Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7209
Peer-review started: January 14, 2022
First decision: March 8, 2022
Revised: April 18, 2022
Accepted: June 14, 2022
Article in press: June 14, 2022
Published online: July 26, 2022
Processing time: 177 Days and 9.6 Hours
Helicobacter pylori (H. pylori) infection is very common and affects a significant proportion of the world population. In contrast, the prevalence of small intestinal bacterial overgrowth (SIBO) in the general population is not well understood. There can be coexistence of both disease states in a given patient and their clinical symptoms may also overlap with one and another. There is no clear clinical guidelines for testing for and treating SIBO in patients with H. pylori infection. This review article explores the available evidence on the relationship between H. pylori infection and SIBO, diagnosis and treatment of these entities and also comments on associated non-gastrointestinal conditions.
Core Tip: This article explores the coexistence of small intestinal bacterial overgrowth (SIBO) in patients with Helicobacter pylori (H. pylori) infection including epidemiology and pathophysiologic mechanisms. It also reviews diagnosis and treatment of these entities and highlights current knowledge gaps and areas of future research. Currently, there are no guidelines for evaluation and management of co-existent SIBO in H. pylori infection.
- Citation: Dharan M, Wozny D. Helicobacter pylori infection and small intestinal bacterial overgrowth–more than what meets the eye. World J Clin Cases 2022; 10(21): 7209-7214
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7209.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7209
The concentration of microbiota increases as we traverse down the gastrointestinal tract, reaching up to 1011 bacteria per gram of stool in the colon. Compared with the colon, the small intestine normally has lower levels of microbial colonization. Excess bacteria in the small intestine that cause gastrointestinal symptoms are known as small intestinal bacterial overgrowth (SIBO). It has been postulated that SIBO occurs due to impaired gastric motility and/or acidity allowing for bacterial multiplication and enhanced colonization[1,2].
During active Helicobacter pylori (H. pylori) infection, gram-negative bacteria hydrolyze urea into ammonia and carbonic acid in the stomach. The ammonia byproduct buffers gastric acid leading to an increase in stomach pH to protect the organism and allow further proliferation. Over time, atrophy of the gastric mucosa occurs permitting further multiplication of the bacteria. The preferred treatment of H. pylori infection includes a course of proton pump inhibitor (PPI) therapy, which further raises gastric pH[3] and antibiotic agents, and may also cause dysbiosis and consequent gastrointestinal symptoms[4,5].
Both mucosal atrophy and gastric pH alterations have been proposed to predispose patients to SIBO[1,2,6]. However, SIBO rates in patients with active or recent H. pylori infection have not been widely studied and no universal guidelines exists regarding testing for the detection of SIBO either concurrently with H. pylori infection or posttreatment[7]. This article highlights available evidence on the relationship between H. pylori infection and SIBO as well as their association with other pathologies.
H. pylori infection affects more than half of the adult population worldwide with a prevalence rate in the United States between 20% and 40%[8]. Due to testing variability, the prevalence of SIBO in the general population is less well understood[9]. Studies have suggested an association of SIBO with altered anatomy, hypochlorhydria, dysmotility, immune deficiencies, small intestinal disease and PPI use[9,10]. The correlation between PPI and SIBO has been well established[11]. One meta-analysis of 19 eligible studies from 1994-2016 included 7055 subjects and found a 3-fold increased risk of SIBO in patients who had received PPI therapy[6].
PPI therapy is often prescribed for patients complaining of dyspepsia, a common complaint known to affect up to 21% of the world’s population[12]. H. pylori infection is also more common in patients with dyspepsia[3,12]. Dyspepsia is often treated with over-the-counter medications, empiric PPI therapy or sometimes antibiotic therapy[13]. Antibiotic therapy is known to disrupt the natural microbiome and predispose patients to dysbiosis[4] and potentially SIBO.
The association of SIBO with H. pylori infection was explored in a 2017 study that tested 109 patients for H. pylori infection and SIBO. Nineteen of 36 or 52.8% of H. pylori infection patients were found to have concurrent SIBO. However, only 16 of 73 or 21.9% of patients without H. pylori infection met the criteria for SIBO. These data suggest that the occurrence of SIBO is 2-fold greater in H. pylori infection patients than in uninfected patients[7]. These findings are supported by a 2018 study that found 53% or 62 of 116 patients with concurrent H. pylori infection and SIBO[14].
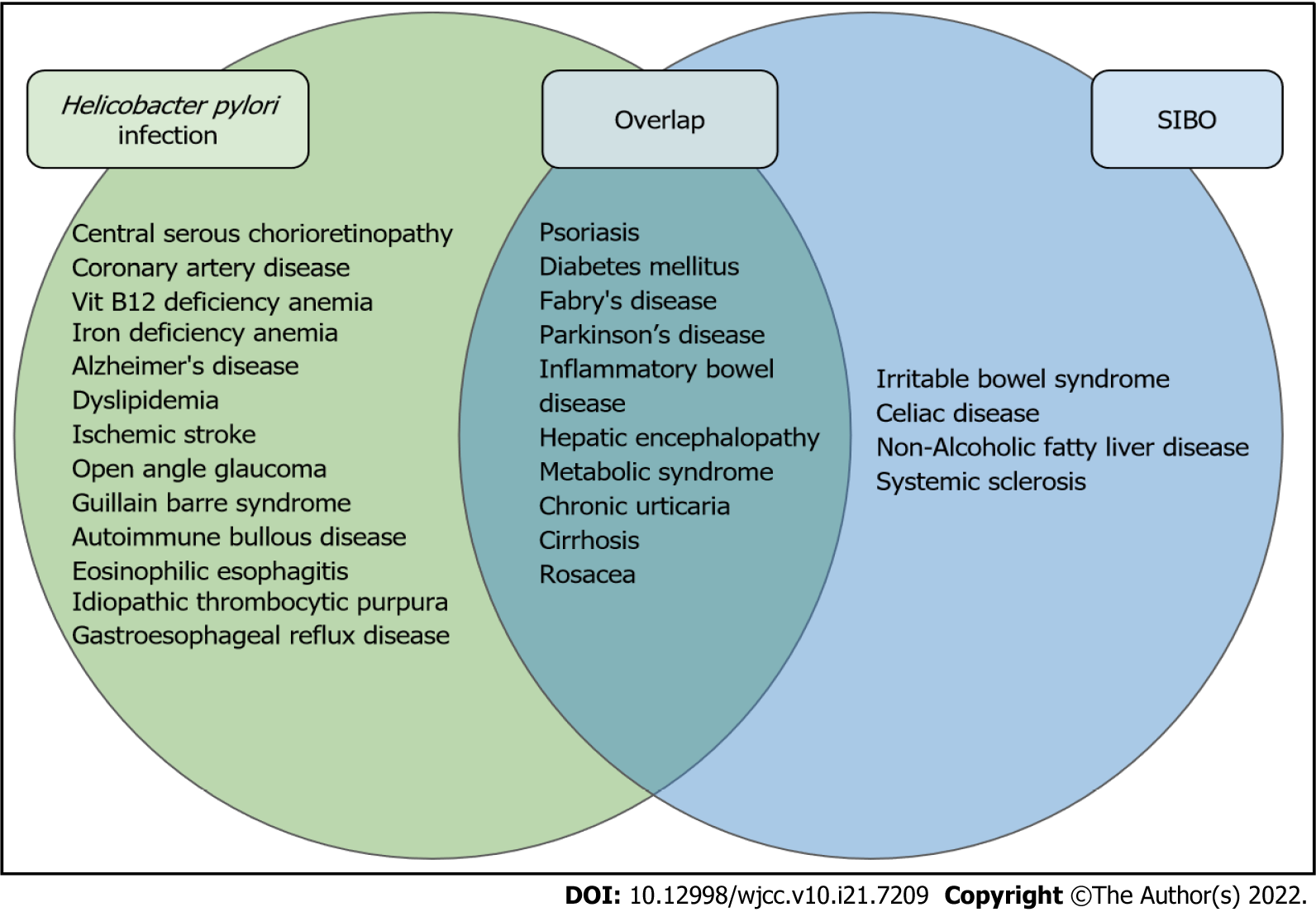
Several studies have reported an association between SIBO, H. pylori infection and a variety of pathologies (Figure 1). In comparison trials, both SIBO and H. pylori infection appear more common in cirrhosis[15], Fabry’s disease[16] and Parkinson's disease[17]. Independent reviews of H. pylori infection and SIBO show overlapping higher incidence in patients with diabetes mellitus, metabolic syndrome, hepatic encephalopathy, chronic urticaria, psoriasis and rosacea when compared to the general population[18-21]. In patients with cirrhosis and hepatic encephalopathy, the eradication of SIBO appears to improve encephalopathic symptoms; however, the treatment of H. pylori infection does not[15]. Inversely, the treatment of H. pylori infection has been documented to improve chronic spontaneous urticaria[18] and rosacea[19] but the treatment of SIBO has not.
In the general population, gastric secretions are strongly acidic with a pH range of 1 to 2. In non-H. pylori infection individuals, daily administration of 20 mg omeprazole has been shown to increase gastric pH by 2 to a pH range of 3 to 4. During H. pylori infection, individuals receiving this same dose of omeprazole showed increased stomach pH by a total of 4 to a pH range of 5 to 6[1]. Within the pH range of 5 to 6, enteric bacterial load can increase by as much as 1000-fold[22]. These bacteria are predominantly gram-negative anaerobes that produce gas with the fermentation of carbohydrates[2]. This gas fermentation allows for the detection of H. pylori infection by the urea breath test and the detection of SIBO by the hydrogen breath test. With that said, both bacterial load and the gas they produce contribute to the nonspecific constellation of gastrointestinal complaints described in SIBO and H. pylori infection.
PPIs are one of the most commonly prescribed medications for the treatment of several gastrointestinal symptoms. Numerous studies have shown an association between PPI use and SIBO[1,6,11]. However, most studies have not found a correlation between the timing of PPI use and SIBO[2].
Antibiotic-induced dysbiosis has been well documented[4]. While the theoretical possibility of SIBO after eradication therapy for H. pylori infection exists, there is a lack of evidence. Interestingly, recurrence of SIBO following antibiotic therapy for the treatment of bacterial overgrowth is well recognized[2]. It remains unclear whether this is due to regrowth of the primary microbiome or due to alteration of the gastrointestinal flora, known as dysbiosis, following antibiotic therapy.
The symptoms of SIBO and H. pylori infection are largely due to malabsorption of nutrients, inflammation and immune activation as a result of a high bacterial load and its byproducts. Although no single symptom is attributed to all cases of bacterial overgrowth, dyspepsia appears to be the most commonly reported in both SIBO[23] and H. pylori infection[3]. In up to two-thirds of patients with SIBO, symptoms include flatulence, bloating, abdominal cramping and diarrhea. Some studies have also reported nausea and constipation[2]. H. pylori infection is also frequently reported with flatulence, bloating, abdominal cramping and nausea[3]. This significant symptom overlap between reported symptoms of SIBO and H. pylori infection might, in some patients, be due to the coexistence of both conditions.
Testing for H. pylori infection is clinically indicated in patients with dyspepsia, unexplained iron deficiency anemia, current or past history of peptic ulcer disease, chronic nonsteroidal anti-inflammatory use, gastric cancer or gastric mucosa-associated lymphoid tissue lymphoma or idiopathic thrombocytopenic purpura[3,12,24,25]. Confirmation of H. pylori infection can be performed directly on biopsy specimens collected during endoscopy, by stool antigen test or by urea breath test. PPI therapy can impair the accuracy of these tests and should be discontinued prior to testing[23]. Stool antigen testing, however, maintains a high level of sensitivity 94% (95%CI: 93-95) and specificity 97% (95%CI: 96-98) regardless of PPI use[25]. Following treatment, clearance of H. pylori infection should be documented after 1 mo by the urea breath test or stool antigen testing[25,26].
Due in large part to a lack of international testing standards for the diagnosis of SIBO, there is a large amount of uncertainty regarding the prevalence of this condition. In 2020, the American College of Gastroenterology (ACG) first published SIBO-related clinical guidelines on diagnosis and treatment. However, evidence behind both testing and treatment for SIBO is currently low and recommendations remain conditional[2].
The diagnosis of SIBO can be made by direct small bowel aspirate or a less invasive hydrogen breath test. The ACG cites a collation study of literature from the North American Consensus for the diagnostic threshold of SIBO on direct small bowel aspirate as a bacterial count of > 103 colony forming units per milliliter[27]. Alternatively, the less invasive hydrogen breath test is performed by ingestion of a fixed quantity of carbohydrate, such as 75 g glucose or 10 g lactulose, and measuring exhaled hydrogen. The recommended diagnostic threshold for SIBO is a rise of at least 20 parts per million (ppm) in exhaled hydrogen above baseline within 90 min of ingestion of either glucose or lactulose[2]. Based on a systematic review, the sensitivity of hydrogen breath testing using lactulose substrate ranges from 31% to 68% and specificity 44% to 100% compared to glucose substrate with a sensitivity range from 20% to 93% and specificity of 30% to 86%[28].
Given the potential for coinfection with both H. pylori infection and SIBO, further research is needed to determine if co-treatment of both pathologies is preferred over first eliminating H. pylori infection before treating SIBO. For the treatment of SIBO, the most widely studied agent remains oral rifaximin. In 2017, a meta-analysis of 32 trials using rifaximin in the treatment of SIBO found the overall success of therapy to be 70.8%[27]. Alternately, studies have proposed the use of amoxicillin-clavulanate, ciprofloxacin, doxycycline, tetracycline, metronidazole, neomycin or trimethoprim-sulfamethoxazole[2]. These alternative antibiotics share some overlap with currently accepted H. pylori infection treatment regimens and might serve as a solution to treating coinfection.
Multiple H. pylori infection treatment regimens are acceptable for initial infection. Considerations such as penicillin allergy, previous macrolide exposure or high local resistance may impact treatment choices[25]. In areas where clarithromycin resistance is low, the ACG 2017 preferred treatment regimen is triple therapy with PPI, clarithromycin and amoxicillin or metronidazole. Where clarithromycin resistance is high, an alternative first-line regimen is a 10-14 d course of bismuth quadruple therapy consisting of bismuth, tetracycline, PPI and metronidazole[3]. A recent meta-analysis from 2021, however, suggests that regardless of local clarithromycin resistance, levofloxacin triple therapy with PPI, amoxicillin and levofloxacin has the highest overall composite eradication rate of 88.5% in Western countries[29]. When primary treatment fails, salvage therapy should be tailored to not include previously attempted antibiotics[25]. Based on current guidelines, bismuth quadruple therapy or levofloxacin-containing regimens are preferred salvage therapy options[3].
However, H. pylori is frequently resistant to metronidazole, with highly variable local resistance rates between 10%-90%[29,30]. One study comparing treatment eradication in H. pylori infection and SIBO coinfection suggests nearly equivalent eradication rates when substituting rifaximin (59.4% eradication) for metronidazole (63% eradication) when using triple therapy[14]. Although these findings suggest that rifaximin-containing regimens are acceptable, further studies are required to determine the best treatment option.
Based on the current literature review, SIBO appears to have an increased prevalence in patients with H. pylori infection compared to the general population[7,14]. While the “test and treat” strategy[13] for H. pylori infection in patients with dyspepsia has been validated, no clear recommendations currently exist for testing/treating SIBO in patients with H. pylori infection. Several extra gastrointestinal conditions appear to be associated with both SIBO[15-18] and H. pylori infection[19-21] and dysbiosis due to the attempted treatment for H. pylori infection may be related to the microbiome-mediated pro-inflammatory state. It is therefore important to recognize the signs and symptoms of H. pylori infection and treat the infection as well as the associated dysbiosis bearing in mind that persistence of gastrointestinal symptoms despite eradication of H. pylori infection could suggest coexisting SIBO.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chojnacki J, Poland; Fujimori S, Japan S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Husebye E. The pathogenesis of gastrointestinal bacterial overgrowth. Chemotherapy. 2005;51 Suppl 1:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Pimentel M, Saad RJ, Long MD, Rao SSC. ACG Clinical Guideline: Small Intestinal Bacterial Overgrowth. Am J Gastroenterol. 2020;115:165-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 252] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 3. | Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 1018] [Article Influence: 127.3] [Reference Citation Analysis (1)] |
| 4. | McDonnell L, Gilkes A, Ashworth M, Rowland V, Harries TH, Armstrong D, White P. Association between antibiotics and gut microbiome dysbiosis in children: systematic review and meta-analysis. Gut Microbes. 2021;13:1-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 142] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 5. | Angelucci F, Cechova K, Amlerova J, Hort J. Antibiotics, gut microbiota, and Alzheimer's disease. J Neuroinflammation. 2019;16:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 284] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 6. | Su T, Lai S, Lee A, He X, Chen S. Meta-analysis: proton pump inhibitors moderately increase the risk of small intestinal bacterial overgrowth. J Gastroenterol. 2018;53:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (2)] |
| 7. | Enko D, Kriegshäuser G. Functional 13C-urea and glucose hydrogen/methane breath tests reveal significant association of small intestinal bacterial overgrowth in individuals with active Helicobacter pylori infection. Clin Biochem. 2017;50:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Peleteiro B, Bastos A, Ferro A, Lunet N. Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Dig Dis Sci. 2014;59:1698-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 228] [Article Influence: 20.7] [Reference Citation Analysis (7)] |
| 9. | Grace E, Shaw C, Whelan K, Andreyev HJ. Review article: small intestinal bacterial overgrowth--prevalence, clinical features, current and developing diagnostic tests, and treatment. Aliment Pharmacol Ther. 2013;38:674-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 10. | Quigley EMM. The Spectrum of Small Intestinal Bacterial Overgrowth (SIBO). Curr Gastroenterol Rep. 2019;21:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 11. | Compare D, Pica L, Rocco A, De Giorgi F, Cuomo R, Sarnelli G, Romano M, Nardone G. Effects of long-term PPI treatment on producing bowel symptoms and SIBO. Eur J Clin Invest. 2011;41:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Du LJ, Chen BR, Kim JJ, Kim S, Shen JH, Dai N. Helicobacter pylori eradication therapy for functional dyspepsia: Systematic review and meta-analysis. World J Gastroenterol. 2016;22:3486-3495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 13. | Chiba N, Van Zanten SJ, Sinclair P, Ferguson RA, Escobedo S, Grace E. Treating Helicobacter pylori infection in primary care patients with uninvestigated dyspepsia: the Canadian adult dyspepsia empiric treatment-Helicobacter pylori positive (CADET-Hp) randomised controlled trial. BMJ. 2002;324:1012-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 154] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Konrad P, Chojnacki J, Gąsiorowska A, Rudnicki C, Kaczka A, Chojnacki C. Therapeutic efficacy of amoxicillin and rifaximin in patients with small intestinal bacterial overgrowth and Helicobacter pylori infection. Prz Gastroenterol. 2018;13:213-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Abid S, Kamran M, Abid A, Butt N, Awan S, Abbas Z. Minimal Hepatic Encephalopathy: Effect of H. pylori infection and small intestinal bacterial overgrowth treatment on clinical outcomes. Sci Rep. 2020;10:10079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Franceschi F, Zampetti A, Gigante G, Gasbarrini A. Helicobacter pylori and small intestinal bacterial overgrowth affect gastrointestinal symptoms in Fabry's disease. Dig Liver Dis. 2015;47:618-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Tan AH, Mahadeva S, Thalha AM, Kiew CK, Yeat CM, Ng SW, Ang SP, Chow SK, Than KM, Hanafi NS, Ibrahim NM, Gibson PR, Fox SH, Lim SY. Helicobacter pylori and small intestinal bacterial overgrowth in Parkinson's disease: Prevalence and clinical significance. J Neurol Sci. 2013;333:e72. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Campanati A, Gesuita R, Giannoni M, Piraccini F, Sandroni L, Martina E, Conocchiari L, Bendia E, Di Sario A, Offidani A. Role of small intestinal bacterial overgrowth and Helicobacter pylori infection in chronic spontaneous urticaria: a prospective analysis. Acta Derm Venereol. 2013;93:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Gravina A, Federico A, Ruocco E, Lo Schiavo A, Masarone M, Tuccillo C, Peccerillo F, Miranda A, Romano L, de Sio C, de Sio I, Persico M, Ruocco V, Riegler G, Loguercio C, Romano M. Helicobacter pylori infection but not small intestinal bacterial overgrowth may play a pathogenic role in rosacea. United European Gastroenterol J. 2015;3:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Gravina AG, Priadko K, Ciamarra P, Granata L, Facchiano A, Miranda A, Dallio M, Federico A, Romano M. Extra-Gastric Manifestations of Helicobacter pylori Infection. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 21. | Losurdo G, Salvatore D'Abramo F, Indellicati G, Lillo C, Ierardi E, Di Leo A. The Influence of Small Intestinal Bacterial Overgrowth in Digestive and Extra-Intestinal Disorders. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Sharma BK, Santana IA, Wood EC, Walt RP, Pereira M, Noone P, Smith PL, Walters CL, Pounder RE. Intragastric bacterial activity and nitrosation before, during, and after treatment with omeprazole. Br Med J (Clin Res Ed). 1984;289:717-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 151] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Chojnacki C, Konrad P, Błońska A, Chojnacki J, Mędrek-Socha M. Usefulness of the hydrogen breath test in patients with functional dyspepsia. Prz Gastroenterol. 2020;15:338-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Lopes AI, Vale FF, Oleastro M. Helicobacter pylori infection - recent developments in diagnosis. World J Gastroenterol. 2014;20:9299-9313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 25. | Crowe SE. Helicobacter pylori Infection. N Engl J Med. 2019;380:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 273] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 26. | Rezaie A, Buresi M, Lembo A, Lin H, McCallum R, Rao S, Schmulson M, Valdovinos M, Zakko S, Pimentel M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am J Gastroenterol. 2017;112:775-784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 395] [Cited by in RCA: 516] [Article Influence: 64.5] [Reference Citation Analysis (1)] |
| 27. | Gatta L, Scarpignato C. Systematic review with meta-analysis: rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Aliment Pharmacol Ther. 2017;45:604-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 28. | Khoshini R, Dai SC, Lezcano S, Pimentel M. A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig Dis Sci. 2008;53:1443-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 29. | Rokkas T, Gisbert JP, Malfertheiner P, Niv Y, Gasbarrini A, Leja M, Megraud F, O'Morain C, Graham DY. Comparative Effectiveness of Multiple Different First-Line Treatment Regimens for Helicobacter pylori Infection: A Network Meta-analysis. Gastroenterology. 2021;161:495-507.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 30. | Gasbarrini A, Gasbarrini G, Pelosini I, Scarpignato C. Eradication of Helicobacter pylori: are rifaximin-based regimens effective? Digestion. 2006;73 Suppl 1:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |