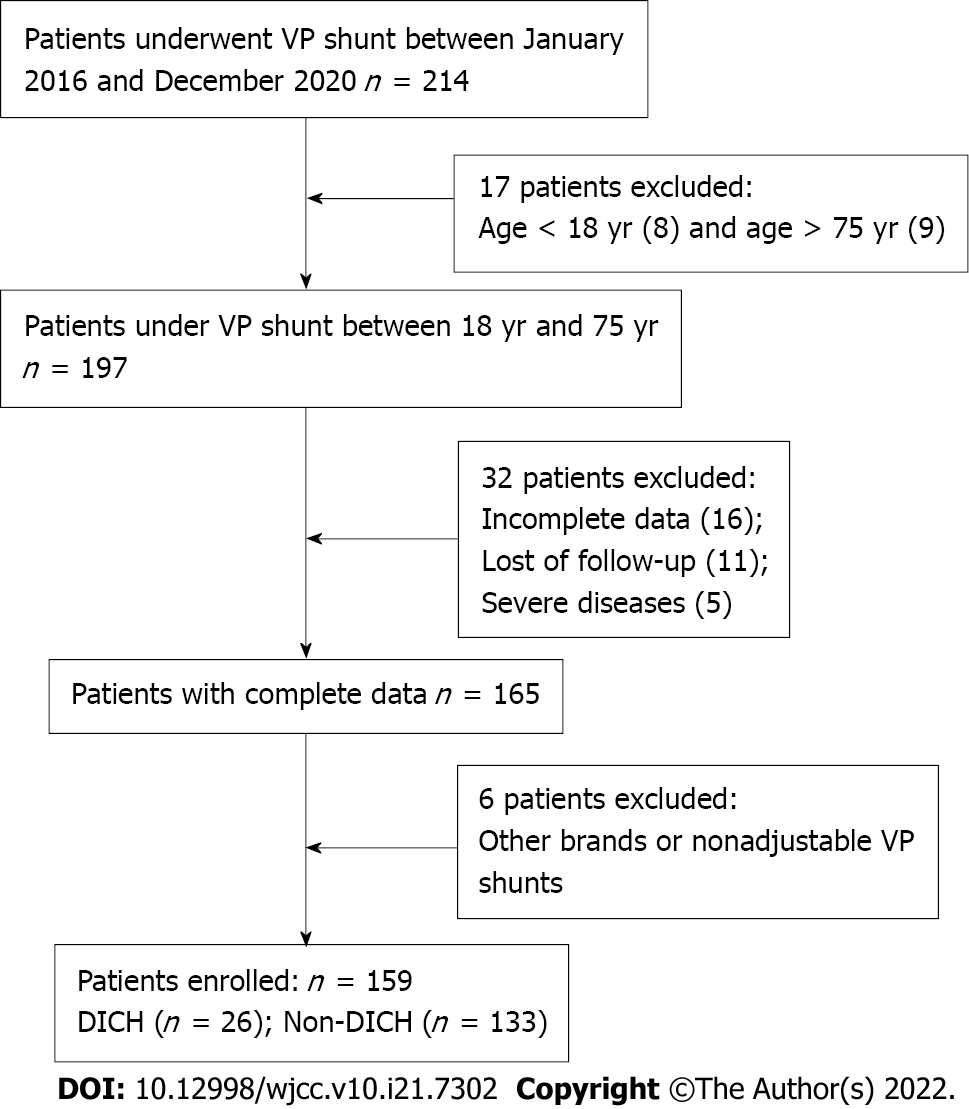
This retrospective study was reviewed and approved by the Ethics Committee of The First Affiliated Hospital of Shantou University Medical College (No. 2019034). All data were anonymously analyzed after the patient provided consent. The medical records of the hydrocephalus patients who received VP shunts at the First Affiliated Hospital of Shantou University Medical College between January 2016 and December 2020 were reviewed. The inclusion criteria were as follows: (1) Aged between 18 years and 75 years; (2) Received a Medtronic Strata Adjustable Pressure VP Shunt with the pressure valve set at 2.0; and (3) Intact clinical data including laboratory tests and radiographic imaging. The exclusion criteria were as follows: (1) A history of severe diseases such as coronary artery atherosclerosis, hepatosclerosis, and coagulation dysfunction; (2) Not followed up for more than 3 mo after treatment; (3) Used other brands of adjustable pressure VP shunts; and (4) Used nonadjustable pressure VP shunts. A flowchart of patient selection is summarized in Figure 1. A retrospective study of 159 patients who met the criteria was retrospectively reviewed in this study.
All medical records were reviewed for parameters including age, sex, primary intracranial lesion, history of surgery [craniotomy, decompression, external ventricular drain (EVD), and cranioplasty], history of hypertension, smoking habit, prior blood transfusion, preoperative lumbar puncture, and routine laboratory examinations. The primary intracranial lesion was classified as traumatic brain injury, intracranial hemorrhage, subarachnoid hemorrhage (SAH) (aneurysm rupture), SAH (Arteriovenous malformation), cerebral infarction, tumor, infection, or primary hydrocephalus. All patients underwent lumbar puncture before VP shunting and cerebrospinal fluid (CSF) pressure and laboratory indicators (CSF protein level, glucose level, and nucleated cells) were recorded in detail. Laboratory examinations, such as routine blood tests, were obtained within 3 d before the operation. The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio were calculated from routine blood results to explore the relationship with DICH. The two new variables were analyzed independently to prevent bias. Other basic diseases, such diabetes and gout, were controlled for.
The details of VP shunt surgery included the operation time, location of the VP shunt (frontal or occipital), and other postoperative complications. An initial cranial CT scan was performed within 24 h after the VP shunt was implanted, and a cranial CT scan was performed on postoperative days 5, 6 and 7. A postoperative emergency CT scan was performed if the patients showed signs of neurological deterioration during hospitalization. The radiographic characteristics that were collected were as follows: Presence of DICH, type of hematoma, volume of hematoma, and brain edema around the catheter. The volume of hematoma was calculated based on CT scan results using the volumetric computer on Advantage Windows 3D Workstation 4.1 (Shantou, China). Interreader variability was determined by analyzing the CT images by two independent radiologists who were blinded to the details of the study. Other postoperative complications, such as infection and shunt obstruction, were also recorded.
We assessed the clinical outcomes at the 3-mo follow-up visit. The general postoperative outcomes were evaluated based on the modified Rankin Scale (mRS) score. The mRS score was divided into two categories: Low (0-2) and high (3-5). In addition, the preoperative mRS score was also recorded to evaluate VP shunt clinical outcomes.
All statistical analyses were performed using statistical software (SPSS Version 23.0, SPSS. IBM Corp., Armonk, NY, United States). Continuous variables are expressed as the means ± SD. Comparisons between the 2 groups were analyzed using the x2 test (or Fisher’s exact test) for categorical data and the t test for continuous data. The relationship between each variable and DICH outcome was analyzed by univariate analysis, followed by multivariate logistic regression analysis. P values < 0.05 were considered statistically significant.
A total of 159 patients were divided into the DICH group (n = 26, 16.4%) or the non-DICH group (n = 133, 83.6%) according to the presence of a new hematoma after the first postoperative CT scan. Table 1 shows the general demographic and clinical characteristics of the patients in this study. For continuous variables, values are expressed as the mean ± SD; for categorical variables, the values are numbers. No significant differences were found for most variables, such as sex and laboratory examination results. A significant difference was found in the history of EVD between these 2 groups (P = 0.004). Regarding radiographic characteristics, a significant difference in the brain edema around the catheter was observed between the 2 groups (P < 0.01).
| Variables | DICH group (n = 26) | Non-DICH group (n = 133) | P value |
| Age (yr) | 51.35 ± 12.08 | 53.89 ± 15.17 | 0.422 |
| Male gender, n (%) | 17 (65.38) | 66 (49.62) | 0.141 |
| Primary intracranial lesion, n (%) | 0.679 | ||
| Traumatic brain injury | 6 (23.07) | 43 (17.29) | |
| Intracranial hemorrhage | 5 (19.23) | 30 (22.56) | |
| SAH (aneurysm rupture) | 10 (38.46) | 29 (21.80) | |
| SAH (AVM rupture) | 0 (0) | 0 (0) | |
| Cerebral infarction | 0 (0) | 0 (0) | |
| Tumor | 2 (7.69) | 8 (6.02) | |
| Infection | 0 (0) | 4 (3.01) | |
| Primary hydrocephalus | 3 (11.54) | 19 (14.29) | |
| Pre-Craniotomy, n (%) | 9 (34.62) | 56 (42.11) | 0.477 |
| Pre-Decompression, n (%) | 8 (30.77) | 50 (37.59) | 0.508 |
| Pre-EVD, n (%) | 16 (61.54) | 42 (31.58) | 0.004a |
| Pre-Cranioplasty, n (%) | 2 (7.69) | 23 (17.29) | 0.350 |
| LP pressure (mmH2O) | 141.54 ± 60.93 | 139.42 ± 64.94 | 0.878 |
| CSF protein (g/L) | 0.56 ± 0.46 | 0.64 ± 0.60 | 0.509 |
| CSF glucose (mmol/L) | 3.87 ± 1.45 | 3.88 ± 1.35 | 0.980 |
| CSF nucleated cells (106/L) | 16.31 ± 20.16 | 11.68 ± 17.15 | 0.223 |
| WBC (109/L) | 8.37 ± 3.10 | 7.97 ± 2.82 | 0.512 |
| Neutrophils (109/L) | 5.55 ± 2.97 | 5.41 ± 2.63 | 0.810 |
| Lymphocyte (109/L) | 1.89 ± 0.55 | 1.75 ± 0.59 | 0.237 |
| NLR | 3.40 ± 2.79 | 3.48 ± 2.20 | 0.869 |
| RBC (1012/L) | 4.04 ± 1.54 | 3.88 ± 0.69 | 0.407 |
| HGB (g/L) | 112.88 ± 15.44 | 114.71 ± 18.15 | 0.631 |
| PLT (109/L) | 276.31 ± 68.61 | 280.92 ± 102.98 | 0.827 |
| PLR | 160.46 ± 69.01 | 184.02 ± 105.17 | 0.275 |
| PT(s) | 11.64 ± 1.24 | 11.40±1.26 | 0.374 |
| INR | 1.01 ± 0.11 | 0.99 ± 0.11 | 0.382 |
| Fib (g/L) | 4.88 ± 7.40 | 3.67 ± 1.60 | 0.416 |
| Potassium (mmol/L) | 3.85 ± 0.42 | 3.81 ± 0.43 | 0.680 |
| Sodium (mmol/L) | 136.68 ± 5.19 | 138.34 ± 5.30 | 0.144 |
| Calcium (mmol/L) | 2.23 ± 0.12 | 2.22 ± 0.17 | 0.796 |
| Blood glucose (mmol/L) | 5.92 ± 1.45 | 6.17 ± 2.34 | 0.591 |
| SBP (mmHg) | 137.46 ± 21.35 | 140.14 ± 24.84 | 0.609 |
| DBP (mmHg) | 86.50 ± 13.22 | 85.71 ± 14.08 | 0.791 |
| Hypertension, n (%) | 10 (38.46) | 49 (36.84) | 0.876 |
| Other basic disease, n (%) | 3 (11.54) | 16 (12.03) | 1.000 |
| Smoker, n (%) | 7 (26.92) | 31 (23.31) | 0.693 |
| Prior Blood transfusion, n (%) | 6 (23.08) | 48 (36.09) | 0.200 |
| Operation time (min) | 73.46 ± 34.58 | 74.35 ± 26.86 | 0.883 |
| Location of VP shunt, n (%) | 0.250 | ||
| Frontal | 23 (88.46) | 101 (75.94) | |
| Occiptal | 3 (11.54) | 32 (24.06) | |
| Other VP Complications, n (%) | 1 (3.85) | 10 (7.52) | 0.801 |
| Brain edema around catheter, n (%) | 15 (57.69) | 34 (25.56) | 0.000a |
| Pre-mRS, n (%) | 0.281 | ||
| Low (0-2) | 8 (30.77) | 56 (42.11) | |
| High (3-5) | 18 (69.23) | 77 (57.89) | |
| Post-mRS, n (%) | 0.024 | ||
| Low (0-2) | 9 | 77 | |
| High (3-5) | 17 | 55 |
Univariate analysis between each variable and the DICH outcome in the original 159 patients revealed that a history of EVD and brain edema around the catheter were significantly correlated with DICH outcome (Table 2). Multivariate analysis showed that these two variables were significantly correlated with DICH outcome: History of EVD [P = 0.045; odds ratio (OR): 2.814; 95%CI: 1.024-7.730] and presence of brain edema around the catheter (P < 0.01; OR: 8.397; 95%CI: 3.043-23.171) (Table 3).
| Variables | DICH group (n = 26) | Non-DICH group (n = 133) | P value |
| Demographics | |||
| Age (yr) | 51.35 ± 12.08 | 53.89 ± 15.17 | 0.420 |
| Male gender, n (%) | 17 (65.38) | 66 (49.62) | 0.145 |
| Primary clinical diagnosis, n (%) | |||
| Traumatic brain injury | 6 (23.07) | 43 (17.29) | |
| Intracranial hemorrhage | 5 (19.23) | 30 (22.56) | |
| SAH (aneurysm rupture) | 10 (38.46) | 29 (21.80) | |
| SAH (AVM rupture) | 0 (0) | 0 (0) | |
| Cerebral infarction | 0 (0) | 0 (0) | |
| Tumor | 2 (7.69) | 8 (6.02) | |
| Infection | 0 (0) | 4 (3.01) | |
| Primary hydrocephalus | 3 (11.54) | 19 (14.29) | |
| Pre-Craniotomy, n (%) | 9 (34.62) | 56 (42.11) | 0.479 |
| Pre-Decompression, n (%) | 8 (30.77) | 50 (37.59) | 0.510 |
| Pre-EVD, n (%) | 16 (61.54) | 42 (31.58) | 0.005a |
| Pre-Cranioplasty, n (%) | 2 (7.69) | 23 (17.29) | 0.233 |
| LP pressure (mmH2O) | 141.54 ± 60.94 | 139.42 ± 64.94 | 0.877 |
| CSF protein (g/L) | 0.56 ± 0.46 | 0.64 ± 0.60 | 0.509 |
| CSF glucose (mmol/L) | 3.87 ± 1.45 | 3.88 ± 1.35 | 0.980 |
| CSF nucleated cells (106/L) | 16.31 ± 20.16 | 11.68 ± 17.15 | 0.229 |
| WBC (109/L) | 8.37 ± 3.10 | 7.97 ± 2.82 | 0.510 |
| Neutrophils (109/L) | 5.55 ± 2.97 | 5.41 ± 2.63 | 0.808 |
| Lymphocyte (109/L) | 1.89 ± 0.55 | 1.75 ± 0.59 | 0.237 |
| NLR | 3.40 ± 2.79 | 3.48 ± 2.20 | 0.868 |
| RBC (1012/L) | 4.04 ± 1.54 | 3.88 ± 0.69 | 0.415 |
| HGB (g/L) | 112.88 ± 15.44 | 114.71 ± 18.15 | 0.629 |
| PLT (109/L) | 276.31 ± 68.61 | 280.92 ± 102.97 | 0.826 |
| PLR | 160.46 ± 69.01 | 184.02 ± 105.17 | 0.276 |
| PT(s) | 11.64 ± 1.24 | 11.40 ± 1.26 | 0.373 |
| INR | 1.01 ± 0.11 | 0.99 ± 0.11 | 0.381 |
| Fib (g/L) | 4.88 ± 7.40 | 3.67 ± 1.60 | 0.183 |
| Potassium (mmol/L) | 3.85 ± 0.42 | 3.81 ± 0.43 | 0.678 |
| Sodium (mmol/L) | 136.68 ± 5.19 | 138.34 ± 5.30 | 0.145 |
| Calcium (mmol/L) | 2.23 ± 0.12 | 2.2238 ± 0.17 | 0.795 |
| Blood glucose (mmol/L) | 5.92 ± 1.45 | 6.17 ± 2.34 | 0.589 |
| SBP (mmHg) | 137.46 ± 21.35 | 140.14 ± 24.84 | 0.607 |
| DBP (mmHg) | 86.50 ± 13.22 | 85.71 ± 14.08 | 0.790 |
| Hypertension, n (%) | 10 (38.46) | 49 (36.84) | 0.876 |
| Other basic disease, n (%) | 3 (11.54) | 16 (12.03) | 0.944 |
| Smoker, n (%) | 7 (26.92) | 31 (23.31) | 0.693 |
| Prior Blood transfusion, n (%) | 6 (23.08) | 48 (36.09) | 0.205 |
| Operation time (min) | 73.46 ± 34.58 | 74.35 ± 26.86 | 0.882 |
| Location of VP shunt, n (%) | 0.170 | ||
| Frontal | 23 (88.46) | 101 (75.94) | |
| Occiptal | 3 (11.54) | 32 (24.06) | |
| Other VP complications, n (%) | 1 (3.85) | 10 (7.52) | 0.508 |
| Brain edema around catheter, n (%) | 15 (57.69) | 34 (25.56) | 0.000a |
| Pre-mRS, n (%) | 0.523 | ||
| 0-2 | 8 (30.77) | 56 (42.11) | |
| 3-5 | 18 (69.23) | 77 (57.89) |
| Variable | P value | OR | 95%CI |
| Age (yr) | 0.162 | 0.975 | 0.942-1.010 |
| Pre-Craniotomy | 0.548 | 0.723 | 0.250-2.085 |
| Pre-EVD | 0.045 | 2.814 | 1.024-7.730 |
| PT(s) | 0.224 | 1.268 | 0.865-1.859 |
| Location of VP shunt | 0.153 | 2.775 | 0.685-11.249 |
| Brain edema around catheter | 0.000 | 8.397 | 3.043-23.171 |
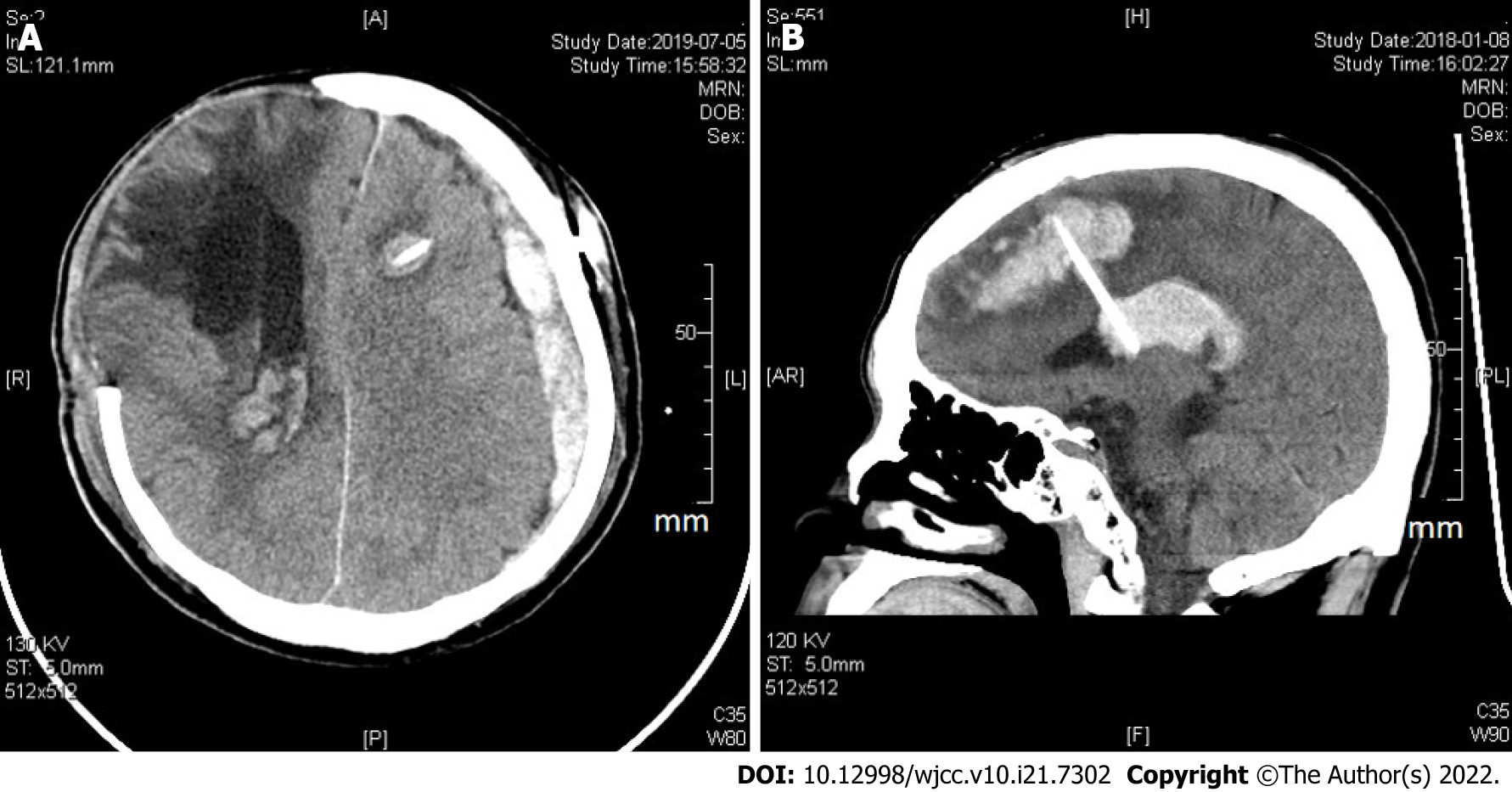
Table 4 shows the clinical data summary of 26 DICH patients after VP shunt. Among these patients, 3 had a subdural hematoma, 9 had an intraventricular hemorrhage, and 14 had an intracerebral hemorrhage around the catheter (Figure 2). No epidural hemorrhage or other types of intracranial hemorrhage were noted. The mean onset day of DICH was 4.19 ± 3.35 d, which ranged from 1 to 11 d. The mean hematoma volume was 10.92 ± 14.53 mL, which ranged from 2 to 56 mL. Two DICH patients with severe neurological deterioration underwent secondary surgical intervention for intracranial hematoma evacuation; 24 patients received conservative treatment because of the low-volume hematoma. A comparison of preoperative mRS scores between the DICH group and the non-DICH group showed a significant difference (P = 0.553). Seventeen of 26 (65%) DICH patients and 55 of 133 (41%) non-DICH patients were included in the high postoperative mRS group. A significant difference was found in the postoperative mRS score at the 3-mo follow-up visit (P = 0.024).
| No. | Age/sex | Primary intracranial lesion | History of EVD | Location of VP shunt | DICH type | Onsetday | Treatment | Pre-mRS | Post-mRS |
| 1 | 50/F | Aneurysm | Y | Frontal | IVH | 2 | Conservative | 1 | 1 |
| 2 | 67/M | ICH | Y | Frontal | ICH around catheter | 3 | Conservative | 3 | 1 |
| 3 | 64/M | Aneurysm | Y | Frontal | IVH | 1 | Conservative | 1 | 1 |
| 4 | 37/F | Tumor | N | Occipital | ICH around catheter | 11 | Conservative | 5 | 4 |
| 5 | 56/M | TBI | Y | Frontal | ICH around catheter | 3 | Conservative | 5 | 5 |
| 6 | 23/M | TBI | N | Frontal | ICH around catheter | 4 | Conservative | 5 | 5 |
| 7 | 63/F | Aneurysm | N | Frontal | ICH around catheter | 7 | Surgery | 4 | 5 |
| 8 | 54/M | Aneurysm | Y | Frontal | ICH around catheter | 3 | Conservative | 5 | 5 |
| 9 | 61/M | Aneurysm | Y | Occipital | ICH around catheter | 5 | Conservative | 2 | 1 |
| 10 | 47/M | ICH | Y | Frontal | ICH around catheter | 7 | Conservative | 5 | 5 |
| 11 | 58/M | Aneurysm | Y | Frontal | IVH | 7 | Conservative | 2 | 2 |
| 12 | 51/F | Aneurysm | N | Frontal | ICH around catheter | 3 | Conservative | 1 | 1 |
| 13 | 58/M | Tumor | N | Frontal | ICH around catheter | 2 | Conservative | 5 | 5 |
| 14 | 58/F | Primary hydrocephalus | N | Frontal | Subdural hematoma | 7 | Conservative | 5 | 5 |
| 15 | 47/M | Primary hydrocephalus | N | Frontal | ICH around catheter | 5 | Conservative | 4 | 4 |
| 16 | 53/M | TBI | Y | Frontal | IVH | 2 | Conservative | 5 | 3 |
| 17 | 42/M | Aneurysm | Y | Frontal | ICH around catheter | 9 | Conservative | 5 | 5 |
| 18 | 32/M | Aneurysm | Y | Occipital | IVH | 1 | Conservative | 2 | 2 |
| 19 | 45/M | TBI | Y | Frontal | ICH around catheter | 1 | Conservative | 5 | 5 |
| 20 | 62/M | ICH | Y | Frontal | Subdural hematoma | 2 | Conservative | 5 | 5 |
| 21 | 47/F | TBI | Y | Frontal | ICH around catheter | 2 | Conservative | 4 | 4 |
| 22 | 56/M | ICH | N | Frontal | IVH | 7 | Conservative | 5 | 4 |
| 23 | 47/M | ICH | N | Frontal | Subdural hematoma | 4 | Surgery | 5 | 5 |
| 24 | 26/F | Primary hydrocephalus | Y | Frontal | IVH | 8 | Conservative | 2 | 2 |
| 25 | 72/F | Aneurysm | Y | Frontal | IVH | 1 | Conservative | 1 | 1 |
| 26 | 59/F | TBI | N | Frontal | IVH | 1 | Conservative | 5 | 5 |
We performed a literature review of retrospective studies on DICH that was associated with VP shunts, and then we summarized these preview studies (Table 5). From these 6 studies, the incidence of DICH ranged from 1.6% to 23.7%. In our study, the incidence of DICH was 16.4%, which corresponded to the incidence range of previous studies. The wide range of incidence may be related to the neglect of minor hematoma volume, lower frequency of postoperative CT scan examinations, and different inclusion and exclusion criteria in different studies[6,19]. We suppose that the real incidence range of DICH will be more accurate with careful surveillance, such as imaging and unified standards, in the future.
| Ref. | Year | DICH | Non-DICH | Number of variables | Proposed risk factors |
| Hudson et al[18] | 2018 | 8 | 72 | 10 | DAPT (P = 0.0001, OR = 31.23, 95%CI: 2.98-327.32) |
| Guo et al[15] | 2017 | 20 | 512 | 8 | Advanced age (P = 0.027, OR = 1.048, 95%CI: 1.005-1.092), craniotomy history (P = 0.025, OR = 3.874, 95%CI: 1.183-12.693), brain edema around the catheter (P < 0.001, OR = 9.056, 95%CI: 3.194-25.675) |
| Gong et al[16] | 2017 | 12 | 742 | 9 | Age ≥ 60 yr (P = 0.0008), prior craniotomy operation (P = 0.0001) and manipulation of the valve system (P = 0.0017) |
| Qian et al[14] | 2017 | 11 | 140 | 18 | Postoperative LMWH therapy (P = 0.026, relative ratio = 4.8, 95%CI: 1.4-17.1) |
| Jang et al[17] | 2018 | 34 | 104 | 9 | Old age (P = 0.037) and delayed PTT (P = 0.032) |
| Li et al[6] | 2021 | 29 | 101 | 21 | Elevated NLRR (P < 0.001, OR = 2.792, 95%CI: 1.747-4.460); history of craniotomy (P = 0.010, OR = 3.394, 95%CI: 1.060-10.869) |
Thirty-seven variables were included to achieve a better comparison between the DICH group and the non-DICH group in our study, which is more than other prior retrospective studies. The analysis of these variables provides a better description of the actual baseline demographic information and clinical characteristics better. In our study, a history of EVD and the presence of brain edema around the catheter were significantly correlated with DICH in a univariate analysis. The selection of covariates for the multivariate analysis was based on previous studies that assessed the risk factors for DICH and our univariate analysis. In these studies, advanced age, craniotomy history, presence of brain edema around the catheter, manipulation of the valve system, location of the shunt (frontal or occipital), delayed partial thromboplastin time, postoperative low-molecular-weight heparin (LMWH) therapy, dual antiplatelet therapy and elevated levels of postoperative NLR and preoperative NLR were found to be positively associated with DICH[18-20]. Considering our available data, 6 variables (age, craniotomy history, EVD history, prothrombin time, location of shunt, and presence of brain edema around catheter) were consequently selected for the multivariate analysis. The variables in the logistic regression multivariate model that were significantly different were EVD history and presence of brain edema around the catheter.
A history of EVD was regarded as an independent risk factor for DICH in our study, which was first reported and not found in previous studies. We also found that the presence of brain edema around the catheter on the first postoperative CT scan increased the risk of DICH, which corresponded with the report by Guo et al[15]. An EVD and VP shunts are inserted using the same frontal approach, which is an invasive brain procedure that may cause neural injury in patients[21]. Some authors have proposed that catheter insertion may lead to a disturbance in venous return or hemostasis of a cortical vein and then contribute to subcortical hemorrhage[15,17,22]. Brain edema around the catheter is regarded as a radiographic sign of vascular erosion and could be used to predict DICH.
Fragile cerebral tissue is considered another underlying mechanism of DICH. Cerebral fragility is not easy to detect and the standard diagnosis is based on features of the fragile arteries surrounding the microbleeds in histological analysis after surgical resection[23]. The microbleed that was confirmed on T2-weighted MR imaging, reflected hemosiderin deposits and could be considered an imaging sign related to fragile cerebral tissue[8,24,25]. In Kwon and Jang[20,26]’s study, two neural tracts (corticoreticular pathway and cingulum) were damaged by an EVD and were confirmed by diffusion tensor imaging parameters (fractional anisotropy and fiber number) and the configuration of the neural tracts[20,26]. The fractional anisotropy value refers to the degree of directionality of water diffusion and represents white matter organization, including the degree of directionality and integrity of white matter microstructures such as axons, myelins, and microtubules[21,27]. This evidence provides an anatomical mechanism to explain cerebral tissue fragility after EVD. Neural injury supposedly occurred in a considerable number of patients with an EVD history[21]. Notably, a history of craniotomy was considered an independent risk factor for DICH in some previous studies[15,16]. The possible mechanism is that craniotomy could contribute to brain fragility and the adhesive arachnoid with small cerebral vessels, which is prone to bleeding after the insertion of a catheter[15,16]. However, a history of craniotomy did not increase the risk of DICH in our research.
Other variables were not risk factors in our study but had statistical significance in other retrospective studies (Table 5). Guo et al[15] pointed out that advanced age might contribute to the high incidence of DICH because of present complications such as hypertension and cerebral amyloid angiopathy. Coagulation dysfunction, antiplatelet therapy, and the use of LMWH are associated with an increased risk of DICH[14,18,19]. Cerebral amyloid angiopathy was believed to contribute to DICH secondary to VP shunt in elderly patients in Wang et al’s research. The discrepancy might be related to the inclusion and exclusion criteria in our study[28]. We included patients aged 18-75 years and excluded patients with coagulation dysfunction to control for confounders and achieve a balanced baseline.
In contrast to the preoperative mRS scores, the postoperative mRS scores for both the DICH group and the non-DICH group were significant in the statistical analysis in our study. Sixty-five percent of DICH patients were involved in the higher postoperative mRS group, which was higher than that of non-DICH patients (41%). This indicates that VP shunt patients with DICH might have worse clinical outcomes. DICH may contribute to severe neurological function deterioration and secondary surgical intervention should be performed in patients with large volume hematoma and intractable intracranial pressure. The average hematoma volume of DICH is 10.92 mL. Only 2 patients with hematomas exceeding 50 mL in volume in our study underwent surgery, and the other 24 patients received conservative treatment. Most DICH patients treated conservatively are asymptomatic due to the low hematoma volume. These observations collectively demonstrate that DICH increases the length of hospital stay and is related to poor clinical outcomes.
Several limitations should be noted in the present study. First, this was a retrospective study that used a multivariate analysis to minimize bias in patient selection. Some confounders mentioned in other studies, such as manipulation of the valve system and same-sided approach as EVD, were not recorded. Second, the low statistical power (0.63) and the small sample size in our study may overestimate the effect measure. The low statistical power increases the likelihood of a false positive result. The small sample was solely comprised of Chinese individuals in a single center. The number of DICH patients was much smaller than that of the non-DICH group. Logistic regression overestimates the OR in studies with small to moderate sample sizes[29]. More samples from different populations and centers should be included in future studies. Third, the follow-up evaluation needs to be replaced by a more objective method to verify the prognosis.
In summary, the incidence of DICH would be more accurate with careful surveillance that includes imaging and unified standards. Our results indicate that a history of EVD and postoperative brain edema around the catheter are associated with a high risk of DICH in VP shunt patients. DICH patients with a high mRS score are vulnerable to poor clinical outcomes.
Delayed intracranial hemorrhage (DICH), one of the high mortality complications in ventriculoperitoneal (VP) shunt patients, has not been fully recognized.
To explore the risk factors of delay intracranial hemorrhage and reduce the incidence of this complication in VP shunt patients.
To explore the potential risk factors and mechanisms of delay intracranial hemorrhage in VP shunt patients.
We collected the demographic and clinical characteristics data of VP shunt patients between January 2016 and December 2020. DICH group and Non-DICH group were compared in a retrospective study.
A history of an external ventricular drain and postoperative brain edema around the catheter were related to a high risk for DICH statistically. There was a significant difference in the postoperative modified Rankin Scale scores at the 3-mo follow-up in these two groups.
A history of an EVD and postoperative brain edema around the catheter were risk factors of DICH VP shunt patients. DICH patients are vulnerable to poor clinical outcomes with a high mRS score.
More samples from different populations and centers should be included in future studies. The follow-up evaluation needs to be replaced by a more objective method to verify the prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kung WM, Taiwan A-Editor: Lin FY, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Katiyar V, Sharma R, Tandon V, Garg K, Narwal P, Chandra PS, Suri A, Kale SS. Comparison of Programmable and Non-Programmable Shunts for Normal Pressure Hydrocephalus: A Meta-Analysis and Trial Sequential Analysis. Neurol India. 2021;69:S413-S419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Mallucci CL, Jenkinson MD, Conroy EJ, Hartley JC, Brown M, Dalton J, Kearns T, Moitt T, Griffiths MJ, Culeddu G, Solomon T, Hughes D, Gamble C; BASICS Study collaborators. Antibiotic or silver vs standard ventriculoperitoneal shunts (BASICS): a multicentre, single-blinded, randomised trial and economic evaluation. Lancet. 2019;394:1530-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 3. | Reddy GK, Bollam P, Caldito G. Long-term outcomes of ventriculoperitoneal shunt surgery in patients with hydrocephalus. World Neurosurg. 2014;81:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 4. | Sun T, Cui W, Yang J, Yuan Y, Li X, Yu H, Zhou Y, You C, Guan J. Shunting outcomes in communicating hydrocephalus: protocol for a multicentre, open-label, randomised controlled trial. BMJ Open. 2021;11:e051127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Wu Y, Green NL, Wrensch MR, Zhao S, Gupta N. Ventriculoperitoneal shunt complications in California: 1990 to 2000. Neurosurgery. 2007;61:557-62; discussion 562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 6. | Li S, Wang H, Li F, Chen M, Chen P. A new inflammatory parameter can predict delayed intracranial hemorrhage following ventriculoperitoneal shunt. Sci Rep. 2021;11:13763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Matsumura A, Shinohara A, Munekata K, Maki Y. Delayed intracerebral hemorrhage after ventriculoperitoneal shunt. Surg Neurol. 1985;24:503-506. [PubMed] |
| 8. | Gold MM, Shifteh K, Valdberg S, Lombard J, Lipton ML. Brain injury due to ventricular shunt placement delineated by diffusion tensor imaging (DTI) tractography. Neurologist. 2008;14:252-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Musali SR, Manne S, Beniwal HK, Butkuri N, Gollapudi PR, Nandigama PK. Delayed Intracerebral Hemorrhage after Placement of a Ventriculoperitoneal Shunt in a Case of Hydrocephalus: A Rare Case Report and Review of Literature. J Neurosci Rural Pract. 2019;10:533-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Ma L, Chen YL, Yang SX, Wang YR. Delayed Intracerebral Hemorrhage Secondary to Ventriculoperitoneal Shunt: A Case Report and Literature Review. Medicine (Baltimore). 2015;94:e2029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Coulibaly O, Dama M, Diallo O, Li G, Sogoba Y, Kanikomo D. Delayed intracerebral and subdural hematomas after ventriculo-peritoneal shunt in a child: A case report and review of the literature. Neurochirurgie. 2016;62:105-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Alcázar L, Alfaro R, Tamarit M, Gómez-Angulo JC, Ortega JM, Aragonés P, Jerez P, Salazar F, del Pozo JM. Delayed intracerebral hemorrhage after ventriculoperitoneal shunt insertion. Case report and literature review. Neurocirugia (Astur). 2007;18:128-133. [PubMed] |
| 13. | Zhou F, Liu Q, Ying G, Zhu X. Delayed intracerebral hemorrhage secondary to ventriculoperitoneal shunt: two case reports and a literature review. Int J Med Sci. 2012;9:65-67. [PubMed] |
| 14. | Qian Z, Gao L, Wang K, Pandey S. Delayed Catheter-Related Intracranial Hemorrhage After a Ventriculoperitoneal or Ventriculoatrial Shunt in Hydrocephalus. World Neurosurg. 2017;107:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Guo L, Chen X, Yu B, Shen L, Zhang X. Delayed Intracerebral Hemorrhage Secondary to Ventriculoperitoneal Shunt: A Retrospective Study. World Neurosurg. 2017;107:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Gong W, Xu L, Yang P, Yu Z, Wang Z, Chen G, Zhang S, Wu J. Characteristics of delayed intracerebral hemorrhage after ventriculoperitoneal shunt insertion. Oncotarget. 2017;8:42693-42699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Jang SY, Kim CH, Cheong JH, Kim JM. Risk Factors of Delayed Intracranial Hemorrhage Following Ventriculoperitoneal Shunt. Korean J Neurotrauma. 2018;14:112-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Hudson JS, Nagahama Y, Nakagawa D, Starke RM, Dlouhy BJ, Torner JC, Jabbour P, Allan L, Derdeyn CP, Greenlee JDW, Hasan D. Hemorrhage associated with ventriculoperitoneal shunt placement in aneurysmal subarachnoid hemorrhage patients on a regimen of dual antiplatelet therapy: a retrospective analysis. J Neurosurg. 2018;129:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Calayag M, Paul AR, Adamo MA. Intraventricular hemorrhage after ventriculoperitoneal shunt revision: a retrospective review. J Neurosurg Pediatr. 2015;16:42-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Kwon YM, Jang SH. Neural injury by frontal approach of external ventricular drainage in stroke patients. Int J Neurosci. 2015;125:742-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Misaki K, Uchiyama N, Hayashi Y, Hamada J. Intracerebral hemorrhage secondary to ventriculoperitoneal shunt insertion--four case reports. Neurol Med Chir (Tokyo). 2010;50:76-79. [PubMed] |
| 22. | Mavridis IN, Mitropoulos A, Mantas C, Karagianni A, Vlachos K. Delayed Intraventricular Hemorrhage following a Ventriculoperitoneal Shunt Placement: Exploring the Surgical Anatomy of a Rare Complication. Case Rep Med. 2017;2017:3953248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Kikuta K, Takagi Y, Nozaki K, Okada T, Hashimoto N. Histological analysis of microbleed after surgical resection in a patient with moyamoya disease. Neurol Med Chir (Tokyo). 2007;47:564-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Naka H, Nomura E, Takahashi T, Wakabayashi S, Mimori Y, Kajikawa H, Kohriyama T, Matsumoto M. Combinations of the presence or absence of cerebral microbleeds and advanced white matter hyperintensity as predictors of subsequent stroke types. AJNR Am J Neuroradiol. 2006;27:830-835. [PubMed] |
| 25. | Imaizumi T, Honma T, Horita Y, Kawamura M, Kohama I, Miyata K, Nyon KS, Niwa J. The number of microbleeds on gradient T2*-weighted magnetic resonance image at the onset of intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2008;17:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Kwon HG, Jang SH. Cingulum injury by external ventricular drainage procedure: diffusion tensor tractography study. Clin Neuroradiol. 2015;25:65-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34:51-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1074] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 28. | Wang XT, Zhang LY, Lv HT, Liu J, Xu YH. Delayed intracerebral hemorrhage after ventriculo-peritoneal shunt procedure: two case reports and a review of literature. Eur Rev Med Pharmacol Sci. 2021;25:6093-6100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Nemes S, Jonasson JM, Genell A, Steineck G. Bias in odds ratios by logistic regression modelling and sample size. BMC Med Res Methodol. 2009;9:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |