Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.6999
Peer-review started: November 1, 2021
First decision: March 23, 2022
Revised: April 6, 2022
Accepted: May 22, 2022
Article in press: May 22, 2022
Published online: July 16, 2022
Processing time: 245 Days and 17.1 Hours
Neonatal hyperbilirubinemia is a common problem faced by pediatricians. The role of genetic factors in neonatal jaundice has been gradually recognized. This study aims to identify genetic variants that influence the bilirubin level in five patients using next-generation sequencing (NGS).
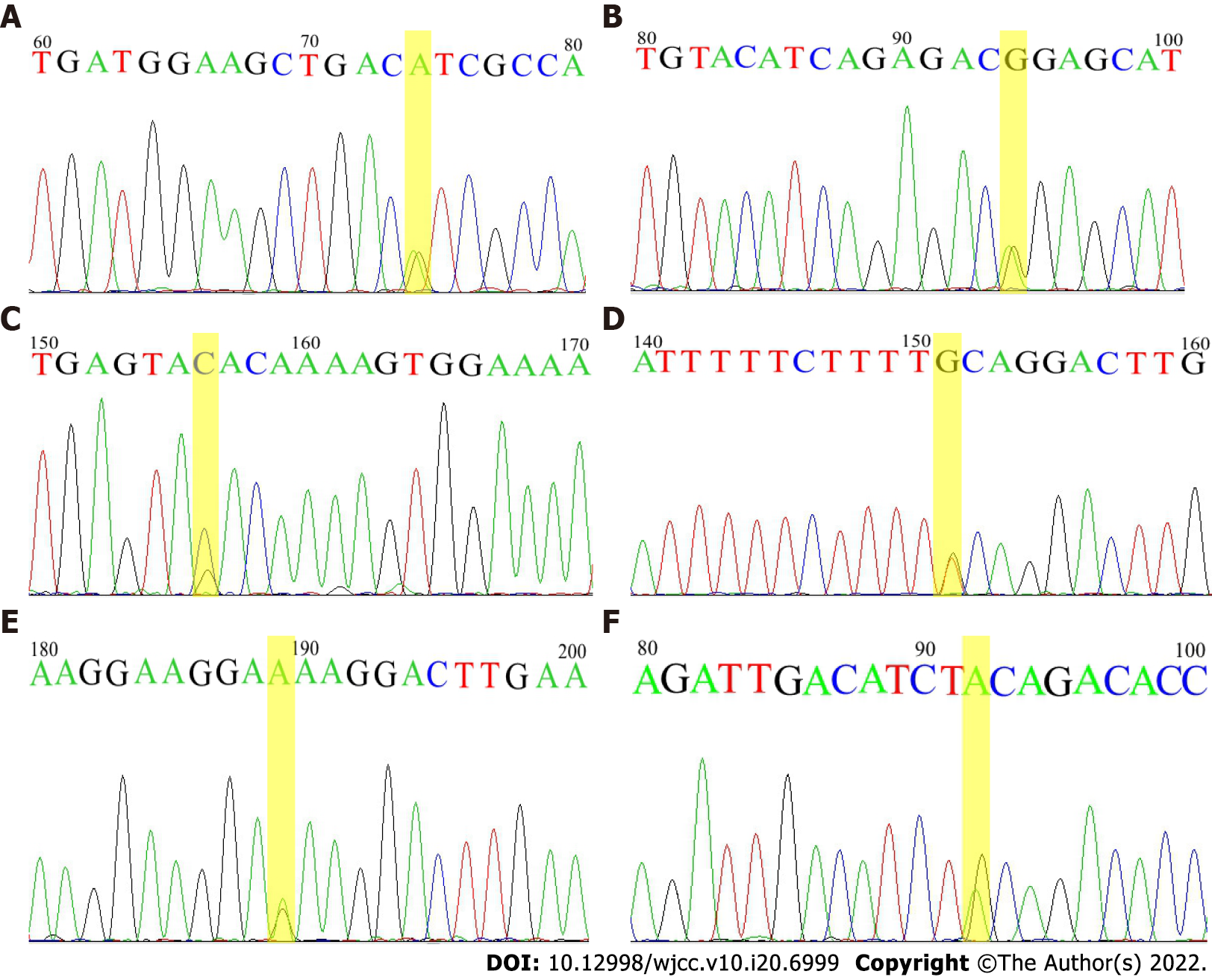
Five neonates with severe hyperbilirubinemia were retrospectively studied. They exhibited bilirubin encephalopathy, hypothyroidism, ABO blood type incompatibility hemolysis, glucose-6-phosphate dehydrogenase (G6PD) deficiency and premature birth, respectively. A customized 22-gene panel was designed, and NGS was carried out for these neonates. Eight variations (G6PD c.G1388A, HBA2 c.C369G, ABCC2 c.C3825G, UGT1A1 c.G211A, SPTB c.A1729G, EPB41 c.G520A, c.1213-4T>G and c.A1474G) were identified in these five neonates. Genetic mutations of these genes are associated with G6PD deficiency, thalassemia, Dubin-Johnson syndrome, Gilbert syndrome, hereditary spherocytosis, and hereditary elliptocytosis. One of the neonates was found to have compound variants of the EPB41 splice site c.1213-4T>G and c.G520A (p.E174K), but no elliptocyte was seen on his blood smear of 4 years old.
Pathological factors of severe neonatal hyperbilirubinemia are complicated. Genetic variants may play an important role in an increased risk of neonatal hyperbilirubinemia, and severe jaundice in neonates may be related to a cumulative effect of genetic variants.
Core Tip: This study has emphasized that for severe hyperbilirubinemia neonates, apart from the predominant glucose-6-phosphate dehydrogenase deficiency and ABO hemolysis, other underlying genetic factors such as thalassemia or red blood cell membrane disorders should be considered, and genetic detection may be taken into consideration for early diagnosis of severe hyperbilirubinemia in neonates.
- Citation: Lin F, Xu JX, Wu YH, Ma YB, Yang LY. Clinical features and genetic variations of severe neonatal hyperbilirubinemia: Five case reports. World J Clin Cases 2022; 10(20): 6999-7005
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/6999.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.6999
Hyperbilirubinemia is one of the most prevalent clinical symptoms in neonates. The main feature of neonatal hyperbilirubinemia is increased bilirubin production, which leads to abnormal bilirubin deposition in the mucosa, skin and organs. Severe hyperbilirubinemia may cause bilirubin encephalopathy and kernicterus and can result in hearing loss, cerebral palsy and disorders of mental development[1]. The importance of genetic contributions in neonatal jaundice has been recognized in recent years[2-5]. We previously demonstrated that the genetic polymorphisms of uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1), solute carrier organic anion transporter family member 1B1 (SLCO1B1), SLCO1B3 and glucose-6-phosphate dehydrogenase (G6PD) contributed to an increased risk of neonatal hyperbilirubinemia, which reflected the complexity of pathological jaundice[6,7].
Next-generation sequencing (NGS) is an effective tool for genomic screening and is widely used for the molecular diagnosis of pediatric diseases. Here, we present five neonates with severe hyperbilirubinemia and use NGS technology to explore the genetic variants.
Case 1: A 5-d-old male neonate was referred to our hospital with chief complaints of vomiting and jaundiced skin for 4 d.
Case 2: A 6-d-old male neonate was referred to our hospital with chief complaint of jaundiced skin for 3 d.
Case 3: A 4-d-old male neonate with chief complaint of jaundiced skin for 1 d.
Case 4: A 28-d old female neonate was admitted to our hospital with chief complaints of yellow skin and weight stagnation for 25 d.
Case 5: A 7-d-old male neonate was admitted to our hospital with chief complaint of yellow skin for 5 d.
Case 1: The neonate was delivered vaginally with a birth weight of 3.15 kg and received formula feeding. His mother was para 2 and gravida 2. He appeared icteric and vomited milk on day 2 after birth. The yellowing of his skin increased gradually. He was admitted on day 5.
Case 2: The patient was a cesarean born neonate with a birth weight of 3.10 kg. He was breastfed. He had a mother of gravida 2 and para 2. He was found with jaundiced skin for 3 d.
Case 3: The neonate was cesarean born to a mother of gravida 1, para 1, and the blood type of his mother was O. His birth weight was 3.30 kg. He was found with yellow skin for 1 d.
Case 4: The neonate was born to a mother of gravida 1 and para 1 by cesarean section, she was breastfed, and did not receive neonatal screening, she showed yellow skin at 4 days of birth, her yellow skin continued to her admission in our hospital (age: 28 d), her body weight did not increased after her birth (birth weight 3.25 kg).
Case 5: The neonate was delivered vaginally because of premature rupture of the membrane, his birth weight was 2.65 kg. His mother was gravida 1 and para 1, he was admitted to our hospital due to yellow skin for 5 d.
The neonates had no other previous medical history.
The neonates had no significant family history.
Case 1: The neonate was found with severe yellow skin and mucous, and abdominal distention were observed during physical examination.
Case 2: Severe yellow skin and mucous were observed during physical examination. His muscle tension and limb activities appeared normal, and he did not show fever or convulsion.
Case 3: Mild yellow skin and yellow sclera.
Case 4: Severe yellow skin and sclera.
Case 5: Severe yellow skin and mild yellow mucous were observed during physical examination.
Case 1: A blood test was performed. His hemoglobin (Hb) was 147g/L, and his total bilirubin (TBIL) increased significantly (719.50 μmol/L, normal upper limit: 20.1 μmol/L), and his G6PD activity was 0.4 (normal range: 1.1-2.5) (Table 1).
| Case ID | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
| Gender | Male | Male | Male | Female | Male |
| Admission time (d) | 5 | 12 | 4 | 28 | 7 |
| Gestational age (wk) | 39 + 5 | 39 | 40 + 3 | 40 | 35 + 4 |
| Birth weight (kg) | 3.15 | 3.1 | 3.3 | 3.25 | 2.65 |
| Mode of delivery | Vaginal | Cesarean | Cesarean | Cesarean | Vaginal |
| Feeding patterns | Formula | Breast | Breast | Formula | Breast |
| Postnatal growth ratardation | + | - | - | + | - |
| Skin and mucous membrane yellowing | + | + | + | + | + |
| Time of jaundice occur after birth (d) | 2 | 3 | 3 | 4 | 2 |
| Maximum TBIL levels (μmol/L)a | 719.5 | 527.87 | 429.6 | 538.7 | 584.4 |
| Age at maximum TBIL (d) | 5 | 12 | 4 | 28 | 7 |
| Phototherapy | + | + | + | + | + |
| RBC transfusion | - | + | - | - | - |
| IVIGb or albumin therapy | + | + | + | - | - |
| Clinical diagnosis | G6PD deficiency; Congenital hypertrophic pyloric stenosis; Neonatal hyperbilirubinemia | G6PD deficiency; Anemia; Bilirubin encephalopathy | ABO blood type incompatibility hemolytic disease | Congenital hypothyroidism; Neonatal hyperbilirubinemia | Premature delivery; Neonatal hyperbilirubinemia |
Case 2: The TBIL level was 360 μmol/L on admission. Phototherapy was carried out three times, and the TBIL level decreased to 260 μmol/L on day 9 after birth. He was released and resumed breastfeeding. However, his skin yellowing gradually increased postdischarge, so he was readmitted on day 12 of life for treatment. He had a TBIL level of 527.87 μmol/L, and a complete blood count revealed a Hb level of 105 g/L. Moreover, the G6PD activity was 0.5.
Case 3: His blood test showed Hb levels of 143 g/L, and he was blood group B and Rh D-positive. Free antibody tests and antibody release tests were positive. He was diagnosed with ABO incompatibility with a TBIL level of 429.6 μmol/L on day 4 after birth, and he had a heterozygous EPB41 c.A1474G (p.T492A) mutation (Table 2).
| Case numbers | Gene name | rs number | cDNA/protein change | Mutationtaster prediction | PolyPhen2 | SIFT | Genotype | Associated disorder |
| Patient 1 | EPB41 | rs201231112 | c.G520A/p.E174K | Disease-causing | Probably damaging | Tolerated | Hetero | Elliptocytosis |
| EPB41 | rs188648724 | c.1213-4T>G | Disease-causing | Hetero | Elliptocytosis | |||
| G6PD | rs72554664 | c.G1388A/p.R463H | Disease-causing | Damaging | Deleterious | Hemiz | G6PD deficiency | |
| Patient 2 | G6PD | rs72554664 | c.G1388A/p.R463H | Disease-causing | Damaging | Deleterious | Hemiz | G6PD deficiency |
| HBA2 | rs41479347 | c.C369G/p.H123Q | Disease-causing | Probably damaging | Deleterious | Hetero | Thalassemia | |
| Patient 3 | EPB41 | rs778090351 | c.A1474G/p.T492A | Disease-causing | Damaging | Deleterious | Hetero | Elliptocytosis |
| Patient 4 | SPTB | rs144668591 | c.A1729G/p.I577V | Disease-causing | Probably damaging | Deleterious | Hetero | Elliptocytosis |
| ABCC2 | rs554976086 | c.C3825G/p.Tyr1275X | Disease-causing | Probably damaging | Deleterious | Hetero | Dubin-Johnson syndrome | |
| Patient 5 | UGT1A1 | rs4148323 | c.G211A/p.G71R | Polymorphism | Probably damaging | Deleterious | Hetero | Gilbert's syndrome |
Case 4: Serological examination showed increased TBIL (538.7 μmol/L) and thyrotropin levels (150 mIu/L, reference 0.35-5.5) with low free triiodothyronine (0.1 pmol/L, reference 3.5-6.5) and free thyroxine (0.60 pmol/L, reference 11.5-22.7) levels. Two heterozygous mutations, SPTB c.A1729G (p.I577V) and ABCC2 c.3825C>G (p.Tyr1275X), were confirmed in this neonate.
Case 5: The TBIL level was 538.7 μmol/L on day 7 after birth.
Case 1: A barium meal examination showed congenital hypertrophic pyloric stenosis (CHPS).
Case 2: Head magnetic resonance imaging showed signal changes on T1WI and T2WI.
Case 1: The neonate was diagnosed with neonatal hyperbilirubinemia and CHPS.
Case 2: He was diagnosed as G6PD deficiency, neonatal hyperbilirubinemia, moderate anemia and bilirubin encephalopathy.
Case 3: The neonate was diagnosed as ABO blood type incompatibility hemolytic disease of newborn.
Case 4: The neonate was diagnosed as hypothyroidism and hyperbilirubinemia.
Case 5: The neonate was diagnosed as neonatal hyperbilirubinemia. This neonate was heterozygous for the UGT1A1 c.G211A (p.G71R) mutation.
Case 1: He received phototherapy for three days (the indication of phototherapy according to the expert consensus for diagnosis and treatment of neonatal hyperbilirubinemia in Chinese Journal of Pediatrics 2014)[8]. Subsequently, he was transferred to the Women and Children's Hospital of Guangdong Province and underwent a surgical operation for CHPS. Two weeks later, he recovered and was discharged. Genetic analysis revealed that he carried the G6PD c.G1388A mutation, and he was also a compound heterozygote for the c.1213-4T>G and c.G520A (p.E174K) mutations of the EPB41 gene (Table 2).
Case 2: He received phototherapy, alkalization of urine, and vitamin K1 supplementation and was subjected to red blood cell (RBC) transfusion (Hb dropping to 95 g/L). The G6PD c.G1388A and HBA2 c.C369G (Hb Westmead) mutations were identified in this neonate (Table 2).
Case 3: The neonate received routine phototherapy.
Case 4: Levothyroxine therapy was initiated (48 μg daily) to adjust the inadequate secretion of thyroxine.
Case 5: The neonate received routine phototherapy.
The five children were followed up, and their intelligence and development were normal.
Pathologic jaundice refers to an abnormal increase in serum bilirubin levels, and a pathological investigation to determine hyperbilirubinemia is important in clinical practice to prevent the adverse reactions of severe jaundice. Routine laboratory test results may fail to explain jaundice severity, and we hypothesized that genetic factors might contribute to the development of severe hyperbilirubinemia. In the present study, we analyzed five neonates who had severe hyperbilirubinemia due to G6PD deficiency, ABO blood type incompatibility hemolysis, premature delivery and hypothyroidism, which were the most common etiological factors of pathological jaundice in the neonatal period. We also confirmed nine gene variants through NGS.
Based on our results, all five neonates carried at least one genetic variant, and the associated disorders included G6PD deficiency, α-thalassemia, hereditary elliptocytosis, hereditary spherocytosis, Dubin-Johnson syndromes and Gilbert’s syndrome[9-12]. A previous study reported a case of neonate that had both G6PD deficiency and a heterozygous variant of the UGT1A1 promoter (TA)6/(TA)7 who developed severe hyperbilirubinemia and bilirubin encephalopathy[13]. Butorac Ahel et al[14] reported a case of infant with unusually severe hyperbilirubinemia due to the coexistence of hereditary spherocytosis and homozygosity for UGT1A1 (TA)7. Our report of 5 patients also confirmed that genetic variations of susceptible genes (Figure 1) contributed to the development of neonatal hyperbilirubinemia and severe jaundice in neonates might be related to a cumulative effect of genetic variants.
Notably, to our knowledge, hereditary elliptocytosis (HE) is a congenital red cell membrane disorder characterized by elliptically shaped cells, and the EPB41 genetic variant is one of the most identified defects. Case 1 was found to have variants for both the EPB41 splice site c.1213-4T>G and c.G520A (p.E174K). In silico predictions concluded that the above two variants were likely to be damaging or possibly damaging. Generally, HE patients with a single heterozygous mutation are often considered to be asymptomatic, but homozygous mutations or compound heterozygous mutations exhibit moderate to severe hemolysis[15]. However, no splenomegaly, reticulocyte level abnormality or hemolytic anemia was observed in this patient after follow-up for 4 years (data not shown). We propose that the two genetic variations may aggravate the jaundice levels in this case. A previous study noted that spherical-shaped red cells were rarely observed in neonates, which was likely due to the immature RBC membranes of neonates[16]. This neonate did not receive a blood smear when he was in the hospital. He received a routine examination at 4 years old, and no elliptocyte was seen on his blood smear. The absence of elliptical-shaped erythrocyte in this patient reflects the high heterogeneity of hereditary elliptocytosis. Moreover, although case 4 was clinically diagnosed with hypothyroidism, no genetic variation associated with hypothyroidism was found (data not shown).
In conclusion, our study emphasized that for neonates with severe hyperbilirubinemia, apart from the predominant G6PD deficiency and ABO blood type incompatibility hemolysis, other underlying genetic factors, such as thalassemia or RBC membrane disorders, should be considered. Furthermore, genetic detection should be considered for the early diagnosis of severe hyperbilirubinemia in neonates.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Govindarajan KK, India; Huang CS, Taiwan A-Editor: Yao QG, China S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Olusanya BO, Kaplan M, Hansen TWR. Neonatal hyperbilirubinaemia: a global perspective. Lancet Child Adolesc Health. 2018;2:610-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 2. | He CH, Qu Y. [Research advances in neonatal hyperbilirubinemia and gene polymorphisms]. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Huang MJ, Lin YC, Liu K, Chang PF, Huang CS. Effects of variation status and enzyme activity for UDP-glucuronosyltransferase 1A1 gene on neonatal hyperbilirubinemia. Pediatr Neonatol. 2020;61:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Zhou JF, Luo JY, Zhu WB, Yang CY, Zeng YL, Qiu XL. Association between genetic polymorphism of heme oxygenase 1 promoter and neonatal hyperbilirubinemia: a meta-analysis. J Matern Fetal Neonatal Med. 2021;34:12-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Li Z, Song L, Hao L. The role of UGT1A1 (c.-3279 T > G) gene polymorphisms in neonatal hyperbilirubinemia susceptibility. BMC Med Genet. 2020;21:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Yang H, Wang Q, Zheng L, Lin M, Zheng XB, Lin F, Yang LY. Multiple Genetic Modifiers of Bilirubin Metabolism Involvement in Significant Neonatal Hyperbilirubinemia in Patients of Chinese Descent. PLoS One. 2015;10:e0132034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Yang H, Wang Q, Zheng L, Zheng XB, Lin M, Zhan XF, Yang LY. Clinical Significance of UGT1A1 Genetic Analysis in Chinese Neonates with Severe Hyperbilirubinemia. Pediatr Neonatol. 2016;57:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Editorial Board of Chinese Journal of Pediatrics; Subspecialty Group of Neonatology, The Society of Pediatrics, Chinese Medical Association. [Experts consensus on principles for diagnosis and treatment of neonatal jaundice]. Zhonghua Er Ke Za Zhi. 2010;48:685-686. [PubMed] |
| 9. | Boo NY, Sin S, Chee SC, Mohamed M, Ahluwalia AK, Ling MM, Ong HK. Genetic Factors and Delayed TSB Monitoring and Treatment as Risk Factors Associated with Severe Hyperbilirubinemia in Term Neonates Admitted for Phototherapy. J Trop Pediatr. 2020;66:569-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Kaplan M, Wong RJ, Stevenson DK. Hemolysis and Glucose-6-Phosphate Dehydrogenase Deficiency-Related Neonatal Hyperbilirubinemia. Neonatology. 2018;114:223-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Li Y, Wu T, Chen L, Zhu Y. Associations between G6PD, OATP1B1 and BLVRA variants and susceptibility to neonatal hyperbilirubinaemia in a Chinese Han population. J Paediatr Child Health. 2019;55:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Shwe S, Boo NY, Ong HK, Chee SC, Maslina M, Ling MMM, Ahluwalia AK. Haemoglobin Constant Spring (HbA2: c.427T>C) and Haemoglobin Adana (HbA2: c.179G>A) in jaundiced Malaysian term neonates with clinically significant hyperbilirubinemia. Malays J Pathol. 2020;42:253-257. [PubMed] |
| 13. | Mukthapuram S, Dewar D, Maisels MJ. Extreme Hyperbilirubinemia and G6PD Deficiency With No Laboratory Evidence of Hemolysis. Clin Pediatr (Phila). 2016;55:686-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Butorac Ahel I, Baraba Dekanic K, Palcevski G, Roganovic J. An Infant With Unusually High Unconjugated Hyperbilirubinemia Due to Coexistence of Hereditary Spherocytosis and Gilbert Syndrome. J Pediatr Hematol Oncol. 2018;40:e127-e128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Ma S, Qin J, Wei A, Li X, Qin Y, Liao L, Lin F. Novel compound heterozygous SPTA1 mutations in a patient with hereditary elliptocytosis. Mol Med Rep. 2018;17:5903-5911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Liu C, Eun HS, Nah H, Lee ST, Choi JR, Kim HO. Newborn hereditary elliptocytosis confirmed by familial genetic testing. Int J Lab Hematol. 2020;42:e20-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |