Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7242
Peer-review started: March 25, 2022
First decision: April 13, 2022
Revised: April 17, 2022
Accepted: June 3, 2022
Article in press: June 3, 2022
Published online: July 26, 2022
Processing time: 108 Days and 5.7 Hours
Gastric cancer (GC) is the second most common cause of cancer-related deaths worldwide. Hepatocyte nuclear factor 4 alpha (HNF4α) that belongs to the nuclear hormone receptor superfamily, is overexpressed in GC tissues, and might be involved in the development of GC by regulating its downstream wingless-related integration site (WNT)/β-catenin signaling.
To clarify the expression of HNF4α/WNT5a/β-catenin signaling proteins in clinical GC tissues.
We immunohistochemically stained pathological blocks of GC and matched para-cancerous tissues. The intensity of HNF4α, WNT5a and β-catenin staining in the tumor cells was determined according to cell rates and staining intensity. The correlations between GC and HNF4α, WNT5a, and β-catenin expression using chi-square and paired chi-square tests. Relationships between double-positive HNF4α and WNT5a expression and types of gastric tumor tissues were assessed using regression analysis. Correlations between HNF4α and WNT5a expression at the RNA level in GC tissues found in the TCGA database were analyzed using Pearson correlation coefficients.
We found more abundant HNF4α and WNT5a proteins in GC, especially in mucinous adenocarcinoma and mixed GC than in adjacent tissues (P < 0.001). Low and high levels of cytoplasmic β-catenin respectively expressed in GC and adjacent tissues (P < 0.001) were not significantly associated with pathological parameters.
The expressions of HNF4α and WNT5a could serve as early diagnostic biomarkers for GC.
Core Tip: Gastric cancer (GC) is the second most common cause of cancer-related deaths worldwide. A precise biopsy biomarker that can predict the progression of GC and change its prognosis is urgently needed. We previously found that hepatocyte nuclear factor 4 alpha (HNF4α)/wingless-related integration site (WNT) signaling is involved in the process of GC in preclinical models. Here, we obtained gastric tumor and para-cancerous tissue sections from Tongji Hospital and investigated the expression of HNF4α, WNT5a, and β-catenin. The expression of HNF4α and WNT5a could serve as an early diagnostic biomarker of GC.
- Citation: Hu Q, Li LL, Peng Z, Yi P. Expression of hepatocyte nuclear factor 4 alpha, wingless-related integration site, and β-catenin in clinical gastric cancer. World J Clin Cases 2022; 10(21): 7242-7255
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7242.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7242
Gastric cancer (GC) is the second most common cause of cancer-related deaths worldwide. Because early symptoms are not obvious, most GCs are diagnosed at a late stage when a radical cure is largely ineffective[1,2]. Thus, to diagnose the disease at an early stage when the treatment is likely to be more effective is critical. Several screening approaches have been proposed, such as serum pepsinogens, serum ghrelin and Gastrin-17. However, further investigation is needed to determine the overall effectiveness of these approaches[3]. Upper gastrointestinal endoscopy is the most sensitive and specific diagnostic screening method, and biopsy tissues can be morphologically examined[4,5]. However, endoscopic diagnosis of early GC is quite challenging because it requires advanced endoscopic techniques, and expert endoscopists who are familiar with current and emerging techniques[4]. Therefore, accurate biopsy biomarkers are needed that can predict the progression and prognosis of GC in para-cancerous or cancerous tissues. Identifying such markers would lead to the development of accurately targeted therapy for GC.
Hepatocyte nuclear factor 4 alpha (HNF4α) is an endoderm-specific zinc-finger transcription factor that has a highly conserved orphan receptor and belongs to the nuclear hormone receptor superfamily[6]. It is found in the liver, kidney, pancreas, stomach, small intestine, and colon[7] and is associated with several types of cancer. The abundance of HNF4α varies in different types of cancer and even reverses. For example, HNF4α is expressed at lower levels in colon carcinoma and hepatocellular carcinoma tissues than in adjacent normal tissues[6,8]. In contrast, it is overexpressed in gastric tumor tissues compared to adjacent normal tissues and associated with a poorer prognosis in patients with GC[9]. Moreover, protein-protein interaction networks have revealed that HNF4α is the most significant node in GC and might be a useful diagnostic marker and therapeutic target[10]. However, a relationship between HNF4α and the pathological classification or demographic characteristics of GC has not been identified.
Wingless-related integration site (WNT) signaling pathways are deregulated in cancers[11]. Nam et al[12] applied PATHOME, a novel algorithm that can sensitively detect expressed pathways in GC gene expression datasets. They found that WNT pathway are involved in the development of primary GC and that WNT5a is a potential target gene[12,13]. A meta-analysis similarly associated WNT5a expression in human GC with aggressiveness and poor prognosis[14]. Anti-WNT5a antibodies can suppress GC metastasis in vivo[15]. Moreover, WNT5a is closely associated with the epithelial-mesenchymal transition (EMT) and participates in GC development by promoting the EMT[16,17].
Hepatocyte nuclear factor 4 alpha is a transcription factor that regulates the transcription of WNT5a by competing with β-catenin for binding to transcription factor 4 (TCF4) and inhibiting WNT/β-catenin signaling in hepatocellular carcinoma cells[6,12,18]. A double-negative feedback mechanism controls WNT/β-catenin signaling and HNF4α expression during EMT regulation, which eventually affects hepatocellular carcinoma development[19]. We previously found that an HNF4α/WNT5a/β-catenin signaling axis in GC cell lines and animal models[20,21], and affects the growth and development of GC[12]. However, the expression of the HNF4α/WNT5a/β-catenin signaling pathway in clinical GC tissues remains unclear. Thus, we investigated the relationship between this pathway and GC in a preclinical model of GC tissues.
In this study, 158 cases of gastric tumoral tissues and 164 cases of adjacent para-cancerous tissues in Tongji hospital were selected. We immunohistochemically stained HNF4α, WNT5a, and β-catenin expression in these tissues. And then we explored relationships between these molecules and the clinicopathological features of GC as well as the sociological features of patients. This is an extension of a series of studies.
Human GC tissues were obtained from patients who presented between 2016 and 2018 at Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology (Wuhan, China). According to the WHO histological classifications, GC comprises signet ring cell carcinoma, and tubular, as well as mucinous adenocarcinoma. We initially selected wax blocks of paraffin-embedded GC and matched para-cancerous tissue blocks (n = 164 each) to reduce the influence of pathological WHO classification of GC on the expression of HNF4α, WNT5a, and β-catenin in GC tissues. However, six small gastric tumor tissues of insufficient quality were excluded. Therefore, we analyzed 158 GC (59 signet-ring cell carcinoma, and 35 tubular, and 64 mucinous adenocarcinomas) and 164 para-cancerous tissues. The GC tissues comprised 97, 46, and 15 samples of intestinal, diffuse mixed types according to the Lauren classification. Pathologists from the Department of Pathology at Tongji Hospital determined the histological classification. The study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Approval no: TJ-IRB20190501).
The paraffin-embedded tissues were cut into 4-µm-thick sections and mounted on triplicate APES-coated slides (AR0001; Boster Biological Technology Co., Ltd., Wuhan, China). The tissues were deparaffinized in xylene (Hubei Jing-Hengye Technology Co., Ltd., Wuhan, China) and rehydrated in graded ethanol (National Medicine Group Chemical Reagent Co., Ltd.). Endogenous peroxidase activity was quenched with a 3% hydrogen peroxide in methanol (National Medicine Group Chemical Reagent Co., Ltd.) at room temperature for 30 min, then the tissues were rinsed in phosphate-buffered saline (PBS) at pH 6.0 after antigen retrieval in 10 mmol/L citrate buffer (pH 6.0) at 94℃ for 8 and 10 min, the slides were immediately cooled for 20 min at room temperature. Nonspecific antigen binding sites were blocked by incubation with wash buffer containing 10% normal goat serum (YJ0130; Shanghai Yanjin Biology Science and Technology Co., Ltd., Shanghai, China) at 37℃ for 30 min. The sections were then incubated overnight at 4℃ with primary antibodies against HNF4α diluted 1:200 (#3113; Cell Signaling Technologies, Danvers, MA, United States), WNT5a diluted 1:80 (abs113167; Abgent, San Diego, CA, United States), and β-catenin diluted 1:100 (#8480; Cell Signaling Technologies). Positive tissues were stained brown with the peroxidase substrate 3-3’-diaminobenzidine tetrahydrochloride (DAB) (Hubei Biossci Biological Co., Ltd., Wuhan, China) and all tissues were lightly counterstained with hematoxylin. Specimens were examined under a light microscope (200× magnification).
The intensity of HNF4α, WNT5a and β-catenin staining in the tumor cells was determined using a single-blind method. The samples were scored as 0, +1 or +2 according to cell rates, and from 0 to +3 according to staining intensity[22]. Cell positivity scores: 0, weakly positive (< 10% of tumor cells emitted faint signals); +1, moderately positive (10%-50% of tumor cells emitted clear signals); +2 strongly positive (> 50% of tumor cells emitted signals). Staining intensity of tumor cells was scored as 0 (negative), +1, +2 or +3 (weakly, moderately, and strongly positive, respectively). The product of the positive cell rate and staining intensity generated the immunohistochemical score, where 0-1 was negative (-), and 2-3, 4, and 6 were mildly (+), moderately positive (++), and strongly (+++) positive, respectively.
All data were analyzed using SPSS v. 20.0 (IBM Corp, Armonk, NY, United States). Relationships between the expression of HNF4α, WNT5a, and β-catenin and the pathological features of GC as well as the sociological features of the patients were analyzed using chi-squared tests. Correlations among HNF4α, WNT5a, and β-catenin expression were evaluated using chi-squared tests of paired comparisons. Relationships between double-positive HNF4α and WNT5a expression and types of gastric tumor tissues were assessed using regression analysis. Correlations between HNF4α and WNT5a expression at the RNA level in GC tissues found in the TCGA database were analyzed using Pearson correlation coefficients. Values with two-tailed P < 0.05 were considered statistically significant.
Among the 158 GC tissues from 101 men and 57 women, 97, 52 and 9 were poorly, moderately, and highly differentiated, respectively. The GC tissues were staged according to the depth of invasion (T), lymph node (N), and distant metastasis (M) (TNM classification). We staged 28, 38, 74, and 18 GC tissues as I, II, III, and IV, respectively (Table 1).
| Groups | n |
| Gender | |
| Male | 101 |
| Female | 57 |
| Age (yr) | |
| ≤ 60 | 97 |
| > 60 | 61 |
| Differentiation | |
| Low | 97 |
| Moderate | 52 |
| High | 9 |
| Invasion depth (T) | |
| T1 | 26 |
| T2 | 20 |
| T3 | 39 |
| T4 | 73 |
| Lymph node metastasis (N) | |
| N0 | 65 |
| N1 | 16 |
| N2 | 32 |
| N3 | 45 |
| Distant metastasis (M) | |
| M0 | 126 |
| M1 | 32 |
| TNM stage | |
| Ⅰ | 28 |
| Ⅱ | 38 |
| Ⅲ | 74 |
| Ⅳ | 18 |
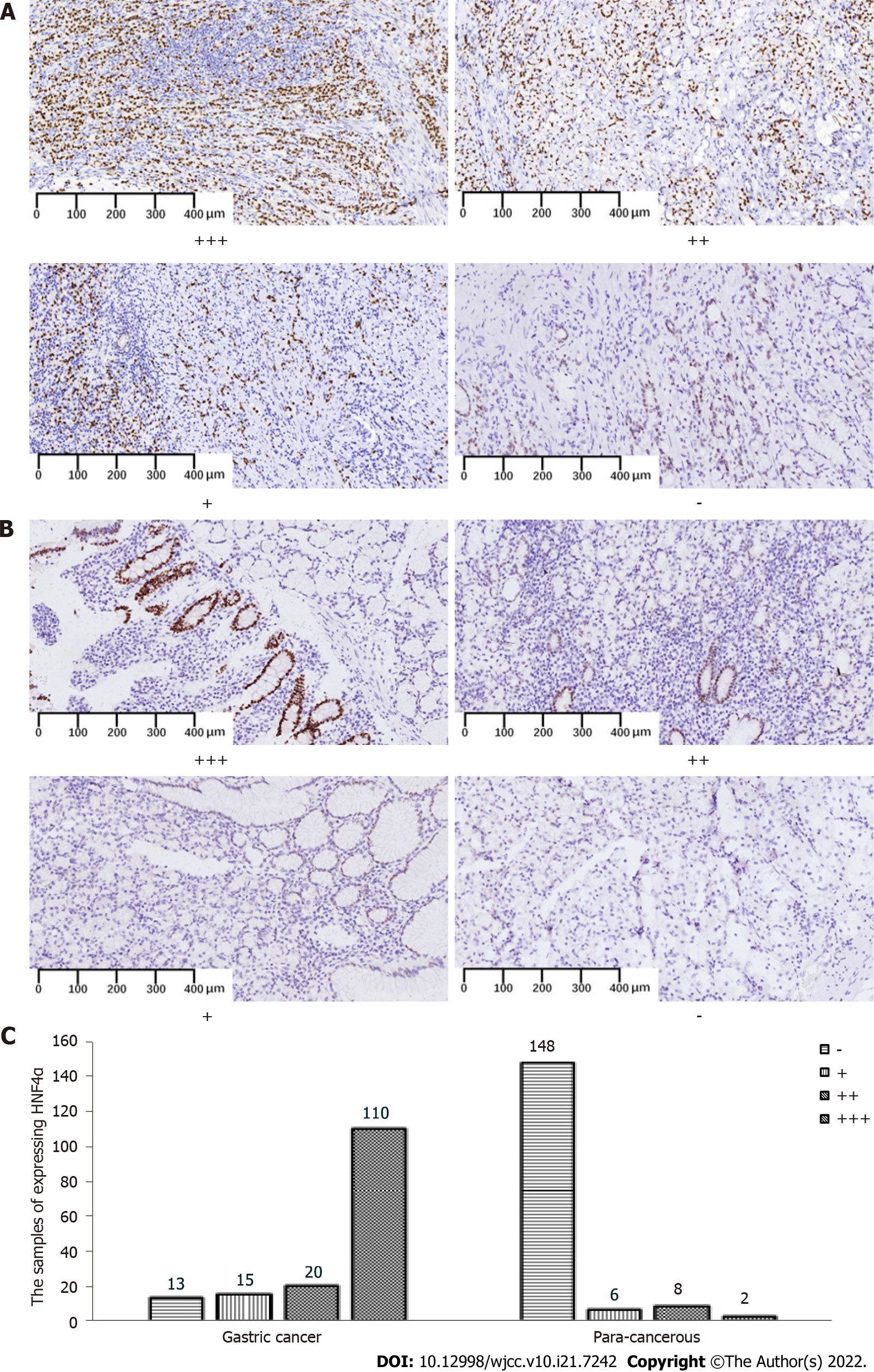
Figure 1A and B shows representative HNF4α expression in different grades of GC and para-cancerous tissues. The positive rates of HNF4α in GC and para-cancerous tissues were 145 (91.7%) of 158 vs. 16 (9.8%) of 164 (P < 0.001 Table 2).
| Groups | n | HNF4α expression | Positive (%) | χ2 | P value | |
| Negative | Positive | |||||
| GC | 158 | 13 | 145 | 91.7 | ||
| PC | 164 | 148 | 16 | 9.8 | 216.5 | 0.000 |
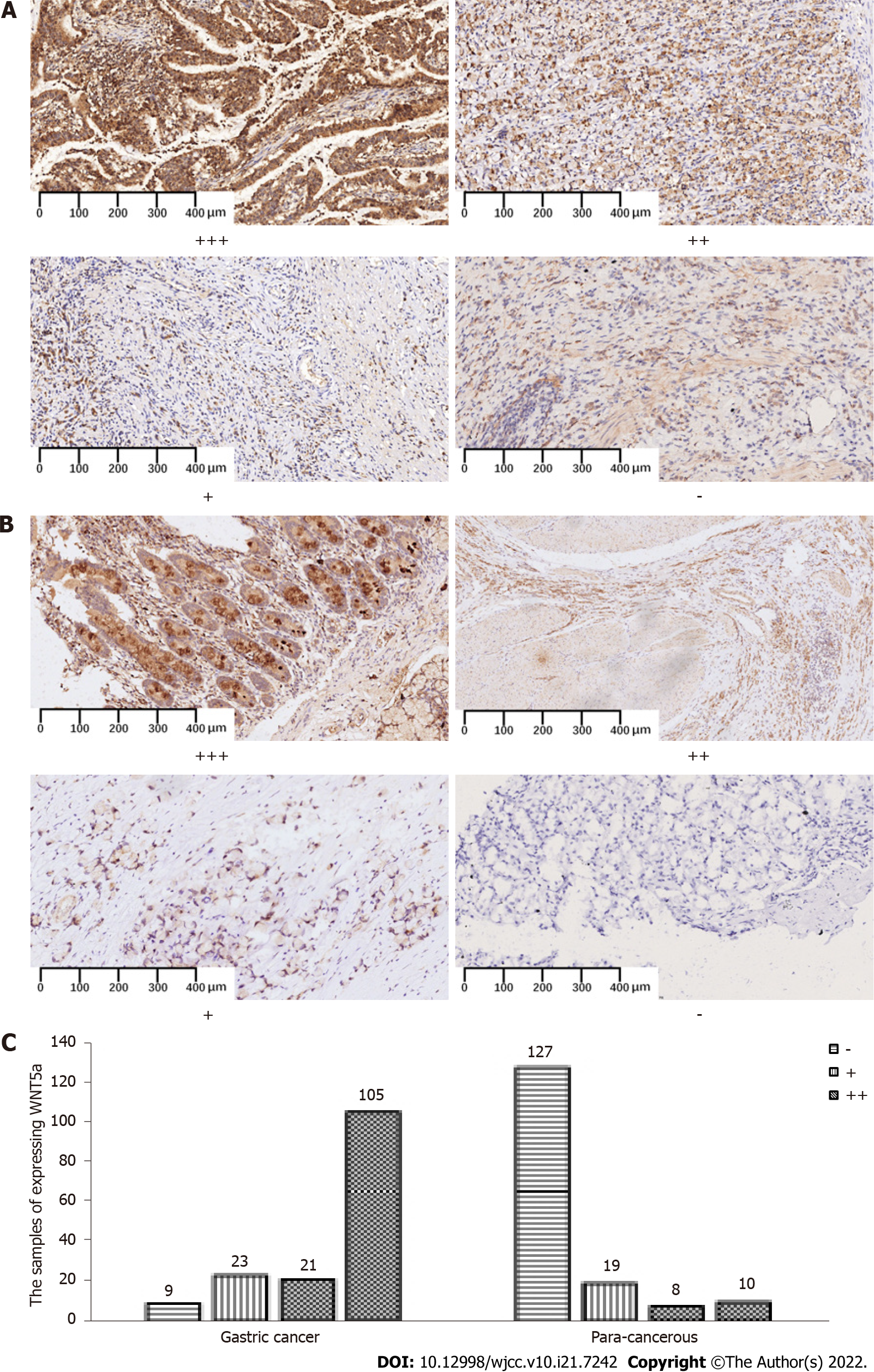
As a ligand for the Frizzled family of atypical G protein-coupled receptors, WNT5a plays a critical role in postnatal cellular functions and development[14]. Both HNF4α and WNT5a, which is a downstream signaling molecule and effector of HNF4α, are involved in GC development. Therefore, we analyzed WNT5a expression in clinical samples[18]. Figure 2 shows WNT5a expression in different grades of gastric tumor and para-cancerous tissues, and cytoplasmic staining. The positive rate of WNT5a expression was 149 (94.3%) of 158 (Table 3) in GC, and significantly decreased in para-cancerous tissues (P < 0.001).
| Groups | n | WNT5a expression | Positive (%) | χ2 | P value | ||
| Negative | Positive | ||||||
| GC | 158 | 9 | 149 | 94.3 | |||
| PC | 164 | 127 | 37 | 22.6 | 169.8 | 0.000 | |
The WHO histologically classifies GC as tubular, signet ring cell, and mucinous adenocarcinoma. Table 4 shows that HNF4α expression was abundant in tubular and mucinous adenocarcinomas and relatively weak in signet ring cell carcinoma (P < 0.001). Conversely, WNT5a expression was more abundant in mucinous, than in tubular adenocarcinoma and signet ring cell carcinoma (P < 0.001). The results of regression analyses showed that tubular GC was more likely to moderately express positive WNT5a (Supplementary Table 1).
The Lauren classification also categorizes GC into intestinal, diffuse, and mixed types. Compared with intestinal and diffuse GC, both HNF4α and WNT5a were strongly positive in mixed GC (Table 5, P < 0.001), but overall, both were abundantly expressed in mucinous and mixed GCs.
We examined pairwise co-expression associations between HNF4α and WNT5a in clinical GC tissue specimens. The χ2 test of paired comparisons found no positive correlations between HNF4α and WNT5a expression in these tissues (χ2 = 1.5, P > 0.05; Table 6), although both were more abundantly expressed in GC, than para-cancerous tissues.
| WNT5a | HNF4α expression | Total | χ2 | P value | |
| Negative | Positive | ||||
| Negative | 8 | 1 | 9 | 1.5 | > 0.05 |
| Positive | 5 | 144 | 149 | ||
| Total | 13 | 145 | 158 | ||
We analyzed the diagnostic accuracy, sensitivity, specificity, and positive and negative predictive rates of HNF4α and WNT5a to further determine their potential roles in diagnosing GC. Table 7 shows that the diagnostic accuracy, sensitivity, specificity, and positive and negative predictive rates of HNF4α were 91.0%, 91.8% 90.2%, 90.1% and 91.9%, respectively. In contrast, the diagnostic accuracy and specificity of WNT5a were relatively low at 85.7% and 77.4%, respectively, but the diagnostic sensitivity reached 94.3%. The positive and negative predictive rates of WNT5a expression were 80.1% and 93.4%, respectively. The expression of HNF4α and WNT5a in GC tissues did not correlate with the patients’ sex and age, or tumor differentiation, invasion depth, distant metastasis, and TNM stage (P > 0.05). In contrast, HNF4α expression was associated with GC differentiation, and the positive rate was the highest among the moderately differentiated GC tissues (P < 0.05) (Supplementary Table 2).
| Diagnostic accurate rate | Sensitivity | Specificity | Positive predictive rate | Negative predictive rate | ||
| HNF4α | 91.0 | 91.8 | 90.2 | 90.1 | 91.9 | |
| WNT5a | 85.7 | 94.3 | 77.4 | 80.1 | 93.4 | |
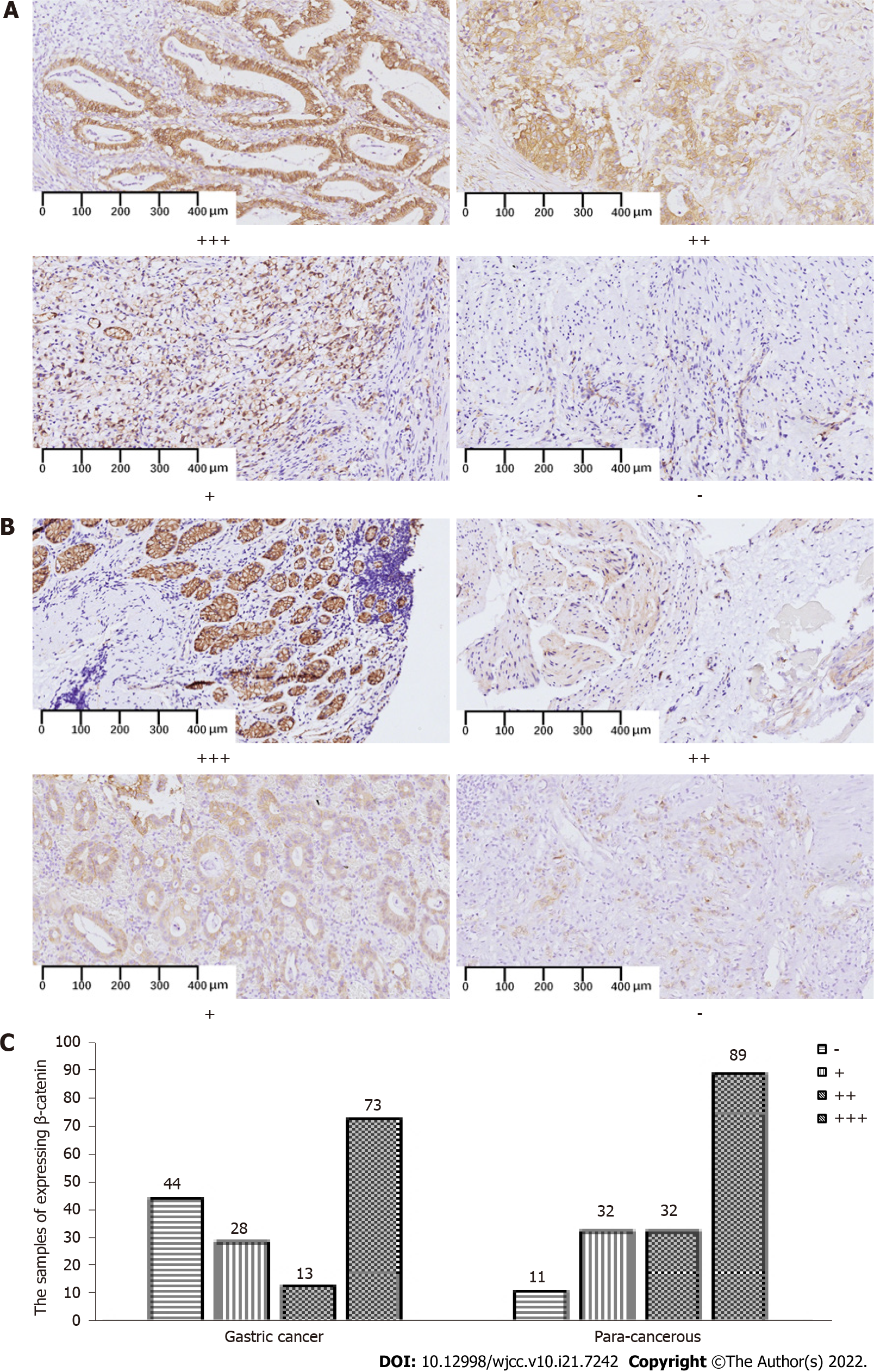
Excessive cytoplasmic β-catenin in the β-catenin-dependent WNT signaling pathway migrates to the nucleus where it acts as a transcription factor and activates a series of downstream signaling pathways. We analyzed whether the canonical β-catenin-dependent or the non-canonical β-catenin-independent WNT pathway participates in clinical gastric carcinogenesis. Figure 3 shows abundant membranous and cytoplasmic β-catenin expression in gastric tumoral and para-cancerous tissues, but the ratio of positive β-catenin expression was significantly lower in the latter 114 (72.2%) of 158 vs. 153 (93.3%) of 164 (P < 0.001; Table 8).
| Groups | n | β-catenin expression | Positive (%) | χ2 | P value | |
| Negative | Positive | |||||
| GC | 158 | 44 | 114 | 72.2 | ||
| PC | 164 | 11 | 153 | 93.3 | 25.394 | 0.000 |
We further analyzed correlations between β-catenin expression in the GC tissues and the clinical demographic features of the patients as well as the histopathological characteristics of GC. The expression of β-catenin was not significantly associated with sex, age, tumor differentiation, depth of invasion, distant metastasis, and TNM stage in these patients (P > 0.05; Supplementary Table 3).
The nuclear transcription factor HNF4α is expressed in the liver, kidney, and intestine[6,23]. It participates in glucose and lipid metabolism and insulin secretion, and is associated with metabolic diseases such as diabetes and obesity[24-26] as well as malignant tumor differentiation via gene regulation[27]. For example, Sugano et al[28] found that HNF4α could serve as a marker for invasive mucinous adenocarcinoma of the lung, and Ma et al[9] found that HNF4α is linked to GC caused by Helicobacter pylori. We previously showed that HNF4α can regulate the proliferation, invasion, and metastasis of GC in vitro and in vivo by modulating the tumorigenic WNT signaling pathway[20]. The present study found that more nuclei stained with HNF4α in GC, than para-cancerous tissues had high diagnostic accuracy, sensitivity, and specificity. Furthermore, HNF4α regulates organic acid metabolism, sustains oncogenic metabolism in GC, and promotes GC development by positively regulating the isocitrate dehydrogenase 1 (IDH1) gene[29]. Moreover, HNF4α enhances multidrug resistance by increasing GC cell apoptosis, thus suppressing therapeutic responses[30]. Therefore, HNF4α can promote GC development by affecting the metabolism and multiple drug resistance of GC cells.
Aberrant WNT signaling often leads to tumor development and WNT5a is a ligand for seven Frizzled family transmembrane receptors, most of which are associated with GC. We previously found significantly increased expression of WNT5a in SGC7901 and MGC803 GC cells and in mice with GC xenografts. Consistent with these results, WNT5a is abundantly expressed in GC tissues, weakly in para-cancerous tissues, and in the cytoplasm of clinical GC sections[12,31]. The selective effects of WNT5a on cancer cells include the promoted invasion but not proliferation of KKLS GC cells, and the stimulation of both in A549 Lung cancer cells[32]. The progression of GC is promoted by WNT5a mainly by increasing the expression of metastatic proteins such as laminin gamma 2 and the EMT[16,33]. However, WNT5a induces the M2 polarization of tumor-associated macrophages through the CaKMII-ERK1/2-STAT3 pathway in CRC, thus promoting the proliferation, migration, and invasiveness of cancer cells[34]. Therefore, abundant WNT5a in gastric tumor tissues might contribute to GC metastasis and invasion by affecting EMT.
We previously found that the WNT5a/β-catenin signaling pathway is cell downstream of HNF4α in GC cells, and the HNF4α/WNT5a signaling affected GC development in GC cell lines and animal models[20,21]. The present study also found more abundant HNF4α and WNT5a expression in GC, than that in adjacent tissues, especially in mucinous adenocarcinoma and mixed GC, and that both had high diagnostic accuracy. However, HNF4α and WNT5a expression did not correlate in GC tissues. We similarly identified a weak correlation (0 < r = 0.11) between HNF4α and WNT5a in GC tissues at RNA level in the Cancer Genome Atlas (TCGA, https://cancergenome.nih.gov/) (Supplementary Figure 1), whereas others have found that HNF4α and WNT5a expression in GC positively correlates at the RNA and protein levels[12,18,20]. Other than the abundant HNF4α expression in moderately differentiated GC, the present study found that neither HNF4α nor WNT5a expression correlates with the demographic and pathological characteristics of GC of sex, age, tumor differentiation degree, invasion depth, distant metastasis and TNM stage.
The present study included tissues from patients who were treated between 2016 and 2018, and follow-up is currently underway. Therefore, we were unable to provide a complete (or 5-year) survival analysis of double positive or double negative HNF4α and WNT5a in GC. However, we analyzed the progression-free (PFS), and overall (OS) survival of 447 patients with HNF4α and WNT5a double positive and negative GC in the TCGA database. We found that the although patients tended to survive longer if they were double negative than double positive, the differences in 5-year OS or PFS did not reach statistical significance (Supplementary Figures 2-5). A retrospective study found that a combi
The WNT/β-catenin pathway is crucial for tissue development and homeostasis in all animal, and its dysregulation is one of the most relevant events linked to cancer development and dissemination[36]. The representative WNT ligand WNT5a can activate both canonical β-catenin-dependent and non-canonical β-catenin-independent WNT pathways[37] that participate in cancer cell migration and invasion by mediating the EMT[23,32,37-43]. Inhibition of the WNT/β-catenin signaling pathway also suppresses GC metastasis[21,44]. We examined β-catenin expression in clinical gastric tumor sections to determine which of the WNT/β-catenin pathways are involved in the development of GC. We found more abundant β-catenin in the membrane and cytoplasm of para-cancerous, than GC tissues, and that this did not correlate with sex and age of patients, or the degree of differentiation, invasion depth, distant metastasis, or TNM stage of tumors. This implies that WNT5a activated the canonical WNT signaling pathway in GC, resulting in the reduction of cytoplasmic β-catenin. However, to confirm that WNT5a regulates the canonical β-catenin-dependent signaling pathway in GC metastasis requires further investigation of β-catenin nuclear staining.
The expression of HNF4α and WNT5a was more abundant in GC, than para-cancerous tissues, whereas that of cytoplasmic β-catenin was lower. The diagnostic accuracy rate of HNF4α and WNT5a for GC was 91.0% and 85.7%, respectively, indicating that both could serve as potential diagnostic tools for GC. To our knowledge, this is the first study to find that HNF4α, WNT5a, and β-catenin proteins are expressed in GC. However, this study is limited by the retrospective design. Because all specimens were pathological paraffin blocks of clinical GC, we could not investigate expression of the HNF4α/WNT5a/β-catenin signaling axis using western blotting or PCR. Therefore, clinical trials are warranted to verify our conclusion.
The authors have previously found in gastric cancer (GC) cell lines and animal models that the expression of hepatocyte nuclear factor 4 alpha (HNF4α) is significantly increased, and it promotes the invasion and metastasis of GC by regulating its downstream wingless-related integration site (WNT)/β-catenin signaling pathway, while negatively regulating the HNF4α/WNT signaling pathway can inhibit the development of GC.
Extending from the preclinical model to clinical GC tissues, we will continue to explore the expression of HNF4α and its downstream WNT/β-catenin signaling pathway in GC tissues, in the hope of providing new treatment ideas for inhibiting the development of GC.
To explore the expression of HNF4α, WNT5a and β-catenin in clinical GC tissues and adjacent tissues, and whether the expression of these three molecules in GC tissues is related.
Screening GC tissues containing each subtype and the adjacent para-cancerous tissues; recording the demographic characteristics of the patients and the pathological type and stage of the GC tissues; and detecting the expressions of HNF4α, WNT5a and β-catenin in the GC tissues and the para-cancerous tissues by immunohistochemistry. The expression levels of these molecules were observed under the microscope and the expression levels were recorded according to the corresponding scoring criteria. Finally, the statistical methods such as analysis of chi-square and paired chi-square were used to analyze the expression differences and correlation of HNF4α, WNT5a and β-catenin in GC tissues and para-cancerous tissues.
Compared with the para-cancerous tissues, the expressions of HNF4α (nuclear staining) and WNT5a (cytoplasmic staining) in GC tissues were significantly increased, while the expression of β-catenin (cytoplasmic staining) was significantly decreased. HNF4α expression was abundant in tubular and mucinous adenocarcinomas and relatively weak in signet ring cell carcinoma; WNT5a expression was more abundant in mucinous, than in tubular adenocarcinoma and signet ring cell carcinoma; both HNF4α and WNT5a were strongly positive in mixed GC, although no positive correlations between HNF4α and WNT5a expression in GC.
Comparing the expression of HNF4α, WNT5a and β-catenin in GC and para-cancerous tissues, we can find the expressions of HNF4α and WNT5a could serve as early diagnostic biomarkers for GC.
It is hopeful that HNF4α and WNT5a can be included into the molecules for the diagnosis of GC.
We would like to thank Yao-Bing Chen for his help, a pathologist of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Martin-Villa JM, Spain; Rojas A, Chile; Shamseldeen AA, Egypt; Tanabe S, Japan A-Editor: Zhu JQ, China S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | De Re V. Molecular Features Distinguish Gastric Cancer Subtypes. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39:1010428317714626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 644] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 3. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1327] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 4. | Pasechnikov V, Chukov S, Fedorov E, Kikuste I, Leja M. Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol. 2014;20:13842-13862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 247] [Cited by in RCA: 303] [Article Influence: 27.5] [Reference Citation Analysis (2)] |
| 5. | Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 727] [Article Influence: 103.9] [Reference Citation Analysis (0)] |
| 6. | Yao HS, Wang J, Zhang XP, Wang LZ, Wang Y, Li XX, Jin KZ, Hu ZQ, Wang WJ. Hepatocyte nuclear factor 4α suppresses the aggravation of colon carcinoma. Mol Carcinog. 2016;55:458-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Duncan SA, Manova K, Chen WS, Hoodless P, Weinstein DC, Bachvarova RF, Darnell JE Jr. Expression of transcription factor HNF-4 in the extraembryonic endoderm, gut, and nephrogenic tissue of the developing mouse embryo: HNF-4 is a marker for primary endoderm in the implanting blastocyst. Proc Natl Acad Sci U S A. 1994;91:7598-7602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 288] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Vuong LM, Chellappa K, Dhahbi JM, Deans JR, Fang B, Bolotin E, Titova NV, Hoverter NP, Spindler SR, Waterman ML, Sladek FM. Differential Effects of Hepatocyte Nuclear Factor 4α Isoforms on Tumor Growth and T-Cell Factor 4/AP-1 Interactions in Human Colorectal Cancer Cells. Mol Cell Biol. 2015;35:3471-3490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Ma L, Zeng J, Guo Q, Liang X, Shen L, Li S, Sun Y, Li W, Liu S, Yu H, Chen C, Jia J. Mutual amplification of HNF4α and IL-1R1 composes an inflammatory circuit in Helicobacter pylori associated gastric carcinogenesis. Oncotarget. 2016;7:11349-11363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Saberi Anvar M, Minuchehr Z, Shahlaei M, Kheitan S. Gastric cancer biomarkers; A systems biology approach. Biochem Biophys Rep. 2018;13:141-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Taciak B, Pruszynska I, Kiraga L, Bialasek M, Krol M. Wnt signaling pathway in development and cancer. J Physiol Pharmacol. 2018;69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 98] [Reference Citation Analysis (0)] |
| 12. | Nam S, Chang HR, Kim KT, Kook MC, Hong D, Kwon CH, Jung HR, Park HS, Powis G, Liang H, Park T, Kim YH. PATHOME: an algorithm for accurately detecting differentially expressed subpathways. Oncogene. 2014;33:4941-4951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Saitoh T, Mine T, Katoh M. Frequent up-regulation of WNT5A mRNA in primary gastric cancer. Int J Mol Med. 2002;9:515-519. [PubMed] |
| 14. | Nam S, Chung JW, Yang JY. WNT5A Correlates with Clinicopathological Characteristics in Gastric Cancer: a Meta-Analysis. Cell Physiol Biochem. 2017;41:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Hanaki H, Yamamoto H, Sakane H, Matsumoto S, Ohdan H, Sato A, Kikuchi A. An anti-Wnt5a antibody suppresses metastasis of gastric cancer cells in vivo by inhibiting receptor-mediated endocytosis. Mol Cancer Ther. 2012;11:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Xue Y, Zhang L, Zhu Y, Ke X, Wang Q, Min H. Regulation of Proliferation and Epithelial-to-Mesenchymal Transition (EMT) of Gastric Cancer by ZEB1 via Modulating Wnt5a and Related Mechanisms. Med Sci Monit. 2019;25:1663-1670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Gao M, Liu L, Yang Y, Li M, Ma Q, Chang Z. LncRNA HCP5 Induces Gastric Cancer Cell Proliferation, Invasion, and EMT Processes Through the miR-186-5p/WNT5A Axis Under Hypoxia. Front Cell Dev Biol. 2021;9:663654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Chang HR, Nam S, Kook MC, Kim KT, Liu X, Yao H, Jung HR, Lemos R Jr, Seo HH, Park HS, Gim Y, Hong D, Huh I, Kim YW, Tan D, Liu CG, Powis G, Park T, Liang H, Kim YH. HNF4α is a therapeutic target that links AMPK to WNT signalling in early-stage gastric cancer. Gut. 2016;65:19-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 19. | Yang M, Li SN, Anjum KM, Gui LX, Zhu SS, Liu J, Chen JK, Liu QF, Ye GD, Wang WJ, Wu JF, Cai WY, Sun GB, Liu YJ, Liu RF, Zhang ZM, Li BA. A double-negative feedback loop between Wnt-β-catenin signaling and HNF4α regulates epithelial-mesenchymal transition in hepatocellular carcinoma. J Cell Sci. 2013;126:5692-5703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Hu Q, Li L, Zou X, Xu L, Yi P. Berberine Attenuated Proliferation, Invasion and Migration by Targeting the AMPK/HNF4α/WNT5A Pathway in Gastric Carcinoma. Front Pharmacol. 2018;9:1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 21. | Wang H, Dong S, Liu Y, Ma F, Fang J, Zhang W, Shao S, Shen H, Jin J. DAB2 suppresses gastric cancer migration by regulating the Wnt/β-catenin and Hippo-YAP signaling pathways. Transl Cancer Res. 2020;9:1174-1184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Fusco N, Rocco EG, Del Conte C, Pellegrini C, Bulfamante G, Di Nuovo F, Romagnoli S, Bosari S. HER2 in gastric cancer: a digital image analysis in pre-neoplastic, primary and metastatic lesions. Mod Pathol. 2013;26:816-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Kanzawa M, Semba S, Hara S, Itoh T, Yokozaki H. WNT5A is a key regulator of the epithelial-mesenchymal transition and cancer stem cell properties in human gastric carcinoma cells. Pathobiology. 2013;80:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Kulkarni RN, Kahn CR. Molecular biology. HNFs--linking the liver and pancreatic islets in diabetes. Science. 2004;303:1311-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Marcil V, Amre D, Seidman EG, Boudreau F, Gendron FP, Ménard D, Beaulieu JF, Sinnett D, Lambert M, Levy E. Hepatocyte nuclear factor 4 alpha polymorphisms and the metabolic syndrome in French-Canadian youth. PLoS One. 2015;10:e0117238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Naiki T, Nagaki M, Shidoji Y, Kojima H, Imose M, Kato T, Ohishi N, Yagi K, Moriwaki H. Analysis of gene expression profile induced by hepatocyte nuclear factor 4alpha in hepatoma cells using an oligonucleotide microarray. J Biol Chem. 2002;277:14011-14019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Chen Z, Gropler MC, Mitra MS, Finck BN. Complex interplay between the lipin 1 and the hepatocyte nuclear factor 4 α (HNF4α) pathways to regulate liver lipid metabolism. PLoS One. 2012;7:e51320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Sugano M, Nagasaka T, Sasaki E, Murakami Y, Hosoda W, Hida T, Mitsudomi T, Yatabe Y. HNF4α as a marker for invasive mucinous adenocarcinoma of the lung. Am J Surg Pathol. 2013;37:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Xu C, Ooi WF, Qamra A, Tan J, Chua BY, Ho SWT, Das K, Adam Isa ZF, Li Z, Yao X, Yan T, Xing M, Huang KK, Lin JS, Nandi T, Tay ST, Lee MH, Tan ALK, Ong X, Ashktorab H, Smoot D, Li S, Ng SC, Teh BT, Tan P. HNF4α pathway mapping identifies wild-type IDH1 as a targetable metabolic node in gastric cancer. Gut. 2020;69:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Ma Y, Wei X, Wu Z. HNF-4α promotes multidrug resistance of gastric cancer cells through the modulation of cell apoptosis. Oncol Lett. 2017;14:6477-6484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66:10439-10448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 348] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 32. | Shojima K, Sato A, Hanaki H, Tsujimoto I, Nakamura M, Hattori K, Sato Y, Dohi K, Hirata M, Yamamoto H, Kikuchi A. Wnt5a promotes cancer cell invasion and proliferation by receptor-mediated endocytosis-dependent and -independent mechanisms, respectively. Sci Rep. 2015;5:8042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Yamamoto H, Kitadai Y, Yamamoto H, Oue N, Ohdan H, Yasui W, Kikuchi A. Laminin gamma2 mediates Wnt5a-induced invasion of gastric cancer cells. Gastroenterology. 2009;137:242-252, 252.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Liu Q, Yang C, Wang S, Shi D, Wei C, Song J, Lin X, Dou R, Bai J, Xiang Z, Huang S, Liu K, Xiong B. Wnt5a-induced M2 polarization of tumor-associated macrophages via IL-10 promotes colorectal cancer progression. Cell Commun Signal. 2020;18:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 35. | Liu X, Xiu LJ, Jiao JP, Zhao J, Zhao Y, Lu Y, Shi J, Li YJ, Ye M, Gu YF, Wang XW, Xu JY, Zhang CA, Liu YY, Luo Y, Yue XQ. Traditional Chinese medicine integrated with chemotherapy for stage IV non-surgical gastric cancer: a retrospective clinical analysis. J Integr Med. 2017;15:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 36. | Koni M, Pinnarò V, Brizzi MF. The Wnt Signalling Pathway: A Tailored Target in Cancer. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 37. | Gencer S, Şen G, Doğusoy G, Bellı AK, Paksoy M, Yazicioğlu MB. β-Catenin-independent noncanonical Wnt pathway might be induced in gastric cancers. Turk J Gastroenterol. 2010;21:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Asem MS, Buechler S, Wates RB, Miller DL, Stack MS. Wnt5a Signaling in Cancer. Cancers (Basel). 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 188] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 39. | Tian S, Hu J, Tao K, Wang J, Chu Y, Li J, Liu Z, Ding X, Xu L, Li Q, Cai M, Gao J, Shuai X, Wang G, Wang L, Wang Z. Secreted AGR2 promotes invasion of colorectal cancer cells via Wnt11-mediated non-canonical Wnt signaling. Exp Cell Res. 2018;364:198-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 40. | Tseng JC, Lin CY, Su LC, Fu HH, Yang SD, Chuu CP. CAPE suppresses migration and invasion of prostate cancer cells via activation of non-canonical Wnt signaling. Oncotarget. 2016;7:38010-38024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 41. | Tian S, Peng P, Li J, Deng H, Zhan N, Zeng Z, Dong W. SERPINH1 regulates EMT and gastric cancer metastasis via the Wnt/β-catenin signaling pathway. Aging (Albany NY). 2020;12:3574-3593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (1)] |
| 42. | Wu Q, Ma J, Wei J, Meng W, Wang Y, Shi M. lncRNA SNHG11 Promotes Gastric Cancer Progression by Activating the Wnt/β-Catenin Pathway and Oncogenic Autophagy. Mol Ther. 2021;29:1258-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 43. | Guo Q, Xu J, Huang Z, Yao Q, Chen F, Liu H, Zhang Z, Lin J. ADMA mediates gastric cancer cell migration and invasion via Wnt/β-catenin signaling pathway. Clin Transl Oncol. 2021;23:325-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Ge Q, Hu Y, He J, Chen F, Wu L, Tu X, Qi Y, Zhang Z, Xue M, Chen S, Zhong J, Wang L. Zic1 suppresses gastric cancer metastasis by regulating Wnt/β-catenin signaling and epithelial-mesenchymal transition. FASEB J. 2020;34:2161-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |