Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6446
Peer-review started: January 24, 2022
First decision: April 7, 2022
Revised: April 29, 2022
Accepted: June 15, 2022
Article in press: June 15, 2022
Published online: July 6, 2022
Processing time: 151 Days and 2 Hours
Colorectal cancer remains a considerable challenge in healthcare nowadays. Approximately 60%-80% of colorectal cancer is caused by intestinal polyps, and resection of intestinal polyps has been proved to reduce the incidence of colorectal cancer. The vast majority of intestinal polyps can be found during colonoscopy and removed endoscopically. Therefore, more attention has been paid to the development of endoscopic resection of intestinal polyps. In this study, we compared the efficacy and safety of cold snare polypectomy (CSP) and hot snare polypectomy (HSP).
To investigate the efficacy and safety of CSP and HSP for colorectal polyps.
Between January and December 2020, 301 patients with colorectal polyps 4-9 mm in diameter were treated with endoscopic therapy in our hospital, and were divided into the CSP group (n = 154) and HSP group (n = 147). The operating time, incidence of bleeding and perforation, use of titanium clips, and complete resection rate were compared between the two groups.
We included 249 patients (301 polyps). No differences in gender, age, and polyp size, location, shape and type were observed between the CSP and HSP groups, and the resection rates in these two groups were 93.4% and 94.5%, respectively, with no significant difference. The use of titanium clips was 15.6% and 95.9%, the operating time was 3.2 ± 0.5 min and 5.6 ± 0.8 min, the delayed bleeding rate was 0% and 2.0%, and delayed perforation was 0% and 0.7%, in the CSP and HSP groups, respectively.
For sessile colorectal polyps < 10 mm, CSP had the same resection rate of impaired tissue integrity as traditional HSP had. The rate of complications was lower in the CSP group. CSP is a safe and effective method for polypectomy.
Core Tip: This is an article about polypectomy for small colorectal polyps, and this is a retrospective study. The objective of this study is to investigate the efficacy and safety of cold snare polypectomy and hot snare polypectomy in the treatment of colorectal polyps with a diameter of 4-9 mm.
- Citation: Meng QQ, Rao M, Gao PJ. Effect of cold snare polypectomy for small colorectal polyps. World J Clin Cases 2022; 10(19): 6446-6455
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6446.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6446
Colorectal cancer is one of the most common malignant tumors of the digestive tract, and has gained more attention in recent years due to its increasing morbidity and mortality[1,2]. Approximately 60%-80% of colorectal cancer is caused by intestinal polyps, and resection of intestinal polyps has been proved to reduce the incidence of colorectal cancer[3]. The vast majority of intestinal polyps can be found during colonoscopy and removed endoscopically. Therefore, more attention has been paid to the development of endoscopic resection of intestinal polyps. Ninety percent of polyps found during colonoscopy are < 10 mm in size[4], and the standard methods used to remove small polyps include biopsy forceps, cold snare polypectomy (CSP), and hot snare polypectomy (HSP). HSP is a popular method that has been used for many years and has a positive therapeutic effect[5]. The principle of HSP is the use a high-frequency current to generate a large amount of localized heat. The polyp is solidified and removed. In clinical practice, the range of injury caused by the current exceeds the range observed during the operation, and complications such as bleeding and perforation occur rapidly postoperatively[6]. Although CSP uses a snare to remove the polyps, without a high-frequency current, the incidence of delayed bleeding and perforation after the operation is low. Therefore, CSP is considered to be a safe operation with a high complete resection rate, and its clinical utilization rate has increased significantly. In 2017, the European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guidelines[7] recom
A total of 249 patients who underwent colorectal polypectomy in the Endoscopic Center of the First Hospital of Jilin University between January and December 2020 were divided into the CSP group (n = 140) and HSP group (n = 109). Inclusion criteria were: (1) Age ≥ 18 years; and (2) Single or multiple polyps with a diameter of 4-9 mm, classified as 0-IS, 0-ISP and 0-IIA polyps using the Paris type classification. Exclusion criteria were: (1) Familial polyposis or inflammatory bowel disease; (2) Coagulation dysfunction or anticoagulant and antiplatelet drug treatment within 1 wk of the study; (3) Intestinal cleaning was insufficient; and (4) Suspected canceration. This research was approved by the Medical Ethics Committee of our institution.
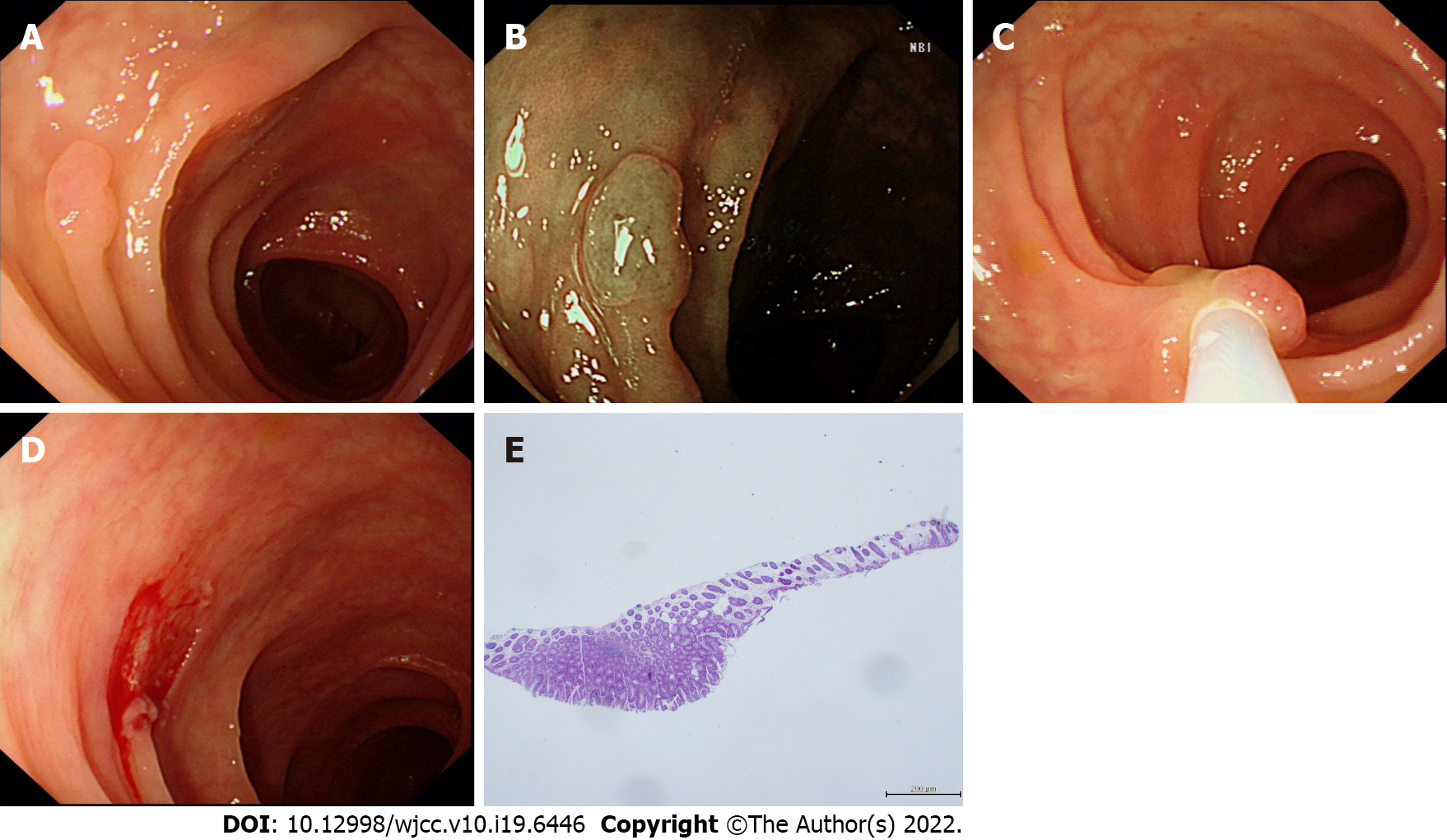
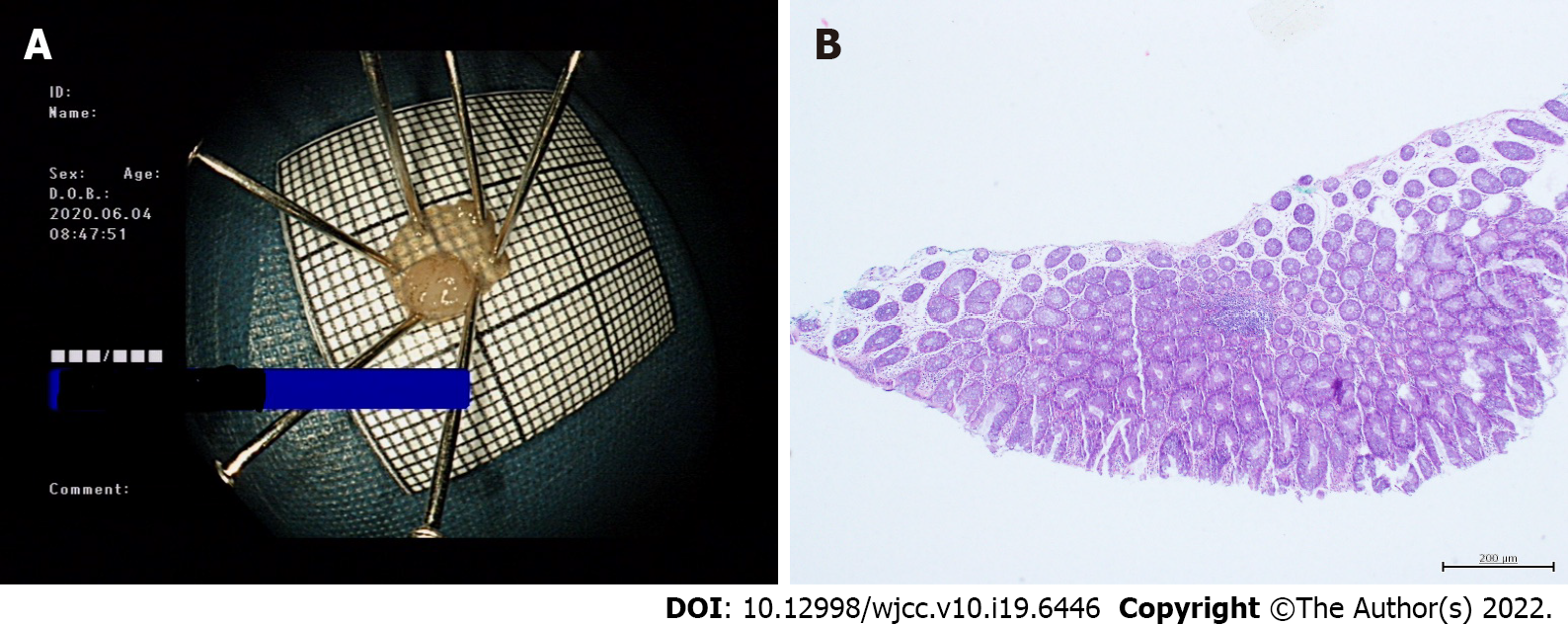
Before the operation, both groups were prepared by consuming a liquid diet for 24 h followed by 2 L oral polyethylene glycol electrolyte solution, and simethicone was used to remove bubbles. Colonoscopy and treatment were performed by two experienced endoscopists with > 5 years of clinical experience. Patients were treated by standard colonoscopy or magnifying endoscopy (CF-H290I, CF-HQ290I or PCF-Q290JI; Olympus, Japan), and colorectal polyps were identified by endoscopy. The location, size and shape of the polyps were recorded, and they were classified by Paris type[8]. The polyp size was estimated according to the open biopsy forceps opening or the fully open snare. In the CSP group, a unique cold snare (CAPTIVATOR II; Boston Scientific, United States) was used to capture the focus and normal tissues with a margin of 1-3 mm. When tightening the snare, it was pressed down toward the intestinal wall. The snare was then quickly tightened to mechanically remove the polyps and surrounding tissues[9,10] (Figure 1). Following an injection of saline at the base of the lesion (depending on the size of the polyp), the snare was used to trap the root of the polyp and was gradually tightened in the HSP group. The polyp was resected by electrocoagulation with a high-frequency electrotome (ERBE VIO200D), and specimens were recovered using a biopsy forceps or suction. According to the size of the wound and the presence or absence of active bleeding, titanium clips were used to clamp the wound. The location, size, quantity, operating time and complications of polypectomy were recorded in detail during the operation. The resected tissues were fixed with formaldehyde solution and then sent for pathological examination. The pathological evaluation of resected lesions was based on World Health Organization standards, and additional biopsy samples of lateral and basal margins were evaluated in detail for tumor involvement.
Observation indicators included the following: general characteristics of the patients and polyps in the two groups; complete polypectomy rate and recovery rate; operating time and use of titanium clips during the operation; and operative complications (including immediate bleeding during the operation, delayed bleeding after the operation, perforation during the operation and delayed perforation). Histologically complete resection was determined by histopathological evaluation of the resected polyp specimen and the biopsy specimen at the lower side of the polyp. Incomplete resection was defined as the presence of pathological tissue in the biopsy specimen, the lower side of the polyp specimen, or at the bottom and periphery of the resection site, and complete histological resection was defined as negative results for the above parameters[11,12]. The polypectomy time referred to the time from the preparation of instruments to the completion of specimen recovery. Immediate bleeding during the operation referred to bleeding for > 30 s after the operation and was stopped by endoscopy[13]. Delayed bleeding after the operation referred to bleeding requiring endoscopic intervention within 2 wk after polypectomy.
All analyses were performed using SPSS for Windows. The measurement data were expressed as the mean ± SD, and comparisons of means between the two groups were carried out using the independent t-test. Numerical data were expressed as n (%), and the χ2 test or Fisher’s exact test was adopted, and the test standard (α) was 0.05.
We enrolled 249 patients with 301 polyps. There were no significant differences in general data such as gender and age between the two groups (Table 1). One hundred and fifty-four polyps were identified in the CSP group and 147 in the HSP group (Table 2). There were no significant differences in polyp size, polyp location, morphological classification and pathological classification between the two groups.
| CSP (n = 140) | HSP (n = 109) | P value | |
| Gender, n (%) | 0.755 | ||
| Male (132) | 73 (52.1) | 59 (54.1) | |
| Female (117) | 67 (47.9) | 50 (45.9) | |
| Age (yr) | 0.268 | ||
| 18-81 (56.1 ± 12.1) | 20-81 (55.4 ± 12.1) | 18-81 (57.1 ± 12.1) |
| CSP (n = 154) | HSP (n = 147) | P value | |
| Size (mm) | 5.8 ± 1.4 | 5.8 ± 1.3 | 0.861 |
| 4-5, n (%) | 68 (44.2) | 59 (40.1) | 0.480 |
| 6-9, n (%) | 86 (55.8) | 88 (59.9) | |
| Location, n (%) | 0.446 | ||
| Right side of colon | 47 (30.5) | 44 (29.9) | |
| Transverse colon | 35 (22.7) | 27 (18.4) | |
| Left side of colon | 48 (31.2) | 43 (29.3) | |
| Rectum | 24 (15.6) | 33 (22.4) | |
| Histology, n (%) | 0.994 | ||
| Tubular adenoma | 120 (79.5) | 115 (79.3) | |
| Villous, n (%) | |||
| Tubular adenoma | 19 (12.6) | 18 (12.4) | |
| Serrated adenoma | 12 (7.9) | 12 (8.3) | |
| Paris type, n (%) | 0.529 | ||
| IS | 109 (72.2) | 103 (71.0) | |
| ISP | 15 (9.9) | 20 (13.8) | |
| IIa | 27 (17.9) | 22 (15.2) |
The CSP and HSP groups both had a high resection rate of impaired tissue integrity, and there was no significant difference between the groups (Table 3). Among the 10 cases of incomplete resection in the CSP group, two had tiny polyps of 4-5 mm, eight had polyps of 6-9 mm, seven had morphological type IIa and three had type I. There was no significant difference in tissue recovery between the two groups. The polyps in both groups in which recovery of tissues failed were all tiny polyps of 4-5 mm, and were located in the transverse colon and left side of the colon. In terms of titanium clip utilization rate and operating time, the CSP group was superior to the HSP group. In the HSP group, titanium clips were mainly used to prevent bleeding and perforation.
| CSP (n = 154) | HSP (n = 147) | P value | |
| Tissue recovery | 151/154 (98.1%) | 145/147 (98.6%) | 0.690 |
| Operation time (min) | 3.2 ± 0.5 | 5.6 ± 0.8 | < 0.001 |
| Usage of titanium clips | 24 (15.6%) | 141 (95.9%) | < 0.001 |
| Complete resection | 141/154 (93.4%) | 137/147 (94.5%) | 0.691 |
The immediate bleeding rate in the CSP group was higher than that in the HSP group (P < 0.05) (Table 4). No delayed bleeding was observed in the CSP group, but there were three cases in the HSP group (P < 0.05). These three patients with delayed bleeding did not bleed after secondary endoscopic therapy and clamping the wound with a titanium clip stopped the bleeding. One case of delayed perforation occurred in the HSP group, and the patient was discharged after conservative treatment. No intraoperative perforations occurred in either group.
| CSP (n = 154) | HSP (n = 147) | P value | |
| Immediate bleeding | 18/154 (11.7%) | 2/147 (1.4%) | < 0.001 |
| Delayed bleeding | - | 3/147 (2.0%) | 0.075 |
| Intraoperative perforation | - | - | - |
| Delayed perforation | - | 1/147 (0.7%) | 0.305 |
CSP was first proposed by Tappero et al[14] in 1992. Due to the short operating time and fewer complications, especially the low incidence of delayed bleeding, CSP has attracted the attention of many endoscopic physicians and has been widely used. In the guidelines for colorectal polypectomy and endoscopic mucosal resection issued by the ESGE in 2017, CSP was recommended for sessile polyps < 10 mm. For colorectal polyps > 10 mm, the safety of CSP requires confirmation[15]. Recently, Murakami et al[16] reported that the local recurrence rate after CSP for lesions < 10 mm and 10–14 mm was 1.4% and 5.4%, respectively; thus, CSP is not recommended for lesions ≥ 10 mm due to high rates of recurrence and malignancy. The ESGE guidelines were followed in our study. Colorectal polyps 4-9 mm in size detected during colonoscopy were randomly allocated to the HSP or CSP group in our study. Based on the patients’ general characteristics (age, gender, indication, preparation status, and endoscope used) and the characteristics of the polyps (location, morphology and size) being similar between the two groups, the rate of complications, complete resection and other results were compared between the CSP and HSP groups.
The most common complication of HSP and CSP is bleeding, which can be divided into immediate and delayed bleeding. Immediate bleeding is a common adverse event after CSP. A large prospective nonrandomized controlled trial by Repici et al[17] reported that the immediate bleeding rate of CSP was 1.8%. Jegadeesan et al[18] reported that the immediate bleeding rate of CSP was higher than that of HSP (6.6% vs 3.3%). In the present study, the immediate bleeding rate of CSP was 11.7%, which was higher than that in the HSP group (1.4%), but was similar to that in several previous studies[17,18]. It is suggested that this adverse event is not important clinically. As most sessile polyps are < 10 mm, there are few major vessels in the roots, bleeding usually occurs in venules or blood capillaries, and there is only minimal bleeding with spontaneous hemostasis a few seconds after mechanical cutting during CSP. Even in rare cases in which bleeding persists, endoscopic clipping is an effective management option. Delayed bleeding is often considered a common adverse event of HSP. Three postprocedural bleeding events occurred in patients who underwent HSP, and bleeding stopped in all patients after the second endoscopic intervention. A prospective study by Suzuki et al[19] observed the wounds after CSP and HSP. Although the size of the wound in the CSP group was larger than that in the HSP immediately after resection, 1 d later, the size of the wound in the CSP group was significantly smaller than that immediately after CSP. The sustained effect of HSP after electrocoagulation increased, suggesting that the wounds in the CSP group healed faster and were more conducive to reduced delayed bleeding compared with those in the HSP group. Surgical intervention or death rarely occurs due to delayed postpolypectomy bleeding[20], but delayed bleeding is thought to increase the risk and difficulty in emergency colonoscopy due to the presence of blood, and poor vision in the colon can lead to hospitalization, blood transfusion and repeated endoscopic hemostasis. The advantage of CSP is that immediate bleeding is easily identified and timely treated, avoiding secondary endoscopic intervention or surgical hemostasis, and obviating additional cost to patients. We found that clips had no advantage in preventing delayed bleeding after CSP, and the utilization rate of clips in the CSP group was only 15.6%. Except for immediate bleeding, clips were not used to prevent delayed bleeding, and no cases of delayed bleeding occurred in the CSP group. These results are similar to those reported by Kawamura et al[21]. Therefore, for sessile polyps < 10 mm, we do not recommend the preventive application of clips during CSP.
Complete resection is now considered a powerful indicator of the quality of colonoscopy. Kawamura et al[21] conducted a prospective, multicenter, randomized controlled, parallel, noninferiority trial in Japan to investigate the success rate of CSP for complete resection of 4-9 mm colorectal adenomatous polyps and compared the success rate with that of HSP. The complete resection rates with CSP and HSP were 98.2% and 97.4%, respectively, with no significant difference between the two groups. They then concluded that HSP and CSP resulted in the same complete resection rate for polyps < 10 mm by consulting the randomized controlled trials in PubMed and the Cochrane library[22]. However, some studies have shown that the complete resection rate was significantly lower with CSP than with HSP[23,24]. This may be explained as follows. First, electrocoagulation was not used with CSP; the resection area may have been inadequate; and residual lesions may have been present. Second, due to the lack of thermocoagulation marks after CSP, the lateral margins of the lesions were unclear, which affected histological evaluation of the specimens. In this study, the complete resection rate was 93.4% in the CSP group and 94.5% in the HSP group. The complete resection rate was high in both groups, and the difference was not significant. In the CSP group, the polyps were removed with the snare extended to normal tissues 1-3 mm away from the edge of the lesion, to ensure adequate margins, and to ensure no problems with histological evaluation of specimens (Figure 2). Some studies reported that the rate of incomplete resection was influenced by polyp size[22], and in this study the result was similar. However, morphological classification was seldom mentioned in previous studies. In this study, there were seven cases of incomplete resection with type IIa morphological classification in the CSP group, and the rate of incomplete resection was significantly higher than that of type IS and ISP. The residual tissue following CSP was in the lateral margins of the defect and not in the bottom margin, which was considered to be related to the morphological classification. The margin was that of a flat type IIa lesion, whose mucosa was similar to the peripheral boundary and was sometimes unclear. Suzuki et al[25] removed 145 lesions with CSP using linked color imaging (LCI), and the residual rate of tumor was 0.7%. They suggested that LCI easily identifies flat colorectal polyps. However, this remains to be confirmed. In recent years, with the development of CSP, some scholars think that CSP is inferior in obtaining submucosal tissue compared with HSP. Ito et al[26] reported that resection depth from muscularis mucosae in CSP versus hot-snare endoscopic mucosal resection was 76 μm versus 338 μm, and that resection of submucosa was achieved in 9% versus 92%, respectively. Shichijo et al[27] prospectively enrolled patients undergoing polypectomy for nonpedunculated polyps of 4-9 mm, and the overall incidence of incomplete mucosal layer resection was 63%. Thus, they concluded that CSP should be used for intraepithelial lesions only, and careful pretreatment evaluation is recommended. To improve the complete resection rate of CSP and improve the submucosal resection rate, some researchers have used submucosal injection before CSP (CSP-SI). Recently, some research on this has been carried out, but the conclusions showed clear differences. Some studies have shown that, compared with conventional CSP, CSP-SI has a significantly higher submucosal resection rate. However, a single-center prospective study by Shimodate et al[28] found that CSP-SI did not improve the resection depth of CSP for colorectal polyps < 10 mm, and the method resulted in lower rates of negative lateral and vertical margins of the resected lesions. Our research was limited to a comparison between conventional CSP and HSP (injection of saline according to the size of the lesion in the HSP group); thus, it was difficult to evaluate the effectiveness and safety of CSP-SI in our study. It has recently been reported that underwater CSP can obtain a higher complete resection rate and a sufficient deep resection compared with conventional CSP[29]. However, further data are required to confirm this. It has also been reported that improvement of the CSP snare may also be necessary to facilitate easy grasp of the lesions together with the submucosal layer[30].
In this study, CSP had an obvious advantage in terms of operating time compared with HSP. The specimen collection rates in the two groups were 98.1% and 98.6%, respectively. We suggest that the specimen collection rate had little to do with the resection method, and was mainly due to the size of the polyps and intestinal cleanliness.
There were some limitations to this study. First, it was a retrospective, single center study. Second, the follow-up data were insufficient. We attempted to obtain follow-up data from the enrolled population within 1 year after operation, but the sample size was too small to analyze. Third, the morphological classification included IS, ISP and IIa lesions, but none of the included patients had type IP polyps. At present, type IP polyps are mainly removed by HSP in our department. We look forward to applying CSP in these patients in future work, in order to better evaluate its safety and effectiveness.
In conclusion, this study revealed that the rate of complete resection with CSP was similar to that with HSP, and CSP resulted in fewer adverse events compared with HSP. Thus, CSP is safe and effective for 4-9 mm colorectal polyps. CSP is worthy of further examination to determine whether it can improve the complete resection rate and resection depth in combination with other endoscopic techniques.
Colorectal cancer remains a considerable challenge in healthcare nowadays and resection of intestinal polyps has been proved to reduce the incidence of colorectal cancer. Cold snare polypectomy (CSP) and hot snare polypectomy (HSP) are the common endoscopic therapy. In this study, we compared the efficacy and safety of CSP and HSP.
HSP is a traditional method that has been used for many years and the incidence of complications is high because of its high frequency electrocoagulation. In recent years, CSP has become the first choice for endoscopists for the advantages of time saving, convenience and less complications. This randomized controlled study was carried out to compare the operating time, incidence of bleeding and perforation, use of titanium clips, and complete resection rate.
To investigate the efficacy and safety of CSP and HSP in the treatment of colorectal polyps with a diameter of 4-9 mm, and to discuss whether it can improve the complete resection rate and resection depth in combination with other endoscopic techniques.
From January 2020 to December 2020, a total of 301 patients with colorectal polyps 4-9 mm in size were treated with endoscopic therapy in our hospital, and were divided into the CSP group (n = 154) and HSP group (n = 147). The operation time, incidence of bleeding and perforation, use of titanium clips and complete resection rate of histology were compared between the two groups.
Two hundred and forty-nine patients (301 polyps) were included in the study. No differences in gender, age, polyp size, location, shape and type were observed between the CSP and HSP groups, and the resection rates in these two groups were 93.4% and 94.5%, respectively, with no significant difference. The use of titanium clips was 15.6% and 95.9%, respectively. The operating time was 3.2 ± 0.5 min and 5.6 ± 0.8 min, the delayed bleeding rate in the two groups was 0% and 2.0%, and delayed perforation was 0% and 0.7%, respectively.
In the treatment of sessile colorectal polyps < 10 mm, CSP has the same resection rate of impaired tissue integrity as HSP, but the delayed bleeding and perforation rate are lower in CSP group. CSP is a safe and effective method for polypectomy.
This study retrospectively analyzed patients with colorectal polyps 4-9 mm in size treated by endoscopy in our hospital from January 2020 to December 2020. A comparison of HSP and CSP was carried including surgical outcome and safety.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Asadzade Aghdaei H, Iran; Katagiri A, Japan S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21368] [Article Influence: 2136.8] [Reference Citation Analysis (3)] |
| 2. | Yusup A, Wang HJ, Rahmutula A, Sayim P, Zhao ZL, Zhang GQ. Clinical features and prognosis in colorectal cancer patients with different ethnicities in Northwest China. World J Gastroenterol. 2013;19:7183-7188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Chakradhar S. Colorectal cancer: 5 big questions. Nature. 2015;521:S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Murino A, Hassan C, Repici A. The diminutive colon polyp: biopsy, snare, leave alone? Curr Opin Gastroenterol. 2016;32:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 5. | Binmoeller KF, Bohnacker S, Seifert H, Thonke F, Valdeyar H, Soehendra N. Endoscopic snare excision of "giant" colorectal polyps. Gastrointest Endosc. 1996;43:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 158] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Takayanagi D, Nemoto D, Isohata N, Endo S, Aizawa M, Utano K, Kumamoto K, Hojo H, Lefor AK, Togashi K. Histological Comparison of Cold versus Hot Snare Resections of the Colorectal Mucosa. Dis Colon Rectum. 2018;61:964-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 765] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 8. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1325] [Article Influence: 60.2] [Reference Citation Analysis (4)] |
| 9. | Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, Levy LC, Toor A, Mackenzie TA, Rosch T, Robertson DJ. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74-80.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 553] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 10. | Hewett DG. Cold snare polypectomy: optimizing technique and technology (with videos). Gastrointest Endosc. 2015;82:693-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Li D, Wang W, Xie J, Liu G, Wang R, Jiang C, Ye Z, Xu B, He X, Hong D. Efficacy and safety of three different endoscopic methods in treatment of 6-20 mm colorectal polyps. Scand J Gastroenterol. 2020;55:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Papastergiou V, Paraskeva KD, Fragaki M, Dimas I, Vardas E, Theodoropoulou A, Mathou N, Giannakopoulos A, Karmiris K, Mpitouli A, Apessou D, Giannikaki L, Karagiannis JA, Chlouverakis G, Paspatis GA. Cold versus hot endoscopic mucosal resection for nonpedunculated colorectal polyps sized 6-10 mm: a randomized trial. Endoscopy. 2018;50:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 13. | Horiuchi A, Nakayama Y, Kajiyama M, Tanaka N, Sano K, Graham DY. Removal of small colorectal polyps in anticoagulated patients: a prospective randomized comparison of cold snare and conventional polypectomy. Gastrointest Endosc. 2014;79:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 14. | Tappero G, Gaia E, De Giuli P, Martini S, Gubetta L, Emanuelli G. Cold snare excision of small colorectal polyps. Gastrointest Endosc. 1992;38:310-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Van Overbeke L, Ilegems S, Mertens G, Mortier L, van Dongen J, Verbeke L, Van Dijck H, Jacomen G. Cold snare endoscopic resection of nonpedunculated colorectal polyps larger than 10 mm. A retrospective series. Acta Gastroenterol Belg. 2019;82:475-478. [PubMed] |
| 16. | Murakami T, Yoshida N, Yasuda R, Hirose R, Inoue K, Dohi O, Kamada K, Uchiyama K, Konishi H, Naito Y, Morinaga Y, Kishimoto M, Konishi E, Ogiso K, Inada Y, Itoh Y. Local recurrence and its risk factors after cold snare polypectomy of colorectal polyps. Surg Endosc. 2020;34:2918-2925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Repici A, Hassan C, Vitetta E, Ferrara E, Manes G, Gullotti G, Princiotta A, Dulbecco P, Gaffuri N, Bettoni E, Pagano N, Rando G, Strangio G, Carlino A, Romeo F, de Paula Pessoa Ferreira D, Zullo A, Ridola L, Malesci A. Safety of cold polypectomy for <10mm polyps at colonoscopy: a prospective multicenter study. Endoscopy. 2012;44:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 18. | Jegadeesan R, Aziz M, Desai M, Sundararajan T, Gorrepati VS, Chandrasekar VT, Jayaraj M, Singh P, Saeed A, Rai T, Choudhary A, Repici A, Hassan C, Fuccio L, Sharma P. Hot snare vs. cold snare polypectomy for endoscopic removal of 4 - 10 mm colorectal polyps during colonoscopy: a systematic review and meta-analysis of randomized controlled studies. Endosc Int Open. 2019;7:E708-E716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Suzuki S, Gotoda T, Kusano C, Ikehara H, Sugita A, Yamauchi M, Moriyama M. Width and depth of resection for small colorectal polyps: hot versus cold snare polypectomy. Gastrointest Endosc. 2018;87:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 20. | Feagins LA. Colonoscopy, Polypectomy, and the Risk of Bleeding. Med Clin North Am. 2019;103:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Kawamura T, Takeuchi Y, Asai S, Yokota I, Akamine E, Kato M, Akamatsu T, Tada K, Komeda Y, Iwatate M, Kawakami K, Nishikawa M, Watanabe D, Yamauchi A, Fukata N, Shimatani M, Ooi M, Fujita K, Sano Y, Kashida H, Hirose S, Iwagami H, Uedo N, Teramukai S, Tanaka K. A comparison of the resection rate for cold and hot snare polypectomy for 4-9 mm colorectal polyps: a multicentre randomised controlled trial (CRESCENT study). Gut. 2018;67:1950-1957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 22. | Kawamura T, Takeuchi Y, Yokota I, Takagaki N. Indications for Cold Polypectomy Stratified by the Colorectal Polyp Size: A Systematic Review and Meta-Analysis. J Anus Rectum Colon. 2020;4:67-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Takeuchi Y, Yamashina T, Matsuura N, Ito T, Fujii M, Nagai K, Matsui F, Akasaka T, Hanaoka N, Higashino K, Iishi H, Ishihara R, Thorlacius H, Uedo N. Feasibility of cold snare polypectomy in Japan: A pilot study. World J Gastrointest Endosc. 2015;7:1250-1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Zhang Q, Gao P, Han B, Xu J, Shen Y. Polypectomy for complete endoscopic resection of small colorectal polyps. Gastrointest Endosc. 2018;87:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 25. | Suzuki T, Kitagawa Y, Nankinzan R, Yamaguchi T. Usefulness of cold polypectomy under linked color imaging. Endosc Int Open. 2020;8:E87-E91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Ito A, Suga T, Ota H, Tateiwa N, Matsumoto A, Tanaka E. Resection depth and layer of cold snare polypectomy versus endoscopic mucosal resection. J Gastroenterol. 2018;53:1171-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 27. | Shichijo S, Takeuchi Y, Kitamura M, Kono M, Shimamoto Y, Fukuda H, Nakagawa K, Ohmori M, Arao M, Iwatsubo T, Iwagami H, Matsuno K, Inoue S, Matsuura N, Nakahira H, Maekawa A, Kanesaka T, Higashino K, Uedo N, Fukui K, Ito Y, Nakatsuka SI, Ishihara R. Does cold snare polypectomy completely resect the mucosal layer? J Gastroenterol Hepatol. 2020;35:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Shimodate Y, Itakura J, Takayama H, Ueno M, Takezawa R, Nishimura N, Mouri H, Sunami T, Hirai R, Yamamoto S, Miyake M, Matsueda K, Yamamoto Y, Mizuno M. Impact of submucosal saline solution injection for cold snare polypectomy of small colorectal polyps: a randomized controlled study. Gastrointest Endosc. 2020;92:715-722.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Maruoka D, Kishimoto T, Matsumura T, Arai M, Akizue N, Ishikawa K, Ohta Y, Kasamatsu S, Taida T, Ishigami H, Okimoto K, Saito K, Nakagawa T, Kato N. Underwater cold snare polypectomy for colorectal adenomas. Dig Endosc. 2019;31:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Horiuchi A, Hosoi K, Kajiyama M, Tanaka N, Sano K, Graham DY. Prospective, randomized comparison of 2 methods of cold snare polypectomy for small colorectal polyps. Gastrointest Endosc. 2015;82:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |