Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6483
Peer-review started: August 19, 2021
First decision: January 10, 2022
Revised: January 24, 2022
Accepted: May 27, 2022
Article in press: May 27, 2022
Published online: July 6, 2022
Processing time: 309 Days and 0.4 Hours
Colorectal cancer is one of the most common cancers worldwide with high mortality and is classified as a single entity, although colon cancer and rectal cancer have largely different diagnoses, treatments, surgical methods, and recurrence rates. ≥ 16-slice spiral computed tomography (SCT) is mostly applied to detect the local stage of colon cancer; however, its diagnostic accuracy and whether it is conducive to distinguishing between high-risk and low-risk colon cancer are unclear.
To systematically review the diagnostic accuracy of ≥ 16-slice SCT for local staging of colon cancer.
Based on the PubMed, EMBASE, Cochrane Library, and Web of Science databases, computers were used to search the literature from the establishment of the database to April 2021, and the results of the diagnostic tests on ≥ 16-slice SCT for local staging of colon cancer were collected according to the inclusion criteria. The data were then extracted and assessed on the basis of the Quality Assessment Checklist of the Institute of Economics of Canada, Reference Citation Analysis (https://www.referencecitationanalysis.com/). Afterward, a meta-analysis was performed using the statistical software Meta-disc 14.0 and Stata 15.0.
Eleven studies that provided data on 1613 subjects with computed tomography diagnostic tests were included in this study. Meta-analysis revealed that the pooled sensitivity, pooled specificity, pooled negative likelihood ratio (LR), pooled diagnostic odds ratio, and area under the fitted receiver operating characteristic (ROC) curve of ≥ 16-slice SCT for colon cancer T staging were 0.67 (95%CI: 0.65-0.70), 0.81 (95%CI: 0.80-0.83), 4.13 (95%CI: 2.66-6.41), 0.39 (95%CI: 0.31-0.49), 10.81 (95%CI: 7.33-15.94), and 0.829, respectively, while the specificity, negative LR, diagnostic odds ratio, and area under the fitted ROC curve of ≥ 16-slice SCT for N staging of colon cancer were 0.54 (95%CI: 0.49-0.59), 0.74 (95%CI: 0.70-0.77), 1.92 (95%CI: 1.36-2.70), 0.67 (95%CI: 0.51-0.87), 3.74 (95%CI: 1.76-7.94), and 0.829 respectively. The sensitivity and specificity of ≥ 16-slice SCT for colon cancer T staging were acceptable, while the sensitivity for colon cancer N staging was relatively low, though its specificity was acceptable.
≥ 16-slice SCT for local staging of colon cancer has good diagnostic value; however, the accuracy needs to be confirmed by further clinical practice.
Core Tip: This systematic review and meta-analysis were based on eleven studies on 1613 patients with computed tomography diagnostic tests. The results indicated that ≥ 16-slice spiral computed tomography, which is most applied in clinical practice, displayed acceptable diagnostic accuracy and good diagnostic value for detecting the local stage of colon cancer. In addition, it is conducive to distinguishing between high-risk and low-risk colon cancer.
- Citation: Liu D, Sun LM, Liang JH, Song L, Liu XP. Diagnostic accuracy of ≥ 16-slice spiral computed tomography for local staging of colon cancer: A systematic review and meta-analysis. World J Clin Cases 2022; 10(19): 6483-6495
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6483.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6483
One of the most common cancers in the world is colorectal cancer[1-3], for which an update on the incidence is provided every 3 years by the American Cancer Society from population-based cancer registrations (as of 2016) and mortality data from the National Center for Health Statistics (as of 2017). These data indicate that colorectal cancer is the third most commonly seen cancer and the third leading cause of cancer death, with the prediction of approximately 147950 new cases of colorectal cancer diagnosed and 53200 deaths recorded by the year 2020[4]. The average annual incidence of colorectal cancer in men and that in women in the United States from 2011 to 2015 were also disclosed to be 45.9/100000 and 34.6/100000, respectively[5]. Classified by the World Health Organization as a single entity, colon cancer and rectal cancer are largely different in their diagnoses, treatments, surgical methods, and recurrence rates[6,7]. The early symptoms of colon cancer patients, such as hematoma, diarrhea, changes in bowel habits, local abdominal pain, and anemia, are easy to ignore, thus leading to approximately 20% of late-stage patients missing the best treatment time over the past 20 years[8]. Characterized by noninvasiveness, high sensitivity and specificity, safety, availability, convenience, and inexpensiveness, a full colonoscopy is the "ideal" screening test for colon cancer, as recommended by the guidelines, and involves varying measurements and strategies with advantages and disadvantages, but is well tolerated, especially under adequate sedation[9]. Full colonoscopy has been shown by a literature review to have a high sensitivity (96%-97%) and specificity (98%), with, aside from other high risks, a perforation rate of 0.1%, bleeding risk of greater than 0.3%, and mortality rate of 0.01%-0.03%[10,11]. Computed tomography (CT) colonography is highly sensitive and cost-effective in detecting colon cancer, and is a technique that applies the optimal bowel wall dilation 3D colon reconstruction (to create a virtual colonoscopy). Elias Nerad et al[12] demonstrated that such an analysis had an excellent sensitivity in detecting colorectal T3-T4, with the CT digits not screened, due to the low lymph node metastasis resulting from the challenge of distinguishing between T1-T3Ab and T3CD-T4. In recent years,≥ 16-slice spiral computed tomography (SCT) have been mostly applied to detect the local stage of colon cancer. This meta-analysis was designed to specifically determine the diagnostic accuracy of ≥ 16-slice SCT used only for colon cancer staging, thus assessing whether ≥ 16-slice SCT is conducive to distinguishing between high-risk and low-risk colon cancer.
PubMed, EMBASE, Cochrane Library, and Web of Science were searched with computers from the establishment of the database to April 2021, with terms such as "colon cancer", "colon tumor", "colorectal cancer", "tumor staging", "computed tomography", and "CT" serving as keywords.
After deleting duplicate publications, reasonable inclusion criteria and exclusion criteria were developed to review the remaining studies. The inclusion criteria were: (1) The purpose of the study was the diagnostic value of computed tomography in the diagnosis of colon cancer; (2) The experimental design was prospective or retrospective; and (3) The inclusion criteria were symptoms, signs, or laboratory tests of colon cancer, with histopathological results serving as the standard for diagnosis of cancer.
The exclusion criteria were: (1) Reviews, case reports, editorials, correspondences, comments, or meeting abstracts/meeting minutes; (2) The number of CT slices was not described in the study and could not be determined, by other means, whether it was ≥ 16-slice CT or < 16-slice CT; (3) Studies without available diagnostic results in a two-by-two table; (4) Studies in languages other than English; and (5) Studies without available full texts.
The data from the literature were independently extracted by two researchers, and were put into a predrawn data table as follows: (1) Basic research information, including the first author, the publication year, the country from which the first author comes, the test time, and the type of the research design; (2) The characteristics of the tested population, including the patients’ age, gender, the number of participants, and the pathological stage; (3) The CT slices; (4) The details of reference standards; (5) The research results, including the number of true positives, false positives, false negatives, and true negatives, for the purpose of determining the accuracy of the diagnoses; and (6) Reference Citation Analysis (https://www.referencecitationanalysis.com/).
The methodological quality of the included studies was assessed by two researchers on the basis of the Quality Evaluation Checklist of the Canadian Institute of Economics (IHE)[13], then cross-checked, and finally an agreement was reached. In light of the misleading scoring of the item conformity[14], the scoring method was not included in the quality evaluation checklist of the IHE methodological case series, and corresponding options for each item were provided instead, leading to the assessment of 13 case series based on the final list. Studies were recommended as acceptable with 14 (70%) or more items in conformity despite the fact that a corresponding quality assessment system had not yet been formulated by the expert group.
The data were analyzed with Meta-disc 14.0 and Stata 15.0. The threshold effect was determined with Meta-disc 14.0. All of the heterogeneity degrees were assessed by the heterogeneity index (I²). The random effects model was adopted in the case of I² > 50%. Conversely, the fixed effects model was applied for the purpose of analyzing indicators such as sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio (DOR,) and area under the curve (AUC). The publication bias of the included literature (≥ 10 articles) was examined by Deeks' test on Stata 15.0.
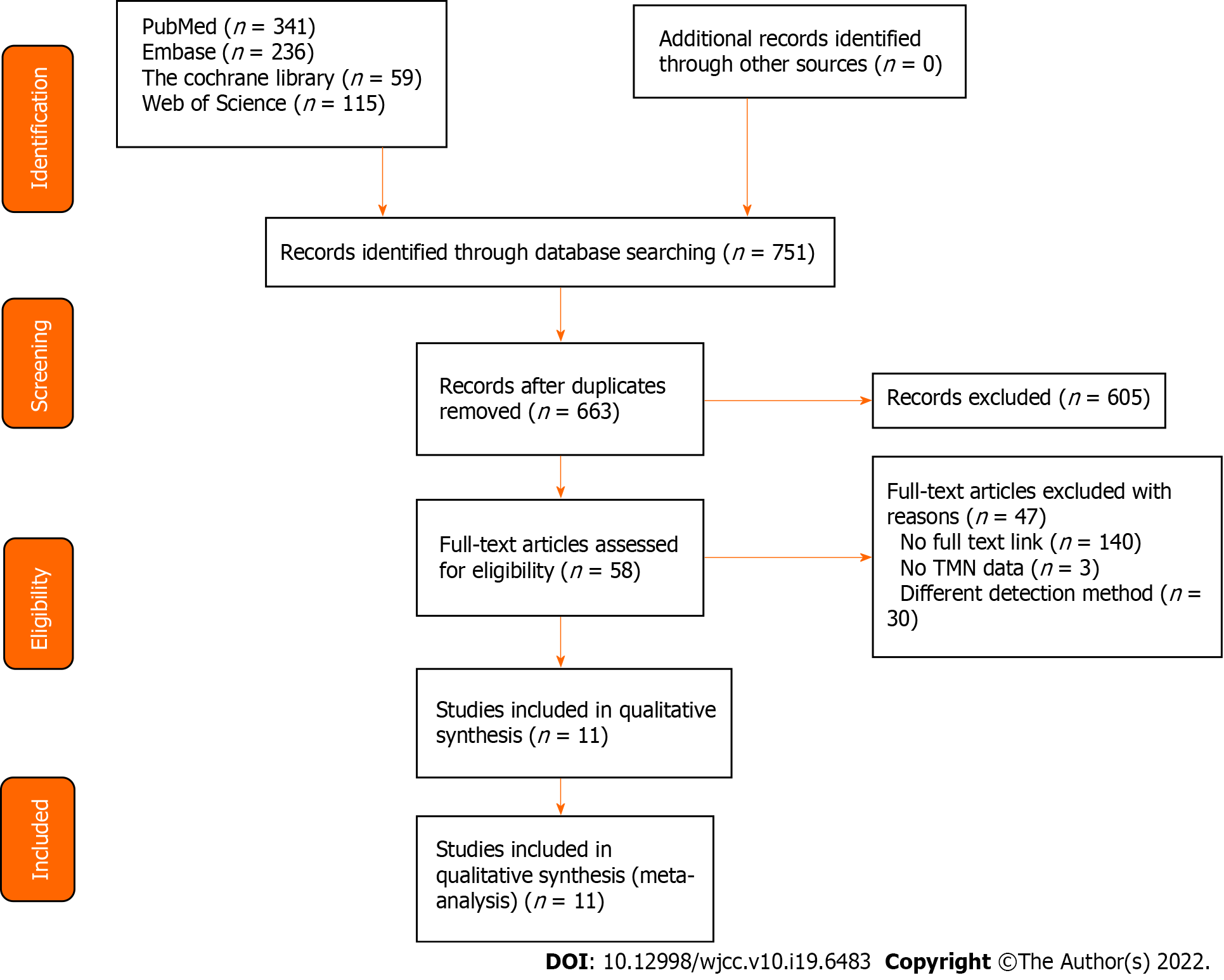
With 663 articles left after deleting duplicates from 751 preliminarily retrieved original articles, a full-text analysis was performed on the basis of the 58 articles remaining after screening by titles and abstracts, leaving 47 articles excluded with full-texts of 14 articles not available, 3 articles without TMN staging descriptions, and 30 articles with different detection methods, resulting in 11 articles finally included in this study (Figure 1)[15-25].
The basic characteristics of the literature included in this study are shown in Table 1-4. A total of 1613 participants were included in the 11 studies, of which three[15,17,24] were on colorectal cancer while the remaining 8[16,18-23,25] were on colon cancer, with 5 of the 11 studies[15,17,18,20] performed with 16-slice CT, 6[16,21-25] with 64-slice CT, and 1[19] with 16-slice (Siemens SOMATON Sensation 16) or 64-slice CT (GE LightSpeed VCT). Four of the eleven [15,20,23,25] were performed as retrospective studies and the remaining 7[16-19,21,22,24] as prospective studies; only 2[19,24] were performed for the follow-up. In terms of the display of diagnostic results, 11 studies showed the diagnostic results of the T staging of colon cancer, among which Rollvén et al[16] reached different judgments on the CT diagnosis results, thus leading to ultimate disagreement, and 4 studies[15,17,19,23] showed the diagnostic results of N staging. With the methodological quality assessment conducted by the IHE quality assessment checklist, the assessment results showed that all 11 included studies met the requirement of more than 14 items (70%), indicating that the quality of the literature included in this study was acceptable.
| Ref. | Year | Nationality | Sample size (case) | Gender (male/female) | Average age | Subjects | Slices of CT scan | Type of research |
| Tezcan et al[15] | 2013 | Turkey | 159 | 115/44 | 60 (28-82) | Colorectal cancer | 16 | Retrospective |
| Rollven et al[16] | 2013 | Stockholm | 29 | 17/11 | 73 ± 11.25 | Colon cancer | 64 | Prospective |
| da Fonte et al[17] | 2012 | Turkey | 25 | 12/13 | 59.8 ± 10 | Colorectal cancer | 16 | Prospective |
| Sibileau et al[18] | 2014 | France | 53 | 27/26 | 70 ± 11.75 | Colon cancer | 16 | Prospective |
| Hunter et al[19] | 2017 | UK | 58 | 34/26 | 69.3 ± 13.6 | Colon cancer | 16 or 64 | Prospective |
| Lee et al[20] | 2014 | Korea | 266 | 154/112 | 63.7 ± 13 | Colon cancer | 16 | Retrospective |
| Maupoey et al[21] | 2019 | Spain | 217 | 128/89 | 70 ± 13.75 | Colon cancer | 64 | Prospective |
| Dighe et al[22] | 2010 | Royal | 84 | 75 ± 10.5 | Colon cancer | 64 | Prospective | |
| Lao et al[23] | 2013 | Taiwan | 152 | 82/70 | 66 ± 5.125 | Colon cancer | 64 | Retrospective |
| Flor et al[24] | 2013 | Italy | 69 | 31/38 | 68 ± 9 | Colorectal cancer | 64 | Prospective |
| Malmstrøm et al[25] | 2018 | Denmark | 501 | 271/230 | 69.4 ± 9.7 | Colon cancer | 64 | Retrospective |
| Ref. | Stage | Number | Histology + | Histology - | TP | FP | FN | TN | Sensitivity (%) | Specificity (%) |
| Tezcan et al[15] | T1/T2 | 159 | 17 | 142 | 13 | 3 | 4 | 139 | 76.47 | 97.89 |
| Tezcan et al[15] | T3 | 159 | 121 | 38 | 116 | 8 | 5 | 30 | 96.00 | 79.00 |
| Tezcan et al[15] | T4 | 159 | 21 | 138 | 17 | 1 | 4 | 137 | 81.00 | 99.00 |
| Rollvén et al[16] | T0-T3ab | 29 | 16 | 13 | 14 | 4 | 2 | 9 | 87.50 | 69.23 |
| Rollvén et al[16] | T3cd-T4 | 29 | 13 | 16 | 9 | 2 | 4 | 14 | 69.23 | 87.50 |
| Rollvén et al[16] | T0-T3ab | 29 | 16 | 13 | 13 | 4 | 3 | 9 | 81.25 | 69.23 |
| Rollvén et al[16] | T3cd-T4 | 29 | 13 | 16 | 9 | 3 | 4 | 13 | 69.23 | 81.25 |
| da Fonte et al[17] | T1/T2 | 25 | 7 | 18 | 4 | 2 | 3 | 16 | 57.10 | 88.90 |
| da Fonte et al[17] | T3 | 25 | 16 | 9 | 14 | 3 | 2 | 6 | 87.50 | 66.70 |
| da Fonte et al[17] | T4 | 25 | 2 | 23 | 2 | 0 | 0 | 23 | 100.00 | 100.00 |
| Sibileau et al[18] | T1/T2 | 53 | 10 | 43 | 6 | 1 | 4 | 42 | 60.00 | 97.67 |
| Sibileau et al[18] | T3 | 53 | 32 | 21 | 26 | 8 | 6 | 13 | 81.25 | 61.90 |
| Sibileau et al[18] | T4 | 53 | 11 | 42 | 9 | 5 | 2 | 37 | 81.82 | 88.10 |
| Hunter et al[19] | T3/T4 | 53 | 42 | 11 | 34 | 7 | 8 | 4 | 81.00 | 36.00 |
| Hunter et al[19] | T1/T2 | 53 | 11 | 42 | 4 | 8 | 7 | 34 | 36.36 | 80.95 |
| Hunter et al[19] | T3/T4 | 53 | 42 | 11 | 29 | 5 | 13 | 6 | 69.00 | 55.00 |
| Hunter et al[19] | T1/T2 | 53 | 11 | 42 | 6 | 13 | 5 | 29 | 54.55 | 69.05 |
| Lee et al[20] | T3 | 266 | 138 | 128 | 138 | 128 | 0 | 0 | 100.00 | 0.00 |
| Lee et al[20] | T4 | 266 | 63 | 203 | 61 | 195 | 2 | 8 | 97.40 | 4.00 |
| Maupoey et al[21] | T1/T2 | 225 | 69 | 156 | 58 | 18 | 11 | 138 | 84.06 | 88.46 |
| Maupoey et al[21] | T3 | 225 | 77 | 148 | 51 | 26 | 26 | 122 | 66.23 | 82.43 |
| Maupoey et al[21] | T4a | 225 | 71 | 154 | 45 | 14 | 26 | 140 | 63.38 | 90.91 |
| Maupoey et al[21] | T4b | 225 | 8 | 217 | 7 | 6 | 1 | 211 | 87.50 | 97.24 |
| Dighe et al[22] | T3 ≥ 5 mm and T4 | 84 | 48 | 36 | 40 | 12 | 8 | 24 | 83.33 | 66.67 |
| Dighe et al[22] | T1/T2 and T3 < 5 mm | 84 | 36 | 48 | 24 | 8 | 12 | 40 | 66.67 | 83.33 |
| Lao et al[23] | T0 or Tis | 153 | 4 | 149 | 2 | 19 | 2 | 130 | 50.00 | 87.25 |
| Lao et al[23] | T1 | 153 | 18 | 135 | 1 | 0 | 17 | 135 | 5.56 | 100.00 |
| Lao et al[23] | T2 | 153 | 25 | 128 | 6 | 27 | 19 | 101 | 24.00 | 78.91 |
| Lao et al[23] | T3 | 153 | 98 | 55 | 74 | 19 | 24 | 36 | 75.51 | 65.45 |
| Lao et al[23] | T4 | 153 | 8 | 145 | 1 | 4 | 7 | 141 | 12.50 | 97.24 |
| Lao et al[23] | T0–Tis, T1–T2 | 153 | 47 | 106 | 33 | 22 | 14 | 84 | 70.21 | 79.25 |
| Lao et al[23] | T3/T4 | 153 | 106 | 47 | 84 | 14 | 22 | 33 | 79.25 | 70.21 |
| Flor et al[24] | T1/T2 | 75 | 17 | 58 | 16 | 17 | 1 | 41 | 96.00 | 71.00 |
| Flor et al[24] | T3/T4 | 75 | 58 | 17 | 41 | 1 | 14 | 16 | 70.69 | 94.12 |
| Malmstrøm et al[25] | T1/T2 | 501 | 163 | 338 | 136 | 141 | 27 | 197 | 83.44 | 58.28 |
| Malmstrøm et al[25] | T3 ≤ 5 mm | 501 | 211 | 290 | 55 | 44 | 156 | 246 | 26.07 | 84.83 |
| Malmstrøm et al[25] | T3 > 5 mm | 501 | 68 | 433 | 34 | 53 | 34 | 380 | 50.00 | 87.76 |
| Malmstrøm et al[25] | T4 | 501 | 59 | 442 | 19 | 19 | 40 | 423 | 32.20 | 95.70 |
| Ref. | Staging | Number | Histology + | Histology - | TP | FP | FN | TN | Sensitivity (%) | Specificity (%) |
| Tezcan et al[15] | N0 | 159 | 75 | 84 | 24 | 0 | 51 | 84 | 32.00 | 100.00 |
| Tezcan et al[15] | N1 | 159 | 46 | 113 | 28 | 23 | 18 | 90 | 61.00 | 80.00 |
| Tezcan et al[15] | N2 | 159 | 38 | 121 | 38 | 46 | 0 | 75 | 100.00 | 62.00 |
| Hunter et al[19] | N1/N2 | 53 | 22 | 31 | 14 | 12 | 8 | 19 | 64.00 | 60.00 |
| Hunter et al[19] | N1/N2 | 53 | 22 | 31 | 16 | 18 | 6 | 13 | 73.00 | 43.00 |
| Lao et al[23] | Nx or N0 | 153 | 76 | 77 | 48 | 34 | 28 | 43 | 63.16 | 55.84 |
| Lao et al[23] | N1 | 153 | 34 | 119 | 8 | 31 | 26 | 88 | 23.53 | 73.95 |
| Lao et al[23] | N2 | 153 | 43 | 110 | 17 | 15 | 26 | 95 | 39.53 | 86.36 |
| Ref. | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | A10 | A11 | A12 | A13 | A14 | A15 | A16 | A17 | A18 | A19 | A20 |
| Tezcan et al[15] | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Unclear | Y | N | Y | Y | N | Y | Y | N | Unclear |
| Rollven et al[16] | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Unclear | Y | N | Y | Y | N | Y | Y | Y | Unclear |
| da Fonte et al[17] | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Unclear | Y | N | Y | Y | N | Y | Y | Y | Unclear |
| Sibileau et al[18] | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Unclear | Y | N | Y | Y | N | Y | Y | Y | Unclear |
| Hunter et al[19] | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Unclear | Y | Y | Y | Y | N | Y | Y | Y | Unclear |
| Sibileau et al[18] | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Unclear | Y | N | Y | Y | N | Y | Y | N | Unclear |
| Maupoey et al[21] | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Unclear | Y | N | Y | Y | N | Y | Y | Y | Unclear |
| Dighe et al[22] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Unclear | Y | N | Y | Y | N | Y | Y | Y | Unclear |
| Lao et al[23] | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Unclear | Y | N | Y | Y | N | Y | Y | N | Unclear |
| Flor et al[24] | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Unclear | Y | Y | Y | Y | N | Y | Y | Y | Unclear |
| Malmstrøm et al[25] | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Unclear | Y | N | Y | Y | N | Y | Y | N | Unclear |
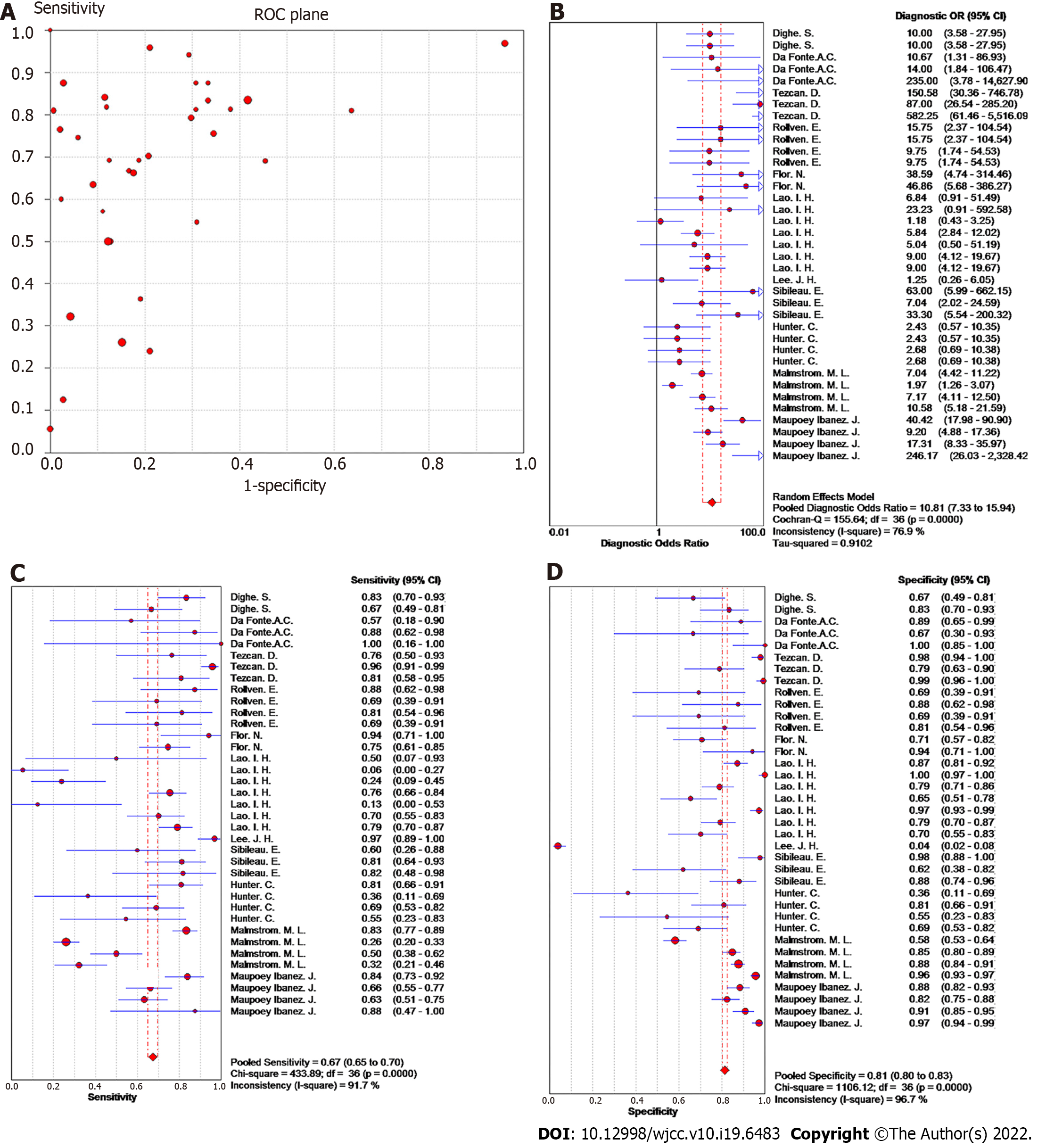
Heterogeneity of meta-analysis of T staging: In a diagnostic meta-analysis, heterogeneity is primarily generated by threshold and nonthreshold effects. In this study, there was no "shoulder-arm" distribution in the "ROC floor plan" results, showing no threshold effect (Figure 2A), no distribution along the same line DOR value as the pooled DOR value of each study in the DOR forest diagram, as well as a Cochran-Q = 155.64 (I2 = 76.9%, P < 0.001), indicating that the heterogeneity was generated by a nonthreshold effect (Figure 2B).
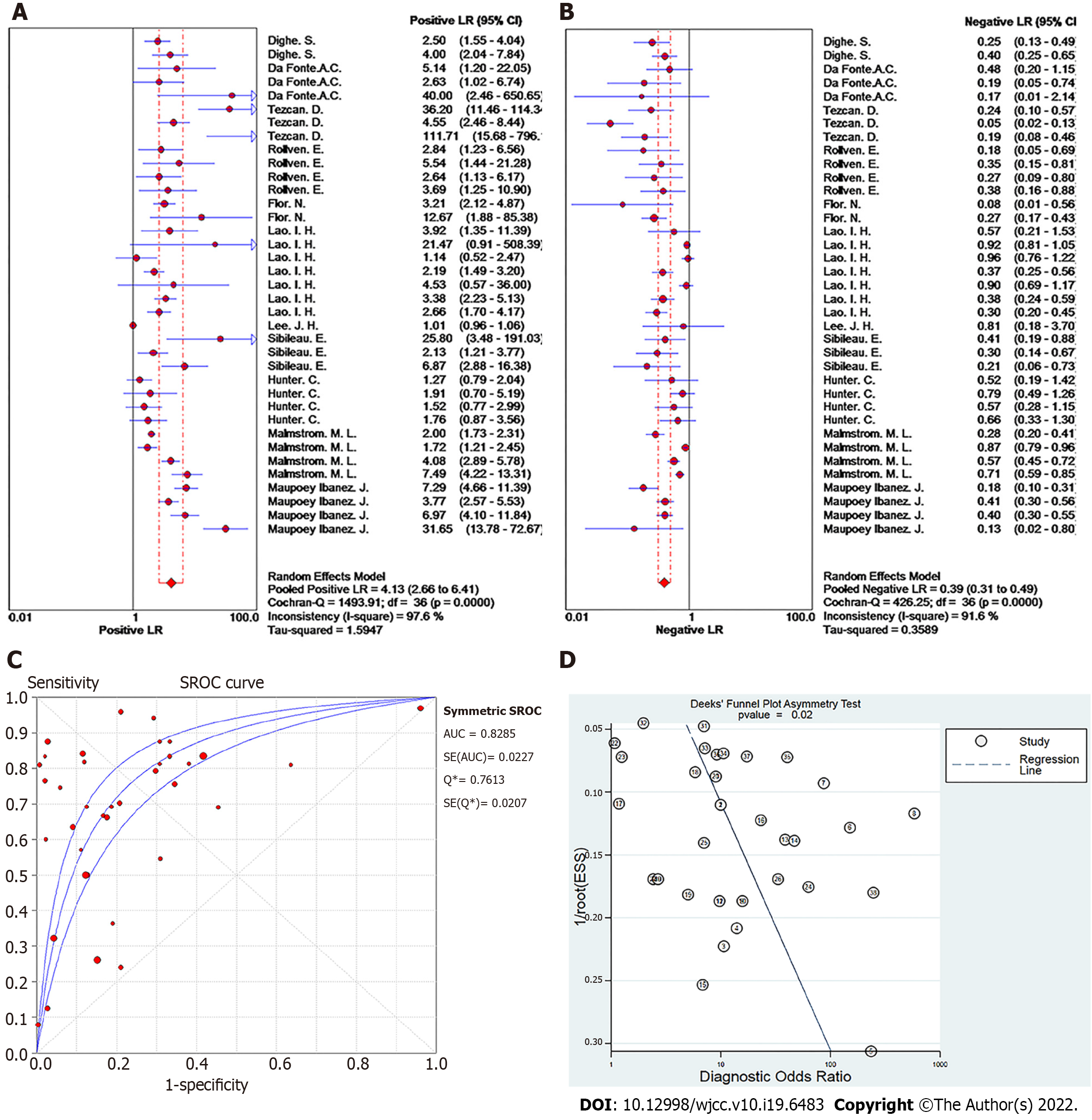
Summary effect size of T staging: The pooled sensitivity of all the studies had an I² = 91.7%, and the sensitivity was combined with the random effects model, which resulted in a pooled sensitivity of 0.67 (95%CI: 0.65-0.70) (Figure 2C). The pooled specificity of the studies had an I² = 96.7%, and the specificity was combined with the random effects model, which resulted in a pooled specificity of 0.81 (95%CI: 0.80-0.83) (Figure 2D). The pooled positive LR of the studies had an I² = 97.6%, and the positive likelihood ratio was combined with the random effects model, which resulted in a pooled positive LR of 4.13 (95%CI: 2.66-6.41) (Figure 3A). The pooled negative likelihood ratio of the studies had an I² = 91.6%, and the negative LR was combined with the random effects model, which resulted in a pooled negative LR of 0.39 (95%CI: 0.31-0.49) (Figure 3B). The pooled DOC of the studies had an I² = 76.9%, and the DOC value was combined with the random effects model, which resulted in a pooled DOC value of 10.81 (95%CI: 7.33-15.94) (Figure 2B). The area under the fitted ROC curve was 0.829 (Figure 3C).
Publication bias of T staging: Deeks' funnel plot asymmetry test was conducted to assess the publication bias of the studies in the meta-analysis, with P = 0.02 as shown in Figure 3D, indicating a significant publication bias in the included literature on the 16-slice CT diagnosis of colorectal cancer T staging.
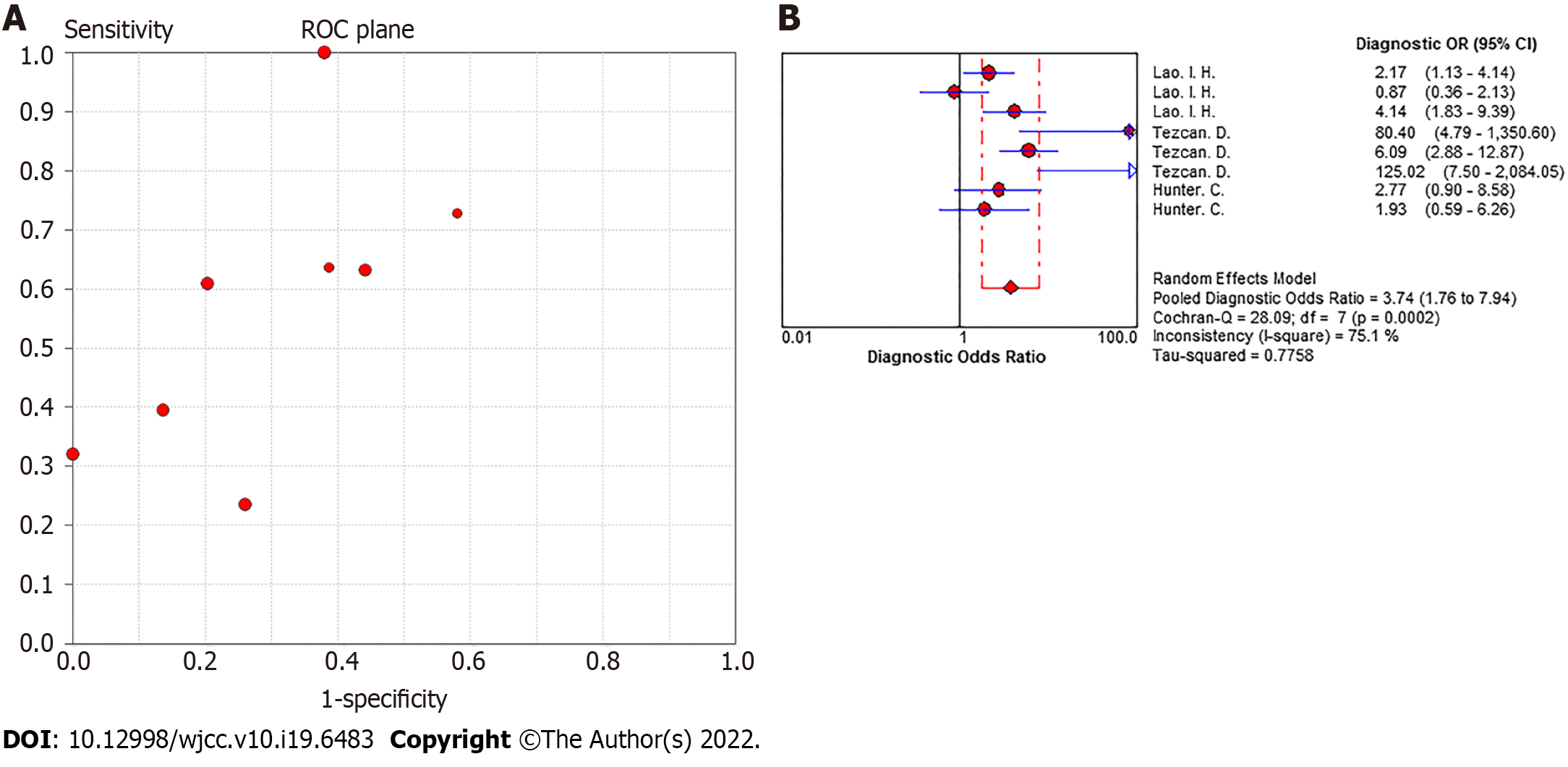
Heterogeneity of meta-analysis of N staging: There was no "shoulder-arm" distribution in the "ROC floor plan" results, showing no threshold effect (Figure 4A), no distribution along the same line of DOR value as the pooled DOR value of each study in the DOR forest diagram, as well as a Cochran-Q = 28.09 (I2 = 75.1%, P < 0.001), indicating that the heterogeneity was generated by a nonthreshold effect. Qualitative details are provided in Figure 4B.
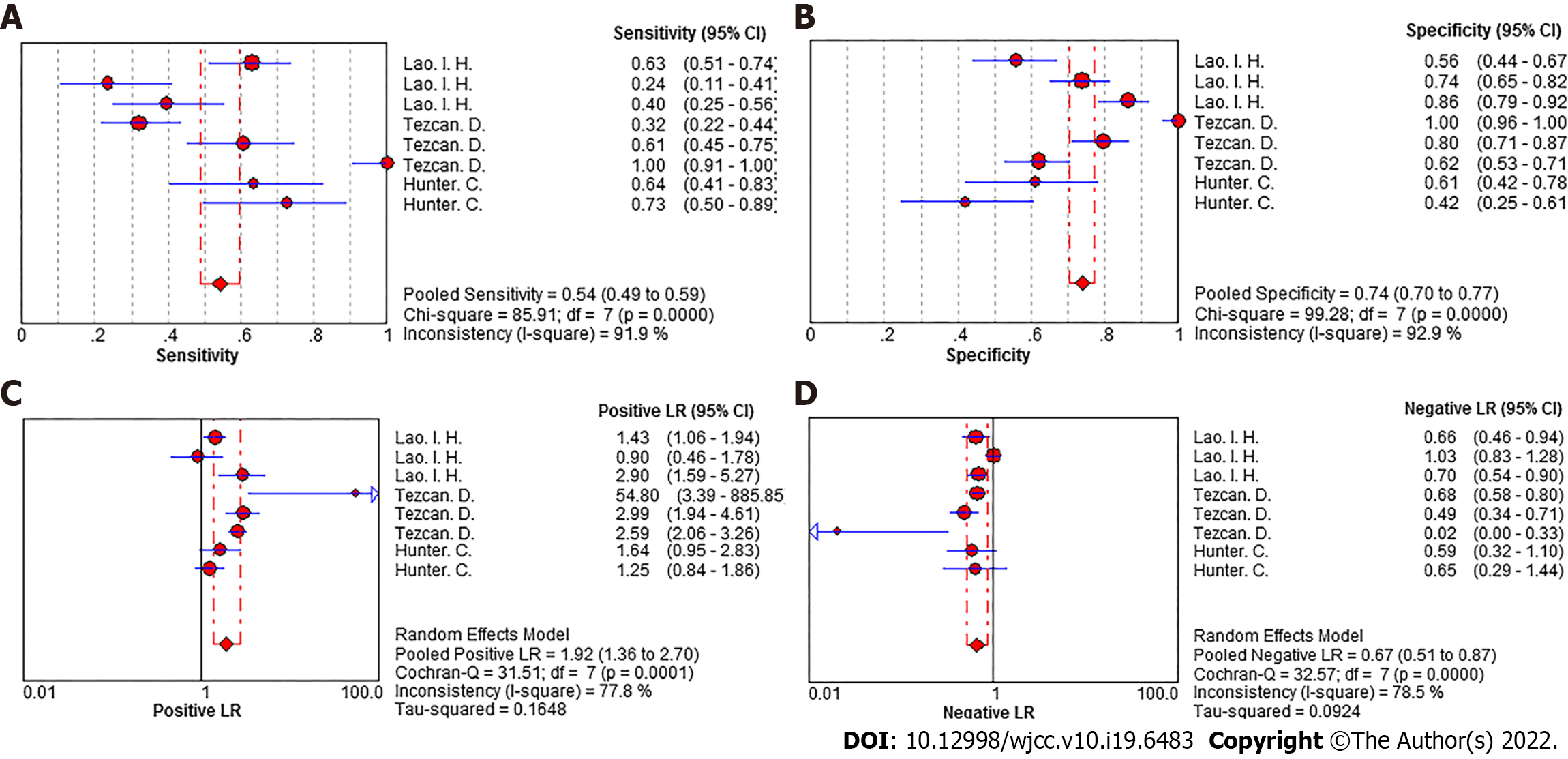
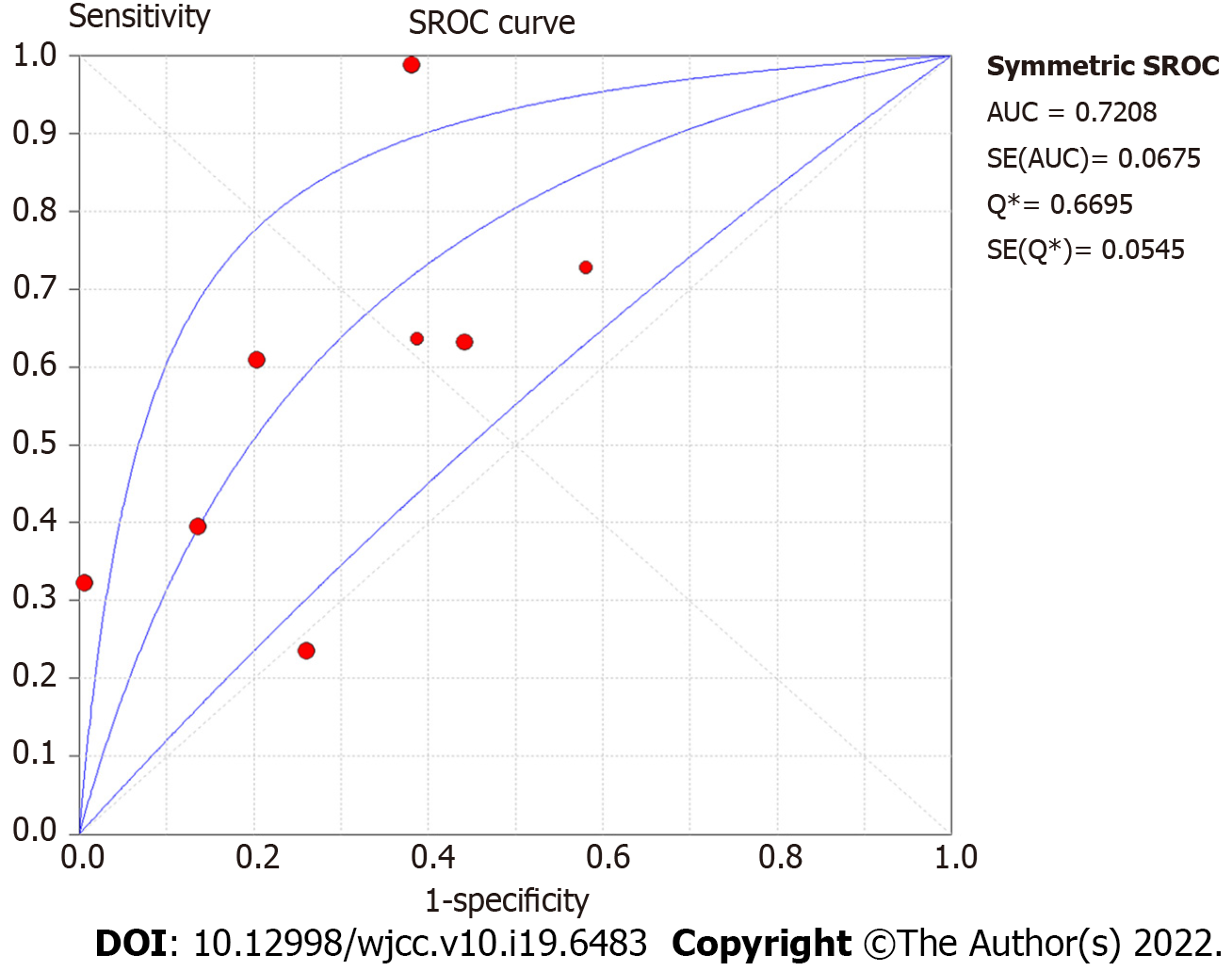
Summary effect size of N staging: The pooled sensitivity of all the studies had an I² = 91.9%, and the sensitivity was combined with the random effects model, which resulted in a pooled sensitivity of 0.54 (95%CI: 0.49-0.59) (Figure 5A). The pooled specificity of the studies had an I² = 92.9%, and the specificity was combined with the random effects model, which resulted in a pooled specificity of 0.74 (95%CI: 0.70-0.77) (Figure 5B). The pooled positive LR of the studies had an I² = 77.8%, and the positive LR was combined with the random effects model, which resulted in a pooled positive likelihood ratio of 1.92 (95%CI: 1.36-2.70) (Figure 5C). The pooled negative LR of the studies had an I² = 78.5%, and the negative LR was combined with the random effects model, which resulted in a pooled negative LR of 0.67 (95%CI: 0.51-0.87) (Figure 5D). The pooled DOC of the studies had an I² = 76.9%, and the DOC value was combined with the random effects model, which resulted in a pooled DOC value of 3.74 (95%CI: 1.76-7.94) (Figure 4B). The area under the fitted ROC curve had an AUC of 0.829 (Figure 6).
Publication bias of N staging: With little literature included (n < 10), the publication bias of the ≥ 16-slice CT diagnosis of colorectal cancer N staging was not assessed.
The staging of colon cancer is mostly performed according to the American Joint Committee on Cancer (AJCC)[26], which distinguishes patients based on the degree of primary tumor invasion (T stage), lymph node status (N stage), and distant spread (M stage). Clinical studies have disclosed that the invasion depth, the presence or absence of lymph node metastasis, and distant metastasis are key factors affecting the clinical prognosis of rectal cancer[27], leading to the conclusion that the application of imaging methods to accurately assess and summarize tumor stage, the surrounding anatomical relationships, and metastasis is of great value for rationally formulating treatment plans and improving clinical prognosis. This study systematically assessed the accuracy of 16-slice SCT in the diagnosis of localized colon cancer.
In this study, ≥ 16-slice SCT were shown to have a relatively low sensitivity of only 67% and specificity of 81% for the diagnosis of colon cancer T staging, with the relatively low sensitivity ascribed mainly to the following factors: (1) In this study, the T stages were divided into subgroups and discussed in more detail, with the diagnostic results of T1 and T2 of the colorectal cancer displayed and T4 discussed only by Lao et al[23], with unsatisfactory sensitivity and no bowel preparation applied; (2) Two independent investigators’ diagnostic results were adopted by Rollvén et al[16], with inconsistent judgments, and in most cases, the treatment plans for different colon cancer T stages would not be changed, since most primary tumor resections in patients were conducted when symptoms such as rectal bleeding or intestinal obstruction were already in existence or might occur in the near future[28,29].
In this study, ≥ 16-slice SCT were shown to have a relatively low sensitivity of only 54% and specificity of 74% for the diagnosis of colon cancer N staging, although there is little literature on the staging reported. During the process of colon cancer treatment, the different lymph node stages will only affect the need for lymph node resection beyond the surgical margin, which largely depends on the existence of distant metastases and/or the presence of clinically relevant complications, and adjuvant chemotherapy is determined based on the presence of metastatic lymph nodes in the resected specimen, rather than on the suspicion of its existence based on imaging techniques[30,31].
The limitations of this study are: (1) This system was explored by only 11 studies (5 using 16-slice CT, 6 using 64-slice CT, and 1 using the combination of the 16- and 64-slice CT), with the different slices of CT divided into subgroups going undiscussed, thus failing to determine whether there is a difference in the detection effect of different slices on the local stage of colon cancer; (2) This system only explored the diagnostic effects of ≥ 16-slice SCT for colon cancer T staging and N staging, without discussion over the more detailed staging; (3) Of the 11 included studies, 4 are retrospective reviews, where only some of the patients were possibly recommended to have a CT test, indicating a low inclusion probability of the patients with early or advanced stage of colon cancer; and (4) There was a significant publication bias in the studies included in this system.
With the detection of tumors by CT depending largely on the sizes of the tumors, some locally advanced tumors that are confirmed by histopathological tests may be too small to be detected by CT. Thus, sensitivity and specificity are of special importance in the development of screening tests because individuals with the diseases are preferred for recruitment. It was revealed in this study that the sensitivity and specificity of ≥ 16-slice SCT for colon cancer T staging and N staging are acceptable, which is indicative of the good diagnostic value of ≥ 16-slice SCT for local staging of colon cancer. The accuracy of these findings will need to be confirmed by further clinical studies.
Colorectal cancer is a common, high-mortality cancer. Classified by the World Health Organization as a single entity, colon cancer and rectal cancer are largely different in their diagnoses, treatments, surgical methods, and recurrence rates. Since early symptoms are easily overlooked, it is important to find a more accurate staging method.
By looking for a method to detect local stages of colon cancer, we hoped to find a method that has better accuracy and can distinguish between high-risk and low-risk colon cancer.
This study aimed to evaluate the diagnostic accuracy of ≥ 16-slice spiral computed tomography (SCT) in detecting local colon cancer staging.
Based on the PubMed, EMBASE, Cochrane Library, and Web of Science databases, computers were used to search the literature from the establishment of the database to April 2021. The results of the diagnostic tests on ≥ 16-slice SCT for local colon cancer staging were collected according to the inclusion criteria, and then the data were extracted and assessed on the basis of the Quality Assessment Checklist of the Institute of Economics of Canada. Afterward, a meta-analysis was performed using the statistical software Meta-disc 14.0 and Stata 15.0.
Eleven studies with a total of 1613 subjects were included. The pooled sensitivity, pooled specificity, pooled negative LR, pooled diagnostic odds ratio, and the area under the fitted receiver operating characteristic curve of ≥ 16-slice SCT for colon cancer T staging and N staging were analyzed. The results revealed that the sensitivity and specificity of ≥ 16-slice SCT for colon cancer T staging were acceptable, while the sensitivity of colon cancer N staging was relatively low, but its specificity was acceptable.
It was revealed in this study that the sensitivity and specificity of ≥ 16-slice SCT for colon cancer T staging are acceptable, while there is a relatively low sensitivity and specificity for colon cancer N staging, which is indicative of the good diagnostic value of ≥ 16-slice SCT for local staging of colon cancer. These findings need to be confirmed in further clinical studies.
In the future, further clinical studies should be carried out to prove the accuracy of 16-slice SCT for local staging of colon cancer.
We would like to thank all authors of the included primary studies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dauyey K, Kazakhstan; Sun C, United States A-Editor: Yao QG, China S-Editor: Xing YX L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Binefa G, Rodríguez-Moranta F, Teule A, Medina-Hayas M. Colorectal cancer: from prevention to personalized medicine. World J Gastroenterol. 2014;20:6786-6808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 245] [Cited by in RCA: 256] [Article Influence: 23.3] [Reference Citation Analysis (3)] |
| 2. | Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3526] [Cited by in RCA: 3657] [Article Influence: 304.8] [Reference Citation Analysis (2)] |
| 3. | International Agency for Research on Cancer. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. World Health Organization Disponible en: http://www-globocan-iarc-fr-2018. |
| 4. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3270] [Article Influence: 654.0] [Reference Citation Analysis (2)] |
| 5. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 12991] [Article Influence: 1443.4] [Reference Citation Analysis (2)] |
| 6. | van de Velde CJ, Aristei C, Boelens PG, Beets-Tan RG, Blomqvist L, Borras JM, van den Broek CB, Brown G, Coebergh JW, Cutsem EV, Espin E, Gore-Booth J, Glimelius B, Haustermans K, Henning G, Iversen LH, Han van Krieken J, Marijnen CA, Mroczkowski P, Nagtegaal I, Naredi P, Ortiz H, Påhlman L, Quirke P, Rödel C, Roth A, Rutten HJ, Schmoll HJ, Smith J, Tanis PJ, Taylor C, Wibe A, Gambacorta MA, Meldolesi E, Wiggers T, Cervantes A, Valentini V; European Registration of Cancer Care. EURECCA colorectal: multidisciplinary mission statement on better care for patients with colon and rectal cancer in Europe. Eur J Cancer. 2013;49:2784-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Tudyka V, Blomqvist L, Beets-Tan RG, Boelens PG, Valentini V, van de Velde CJ, Dieguez A, Brown G. EURECCA consensus conference highlights about colon & rectal cancer multidisciplinary management: the radiology experts review. Eur J Surg Oncol. 2014;40:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6:29765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 681] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 9. | Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol. 2021;116:458-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 458] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 10. | Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, Nagtegaal ID, Beets-Tan RG, Arnold D, Ciardiello F, Hoff P, Kerr D, Köhne CH, Labianca R, Price T, Scheithauer W, Sobrero A, Tabernero J, Aderka D, Barroso S, Bodoky G, Douillard JY, El Ghazaly H, Gallardo J, Garin A, Glynne-Jones R, Jordan K, Meshcheryakov A, Papamichail D, Pfeiffer P, Souglakos I, Turhal S, Cervantes A. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1107] [Article Influence: 85.2] [Reference Citation Analysis (1)] |
| 11. | Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2286] [Cited by in RCA: 2430] [Article Influence: 270.0] [Reference Citation Analysis (31)] |
| 12. | Nerad E, Lahaye MJ, Maas M, Nelemans P, Bakers FC, Beets GL, Beets-Tan RG. Diagnostic Accuracy of CT for Local Staging of Colon Cancer: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol. 2016;207:984-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 13. | Moga C, Guo B, Schopflocher D, Harstall C. Development of a quality appraisal tool for case series studies using a modified Delphi technique. Edmonton AB: Institute of Health Economics. Canada, 2012. |
| 14. | Greenland S. Quality scores are useless and potentially misleading. Am J Epidemiol. 1994;140:300-301. |
| 15. | Tezcan D, Türkvatan A, Türkoğlu MA, Bostancı EB, Sakaoğullları Z. Preoperative staging of colorectal cancer: accuracy of single portal venous phase multidetector computed tomography. Clin Imaging. 2013;37:1048-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Rollvén E, Holm T, Glimelius B, Lörinc E, Blomqvist L. Potentials of high resolution magnetic resonance imaging vs computed tomography for preoperative local staging of colon cancer. Acta Radiol. 2013;54:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | da Fonte AC, Chojniak R, de Oliveira Ferreira F, Pinto PN, dos Santos Neto PJ, Bitencourt AG. Inclusion of computed tomographic colonography on pre-operative CT for patients with colorectal cancer. Eur J Radiol. 2012;81:e298-e303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Sibileau E, Ridereau-Zins C, Vanel D, Pavageau AH, Bertrais S, Metivier-Cesbron E, Venara A, Aubé C. Accuracy of water-enema multidetector computed tomography (WE-MDCT) in colon cancer staging: a prospective study. Abdom Imaging. 2014;39:941-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Hunter C, Siddiqui M, Georgiou Delisle T, Blake H, Jeyadevan N, Abulafi M, Swift I, Toomey P, Brown G. CT and 3-T MRI accurately identify T3c disease in colon cancer, which strongly predicts disease-free survival. Clin Radiol. 2017;72:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Lee JH, Lee MR. Positron emission tomography/computed tomography in the staging of colon cancer. Ann Coloproctol. 2014;30:23-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Maupoey Ibáñez J, Pàmies Guilabert J, Frasson M, Boscà Robledo A, Giner Segura F, García-Granero Ximénez E. Accuracy of CT colonography in the preoperative staging of colon cancer: a prospective study of 217 patients. Colorectal Dis. 2019;21:1151-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Dighe S, Blake H, Koh MD, Swift I, Arnaout A, Temple L, Barbachano Y, Brown G. Accuracy of multidetector computed tomography in identifying poor prognostic factors in colonic cancer. Br J Surg. 2010;97:1407-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Lao IH, Chao H, Wang YJ, Mak CW, Tzeng WS, Wu RH, Chang ST, Fang JL. Computed tomography has low sensitivity for the diagnosis of early colon cancer. Colorectal Dis. 2013;15:807-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Flor N, Ceretti AP, Mezzanzanica M, Rigamonti P, Peri M, Tresoldi S, Soldi S, Mangiavillano B, Sardanelli F, Cornalba GP. Impact of contrast-enhanced computed tomography colonography on laparoscopic surgical planning of colorectal cancer. Abdom Imaging. 2013;38:1024-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Malmstrøm ML, Brisling S, Klausen TW, Săftoiu A, Perner T, Vilmann P, Gögenur I. Staging with computed tomography of patients with colon cancer. Int J Colorectal Dis. 2018;33:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88:1739-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Nong LH, Bing-Feng LU. Advances in image evaluation of colon carcinoma. Zhongguo Linchuang Yixue Yingxiang Zazhi. 2017;10:193-196. |
| 28. | Mauchley DC, Lynge DC, Langdale LA, Stelzner MG, Mock CN, Billingsley KG. Clinical utility and cost-effectiveness of routine preoperative computed tomography scanning in patients with colon cancer. Am J Surg. 2005;189:512-7; discussion 517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Smith NJ, Bees N, Barbachano Y, Norman AR, Swift RI, Brown G. Preoperative computed tomography staging of nonmetastatic colon cancer predicts outcome: implications for clinical trials. Br J Cancer. 2007;96:1030-1036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A; Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2653] [Cited by in RCA: 2732] [Article Influence: 130.1] [Reference Citation Analysis (0)] |
| 31. | De Gramont A, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Marceau-Suissa J, Lorenzato C. Oxaliplatin/5FU/LV in the adjuvant treatment of stage II and stage III colon cancer: efficacy results with a median follow-up of 4 years. J Clin Oncol. 2005;23:3501-3501. |