Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.6960
Peer-review started: September 26, 2021
First decision: March 14, 2022
Revised: March 16, 2022
Accepted: May 26, 2022
Article in press: May 26, 2022
Published online: July 16, 2022
Processing time: 281 Days and 2.5 Hours
This paper introduces a case of recurrent keratoacanthoma (KA). KA is a self-healing disease. Recurrence after surgical resection is rare. In this case, the local application of retinoic acid ointment after the second operation achieved a good prognosis after 2 years of follow-up.
A 76-year-old male patient was admitted to the hospital for "lower lip rupture and scab for 3 mo". Treatment: A rectangular incision was made in the healthy tissue about 3 mm outside the periphery of the lower lip mass, and a modified Bernard sliding flap was designed to completely remove the mass. Pathology showed (lower lip) KA. When the patient returned 6 mo after surgery, the middle mucosa of the lower lip had a bulge with a diameter of about 0.5 cm. The boundary was still clear, the surface was ulcerated. A recurrence of lower lip KA was suspected and a fan-shaped incision was performed in the healthy tissue about 5 mm outside the lesion to completely resect. Pathological showed lower lip KA had recurred. Topical application of tretinoin cream was applied once a day for 3 mo. The lower lip wounds were clean at the 2-year postoperative follow-up and the mucosa was normal.
Adjuvant retinoic acid treatment after KA surgical resection can achieve good results.
Core Tip: Keratoacanthoma of the lower lip is a rare benign tumour with unique clinical and pathological characteristics which are very similar to those of well-differentiated squamous cell carcinoma of the lower lip. This article reports on an elderly male patient with recurrent lower lip keratoacanthoma, describing its clinical and pathological characteristics and treatment measures. Through reviewing the relevant literature and analysing the causes of the disease, clinical manifestations, pathological characteristics, treatment options, and causes of recurrence, it is possible to further deepen the understanding of the disease and help determine the diagnosis and treatment.
- Citation: Liu XG, Liu XG, Wang CJ, Wang HX, Wang XX. Lower lip recurrent keratoacanthoma: A case report. World J Clin Cases 2022; 10(20): 6960-6965
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/6960.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.6960
This study presents the case of an elderly male patient with recurrent lower lip keratoacanthoma (KA), describing its clinical and pathological characteristics and treatment measures. This patient experienced a recurrence of a previous KA which was successfully treated with surgical resection. Local tretinoin cream was used after the second operation. No recurrence was seen at the 2-year follow-up and the cosmetic results were excellent. We believe that our study makes a significant contribution to the literature given the similar features of KA and squamous cell carcinoma. Our case study may therefore help clinicians find features and tests to make this distinction clearer in the clinical setting. Further, KA rarely recurs. In this case, tretinoin ointment was applied locally after the second surgical resection, and no recurrence occurred. Tretinoin as an anti-keratosis drug has a good effect on preventing recurrence after KA surgery.
Lower lip rupture and scab for 3 mo.
Three months ago, the patient felt that there was a "rice grain" size ulcer on the lower lip, and local itching associated with discomfort. No treatment was given. Recent one month, the lesions have gradually increased, highlighting the mucosal surface of the lower lip, accompanied by local pain. After the application of erythromycin ointment, the pain symptoms were reduced, and the size of the lesion remained unchanged.
The patient was in good health, denied a history of lower lip trauma and infection, denied a history of food and drug allergy, and denied a history of family genetic disease.
The patient had a smoking and drinking history of 6 cigarettes and 100 mL, respectively, for 20 years.
An irregular bulge with a size of about 2.0 cm × 3.0 cm could be seen in the middle of the lower lip mucosa. The boundary was still clear, the surface was irregular and rough, with yellow-white exuding scabs, some local covered blood scabs, and a hard texture. There was also local tenderness, with the outer lesion border close to the border between the lips and skin, the inner border about 1 cm away from the vestibular sulcus, and local scabs on both sides. Palpable swollen lymph nodes were detected in the submandibular area on both sides, which were movable, tender, and about 1.0 cm in diameter at most.
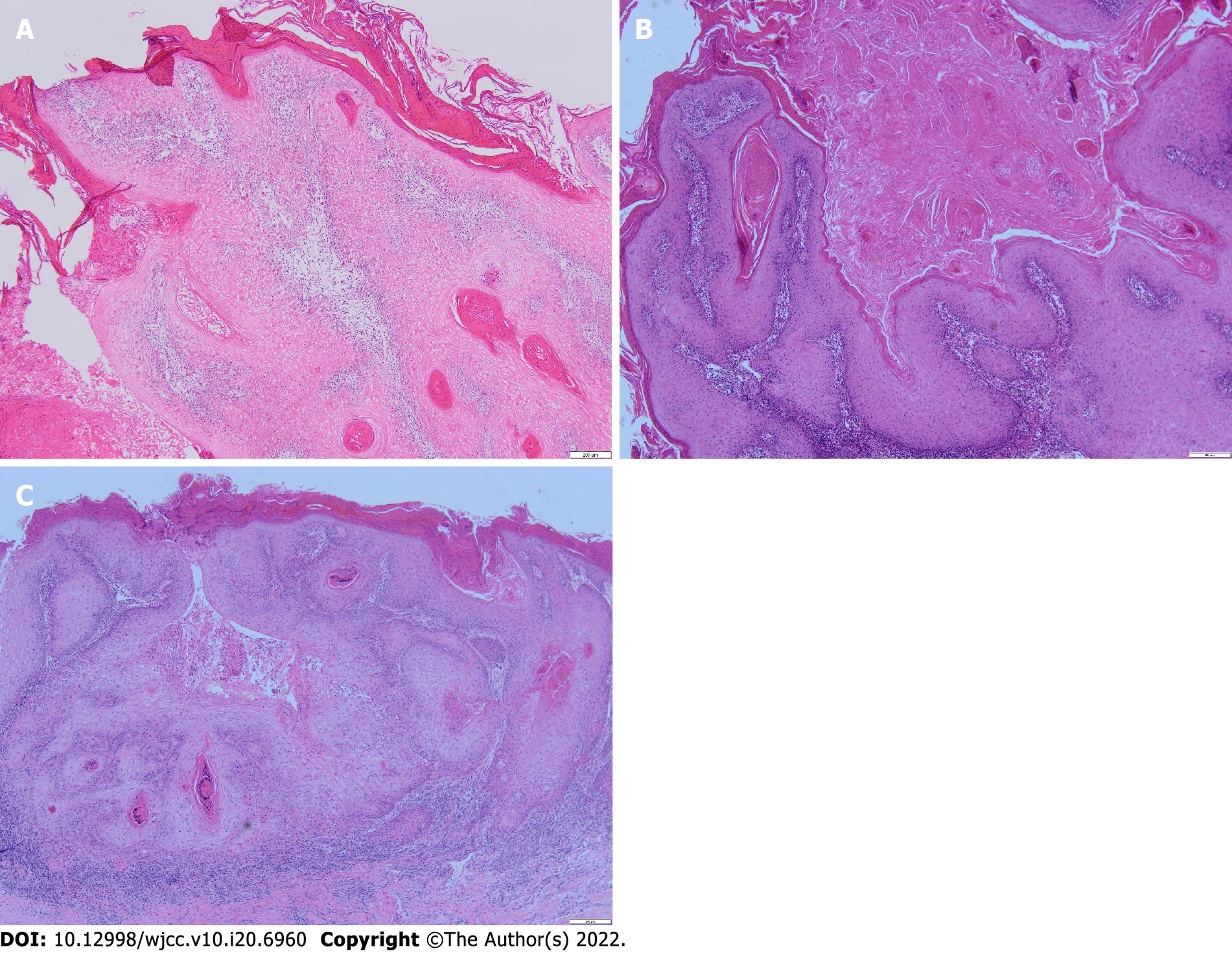
A rapid pathology examination during the operation revealed that the lesion was a KA. Postoperative routine pathology showed lower lip stratified squamous epithelium hyperplasia, local enlarged epithelial spikes, malkeratosis, and a large amount of epithelial keratinization. There was also infiltration of the lamina propria by lymphocytes and plasma cells. Tip (lower lip) KA (Figure 1).
Lower lip recurrent keratoacanthoma.
A rectangular incision was made in the healthy tissue about 3 mm outside the periphery of the lower lip mass, and a modified Bernard sliding flap was designed to completely remove the mass, then, topical application of tretinoin cream was applied once a day for 3 mo.
There were no abnormalities in the follow-up visits 1, 3, 6, and 12 mo postoperatively. The lower lip wounds were clean at the 2-year postoperative follow-up and the mucosa was normal.
KA was first described by Jonathon Hutchinson in 1889 as a crater-like facial ulcer. In the field of skin pathology, there is no consensus on the pathological nature of KA. Although KA has clinical and histological features similar to squamous cell carcinoma (SCC), it is also considered to be a subtype of SCC, but even if KA is classified as a malignant tumour, its self-limiting nature and low likelihood of distant metastasis is very low, so that diagnosis of benign tumour is more inclined[1]. On microscopy, SCC shows pleomorphism of cells and nuclei and abnormal mitosis, which are different from KA. A large number of reports are more inclined to the biological behaviour of KA to be more like that of benign tumours[2].
The cause of KA remains unknown. Sunlight exposure and UV radiation accumulation are considered to be the main risk factors for KA[3]. Related causes include viral infections, especially human papillomavirus[2], reduced immune function[4], trauma[5], and genetic factors[6]. Ramos et al[7] suggested that the KA growth cycle is similar to the life cycle of hair follicles, hinting at the possibility that KA may be derived from hair follicle cells. Kamath et al[3] found that KA cells can differentiate into cells similar to those of the outer root sheath of hair follicles, further supporting the possibility that skin KA originates from hair follicles. Since the lip mucosa lacks the hair follicle structure of the skin, lip KA may be derived from other structures of the lip mucosa. Chauhan et al[8] reported that lip KA originate from ectopic sebaceous glands or surface epithelial cells. Wagner et al[9] analysed keratin and other related biomarkers by immunohistochemical and histochemical methods and concluded that KA tissue occurs on the outer root sheath of skin hair follicles, while in the lip mucosa it occurs in epithelial cells.
As a rare benign tumour, KA is more common in light-skinned people, and is more common in middle-aged and elderly people, especially those over 40 years old. It is more common in men, and it occurs in exposed areas, especially the head, face, and limbs, but it is rarely seen on the lips[5]. According to estimates by the World Health Organization, less than 8% of KAs occur on the lips[10]. KA is clinically divided into 4 types, the most common being the single type, while the multiple, marginal eccentric, and generalized rash types are rare. In this article, we introduce the clinical and pathological characteristics of a single case of KA of the lower lip. The clinical evolution of KA is divided into three stages: proliferation, maturation, and decomposition, which is the natural KA process of from onset to regression.
The lesions were small papules or dome-shaped nodules at first, and the central nodules were filled with keratin. Later, they formed crater-like bulges with keratinous plugs. The diameters of the lesions vary. They often grow rapidly and reach the peak size within 2 to 3 mo, after which a slow healing process starts, which lasts for more than 2-5 wk, and then the lesions gradually subside. Some lesions subside on their own within about 1 year, leaving local atrophy or a lighter pigmented scar[6]. Some scholars believe that the self-resolution of KA is related to apoptosis[11]. KA usually manifests as an isolated lesion, but it may also occur with other diseases, such as Muir-Torre syndrome[12], sebaceous nevus[13], Grzybowski syndrome[14], and cutaneous papillomatosis[6]. KA may be related to multiple system syndromes, and appropriate systemic examinations should be performed on patients with KA.
Histopathologically, the typical manifestation of KA is through tumour nodular protrusions with crater-like, lamellar, or whirlpool-like keratinous material filling inside, and epithelial cells on both sides bulging upwards in a lip or neck ring shape, and horny cysts can be seen. Insufficiency or hyperkeratosis, the hyperplastic keratinocytes of the spinous layer and basal layer are pale and transparent, the cytoplasm is rich and lightly stained and has an eosinophilic ground glass-like appearance, cell proliferation is active at the bottom, and occasional cell abnormalities and pathological mitotic figures are seen at the base. There is also visible infiltration of inflammatory cells such as neutrophils, lymphocytes, and eosinophils[6,15].
Lower lip KA and SCC have very similar histopathology, making it difficult to distinguish between them. Therefore, immunohistochemistry is needed to distinguish between the two. More specifically, the expression of lectin, vascular cell adhesion molecules (CD-106), intercellular adhesion molecules (CD-54), angiotensin type 1 receptors, and syndecan-1 expression, as well as MIB-1 immunohistochemistry and ultrastructural characteristics are all helpful to distinguish KA from SCC[6].
KA is a benign tumour. Given its self-limiting biological behaviour, Ramos et al[7] suggest that no intervention is needed. However, since the potential size of the lesion is unpredictable, its development may affect the function of adjacent tissues and organs. If local large-scale damage is easy to continue Infection, the scars remaining after the lesions subside may affect the local anatomical function and may even cause squamous cell carcinogenesis. It is clinically considered that it is not feasible to wait for KA to subside on its own. Surgical treatment is the first choice for KA[1,15]; however, surgical resection is destructive, especially when the lesion is located in an aesthetically or functionally important area. Other treatment methods such as laser treatment, radiotherapy, cryotherapy, oral tretinoin drugs, intralesional injection of methotrexate (bleomycin, Triamcinolone acetonide, 5-fluorouracil, interferon a-2a), photodynamic therapy, topical imiquimod cream, etc. can all be used according to the disease condition[1,8,16-18]. Laser and cryotherapy are effective for multiple KAs, especially when small, but they can cause functional and cosmetic defects and make histopathological examination impossible. Radiotherapy can be used as an adjuvant treatment to prevent recurrence after surgical treatment, but radiotherapy will increase the tendency of local tissue to become cancerous. Oral retinoic acid can be considered for patients with multiple KA or with a preventive intent after recurrent KA. Local injection of methotrexate, bleomycin, and triamcinolone acetonide can be used for lesions that cannot be surgically removed. Topical 5% imiquimod cream provides a new treatment method for patients who cannot tolerate surgical treatment and who are unwilling to receive injection treatment. Two thirds of patients can achieve good results after 4-6 wk of application, but the effects should be closely monitored so that treatment is changed if needed[16].
In this case, the lower lip lesion tissue was surgically removed for the first time, and the lower lip KA recurred in situ 6 mo later. A second surgery was performed, and local tretinoin ointment was applied after the operation. At the 2-year follow-up visit, the lesion did not recur. Surgical resection should be the first choice for the treatment of KA, and local enlarged resection can effectively prevent recurrence. To ensure complete removal of the diseased tissue, intraoperative frozen section edge control is necessary[19]. The clinical application of Mohs microsurgery can locate the edge of the tumour, so as to more accurately remove the diseased tissue, obtain a clear edge with the smallest surgical defect, and reduce disease recurrence[20]. Hadley et al[21] Although KA usually subsides spontaneously, up to 20% of KA cases can show nerve, blood vessel, or intravascular invasion, which can easily lead to recurrence. Moss et al[17] reported that the recurrence rate of KA is about 8%. According to some studies, KA often tends to worsen each time it relapses, and it can easily transform into well-differentiated SCC[20]. In this case, the patient had the same pathological characteristics in the first and in the recurring lesion. In addition to the recurrence of KA after surgical resection, the trauma of the operation itself is also the cause of KA[21]. For recurrent KA, combined treatment measures are thus necessary, including surgical resection combined with radiotherapy and injection of triamcinolone acetonide, YAG laser combined with local injection of 5-fluorouracil, and local application of imiquimod after surgical treatment, which can achieve good results[18,22,23]. In this case, surgical resection and topical application of tretinoin cream were used for recurrent KA, which effectively prevented disease recurrence.
KA is a benign tumour with clinical and pathological features similar to those of SCC. Based on a case of recurrent lower lip KA combined with a literature review, this article summarized the clinical and pathological characteristics of lower lip KA and analysed the cause of the patient's recurrence, deepening the understanding of the disease and proving useful information for clinical diagnosis and treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Eccher A, Italy; Rattan V, India S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Liang X, Lin S, Yan J. Photodynamic therapy for keratoacanthoma on the upper lip. Photodiagnosis Photodyn Ther. 2020;30:101798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Savage JA, Maize JC Sr. Keratoacanthoma clinical behavior: a systematic review. Am J Dermatopathol. 2014;36:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Kamath P, Pereira T, Chande M, Shetty S. Keratoacanthoma of the lip: A case report with emphasis on histogenesis. J Oral Maxillofac Pathol. 2017;21:115-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Kulkarni RP, Plaisier S, Ra SH, Li X, Lee DJ, Hillman JD, Binder SW. Genetic profiling of BRAF inhibitor-induced keratoacanthomas reveals no induction of MAP kinase pathway expression. J Invest Dermatol. 2013;133:830-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Pattee SF, Silvis NG. Keratoacanthoma developing in sites of previous trauma: a report of two cases and review of the literature. J Am Acad Dermatol. 2003;48:S35-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30:326-33; discussion 333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Ramos LM, Cardoso SV, Loyola AM, Rocha MA, Durighetto-Júnior AF. Keratoacanthoma of the inferior lip: review and report of case with spontaneous regression. J Appl Oral Sci. 2009;17:262-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Chauhan A, Chaudhary S, Agnihotri PG, Aadithya B. A solitary crateriform ulcer of the lower lip: a case report with review of literature. Indian J Dermatol. 2011;56:435-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Wagner VP, Martins MD, Dillenburg CS, Meurer L, Castilho RM, Squarize CH. Histogenesis of keratoacanthoma: histochemical and immunohistochemical study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Dillenburg CS, Martins MD, Meurer L, Castilho RM, Squarize CH. Keratoacanthoma of the Lip: Activation of the mTOR Pathway, Tumor Suppressor Proteins, and Tumor Senescence. Medicine (Baltimore). 2015;94:e1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Ra SH, Su A, Li X, Zhou J, Cochran AJ, Kulkarni RP, Binder SW. Keratoacanthoma and squamous cell carcinoma are distinct from a molecular perspective. Mod Pathol. 2015;28:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Schwartz RA, Torre DP. The Muir-Torre syndrome: a 25-year retrospect. J Am Acad Dermatol. 1995;33:90-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 241] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Beer GM, Widder W, Cierpka K, Kompatscher P, Meyer VE. Malignant tumors associated with nevus sebaceous: therapeutic consequences. Aesthetic Plast Surg. 1999;23:224-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Parry F, Saunière D, Huertas Diaz DL, Dandurand M. [Generalized eruptive keratoacanthomas of Grzybowski: A case report followed over 11 years]. Ann Chir Plast Esthet. 2017;62:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Zargaran M, Baghaei F. A clinical, histopathological and immunohistochemical approach to the bewildering diagnosis of keratoacanthoma. J Dent (Shiraz). 2014;15:91-97. [PubMed] |
| 16. | Pancevski G, Pepic S, Idoska S, Tofoski G, Nikolovska S. Topical Imiquimod 5% as a Treatment Option in Solitary Facial Keratoacanthoma. Open Access Maced J Med Sci. 2018;6:531-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Moss M, Weber E, Hoverson K, Montemarano AD. Management of Keratoacanthoma: 157 Tumors Treated With Surgery or Intralesional Methotrexate. Dermatol Surg. 2019;45:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Feldmeyer L, Szeverényi I, Mandallaz M, Lane EB, Hohl D. Late-Onset Multiple Self-Healing Squamous Epithelioma Ferguson-Smith Recurrence Induced by Radiotherapy. Case Rep Dermatol. 2016;8:344-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Donaldson MJ, Sullivan TJ, Whitehead KJ, Williamson RM. Periocular keratoacanthoma: clinical features, pathology, and management. Ophthalmology. 2003;110:1403-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Juhász MLW, Marmur ES. A Multiple Recurrent Keratoacanthoma of the Lower Leg After Repeated Wide-Excision and Mohs Micrographic Surgery. Dermatol Surg. 2018;44:1028-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Hadley JC, Tristani-Firouzi P, Florell SF, Bowen GM, Hadley ML. Case series of multiple recurrent reactive keratoacanthomas developing at surgical margins. Dermatol Surg. 2009;35:2019-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Thiele JJ, Ziemer M, Fuchs S, Elsner P. Combined 5-fluorouracil and Er:YAG laser treatment in a case of recurrent giant keratoacanthoma of the lower leg. Dermatol Surg. 2004;30:1556-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Kurien A, Henderson C, Lee S. Recurrent keratoacanthoma with vascular invasion: a diagnostic and management dilemma. Australas J Dermatol. 2009;50:194-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |