Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6289
Peer-review started: December 26, 2021
First decision: March 7, 2022
Revised: March 20, 2022
Accepted: April 24, 2022
Article in press: April 24, 2022
Published online: June 26, 2022
Processing time: 172 Days and 20.4 Hours
Left-dominant arrhythmogenic cardiomyopathy (LDAC) is a relatively rare disease characterized by poor prognosis that exacerbates the incidence of sudden cardiac death and ventricular arrhythmias. Clinically, LDAC is constantly overlooked or misdiagnosed as myocardial infarction, myocarditis, and dilated cardiomyopathy, owing to atypical and nonspecific clinical manifestations at an early stage.
A 57-year-old woman was diagnosed with sinus bradycardia and chronic bifascicular block during a health check. She occasionally experienced mild chest pain and paroxysmal palpitation during activity in the past 2 years. Comprehensive auxiliary examinations, including electrocardiogram, echocardiography, coronary computerized tomography angiography, and magnetic resonance imaging, revealed that she had LDAC instead of congenital ventricular diver
Based on this case, clinicians need to be aware of LDAC in patients with localized left ventricular lesions and multiple electrocardiographic abnormalities. Mul
Core Tip: Left-dominant arrhythmogenic cardiomyopathy is a relatively rare disease, characterized by poor prognosis. We present a case with a dilated left ventricle that manifested with reduced ejection fraction, multiple outpouchings, left chest leads low voltage, and fragmented QRS. Multimodality cardiovascular imaging diagnosed the patient with left-dominant arrhythmogenic cardiomyopathy instead of congenital ventricular diverticulum. This case alerts clinicians to be aware of left-dominant arrhythmogenic cardiomyopathy in patients with localized left ventricular lesions and multiple electrocardiographic abnormalities.
- Citation: Zhang X, Ye RY, Chen XP. Dilated left ventricle with multiple outpouchings — a severe congenital ventricular diverticulum or left-dominant arrhythmogenic cardiomyopathy: A case report. World J Clin Cases 2022; 10(18): 6289-6297
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6289.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6289
Left-dominant arrhythmogenic cardiomyopathy (LDAC) is a non-hypertrophic, non-hypertensive, and non-valvular progressive cardiomyopathy with fibrofatty myocardium infiltration that prominently occurs in the left ventricle[1]. LDAC might present with ventricular outpouching, which makes it difficult to differentiate from congenital ventricular diverticulum from the echocardiography[2]. Here, we present a case of LDAC with left chest leads low voltage, fragmented QRS (f-QRS), left ventricle (LV) dilation, LV systolic impairment, LV outpouchings, and late gadolinium enhancement of LV myo
A 57-year-old woman, presenting with a dilated left ventricle, reduced ejection fraction, and chronic bifascicular block, was referred to the cardiology department, West China Hospital, on December 10, 2019.
She occasionally experienced mild chest pain and paroxysmal palpitation during activity in the past 2 years. Her exercise capacity was also mildly reduced. She did not manifest symptoms of fatigue, dizziness, syncope, peripheral edema, and abdominal distention.
The patient had neither prior medical comorbidities nor addictions.
Her father had a premature sudden cardiac death at the age of 40.
The patient showed good nutrition, active position, clear mind, fluent language, and was cooperative in examination. Examinations on her whole skin and mucous membrane revealed no yellow staining, cyanosis, and bleeding spots. She had a blood pressure and resting heart rate of 120/68 mmHg and 60 beats per min, respectively, and auscultation of both lungs was normal with neither dry nor wet rales. The apical pulse was located 0.5 cm lateral to the midclavicular line on the left side of the fifth rib, while her heart rhythm was regular. The first and second heart sounds were basically normal, without extra and splitting of the heart sound. No valvular murmurs were detected in any of the auscultation areas. In addition, she did not exhibit any physical signs of heart failure, including edema, ascites, jugular venous distention, and hepatojugular reflux.
Results from the routine blood test and plasma biochemical examinations, including kidney and liver function, glucose, lipid, and electrolyte, were normal. Similarly, thyroid function, kappa and lambda urine free light chains as well as coagulation profile and autoimmune antibodies were also within the normal range. The plasma N-terminal fragment of the pro-brain natriuretic peptide was 325 ng/L.
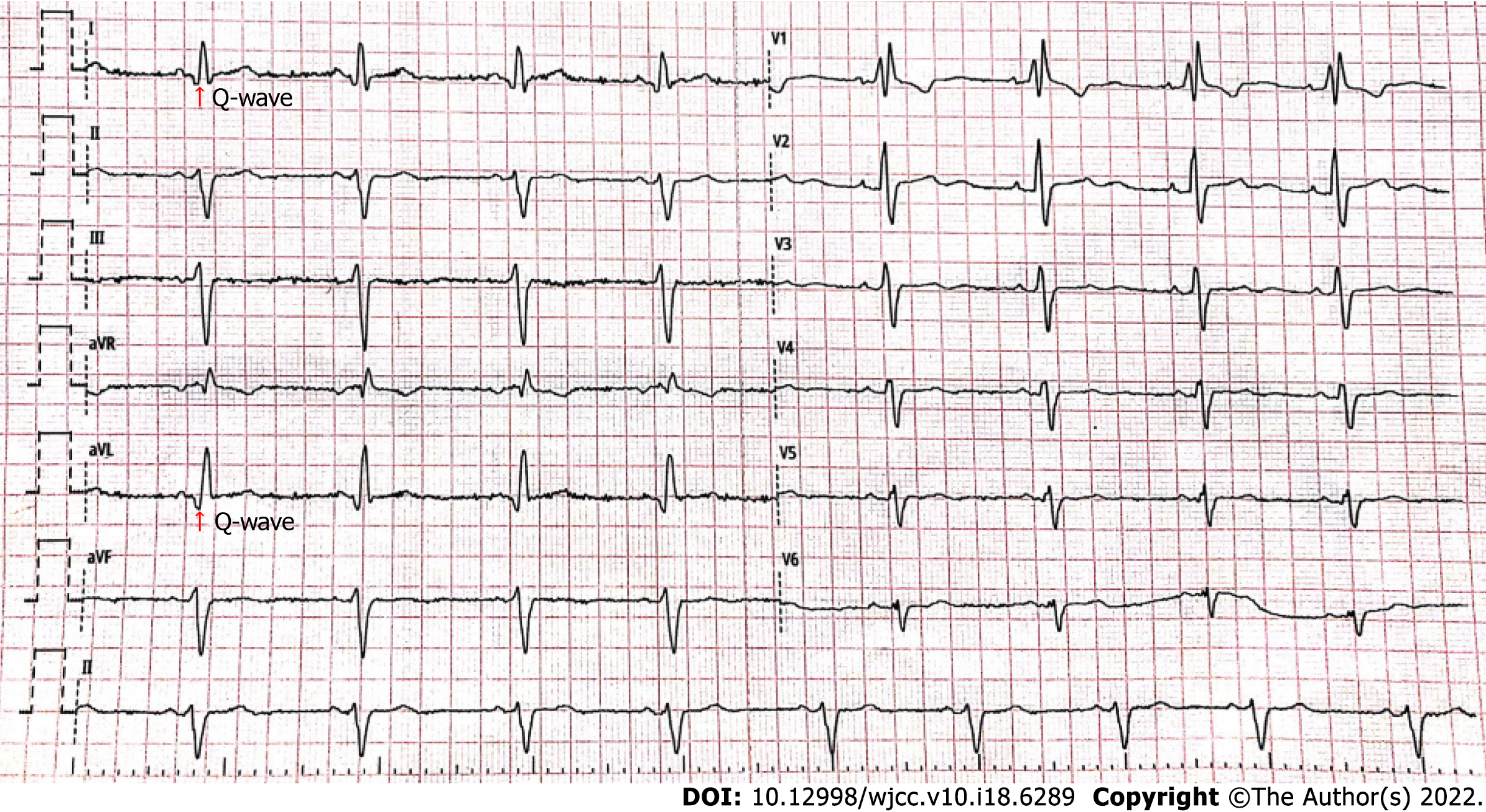
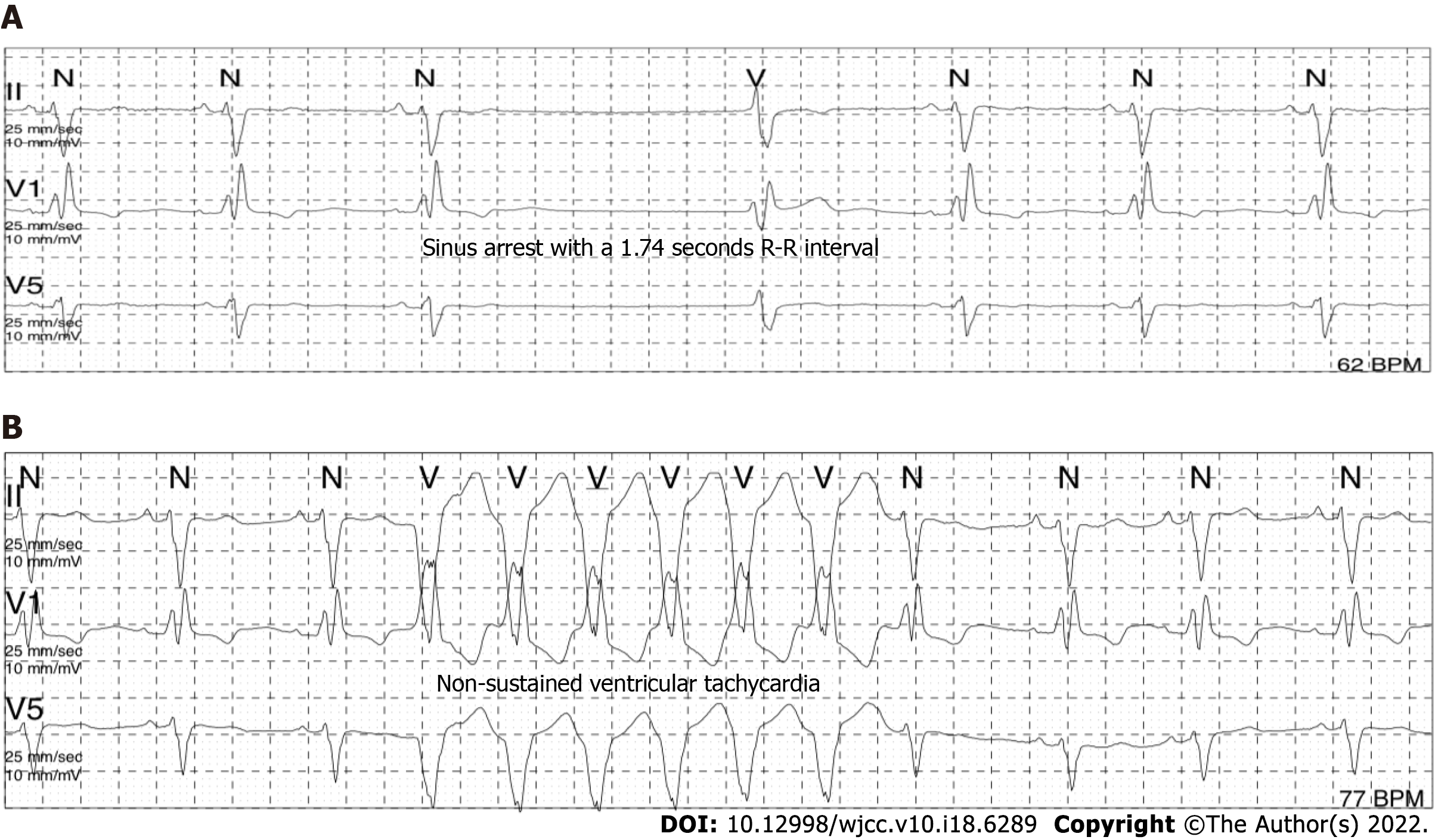
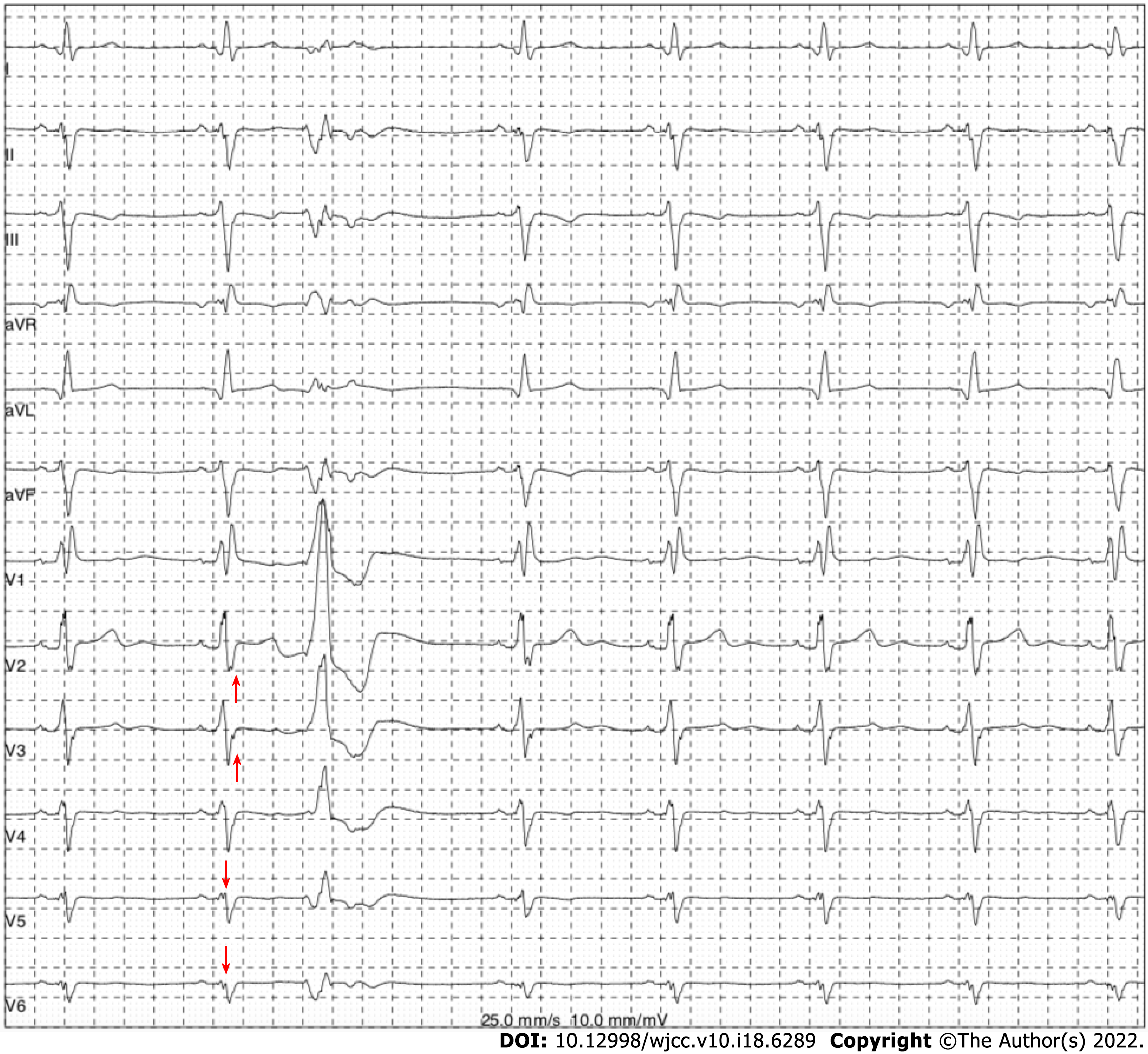
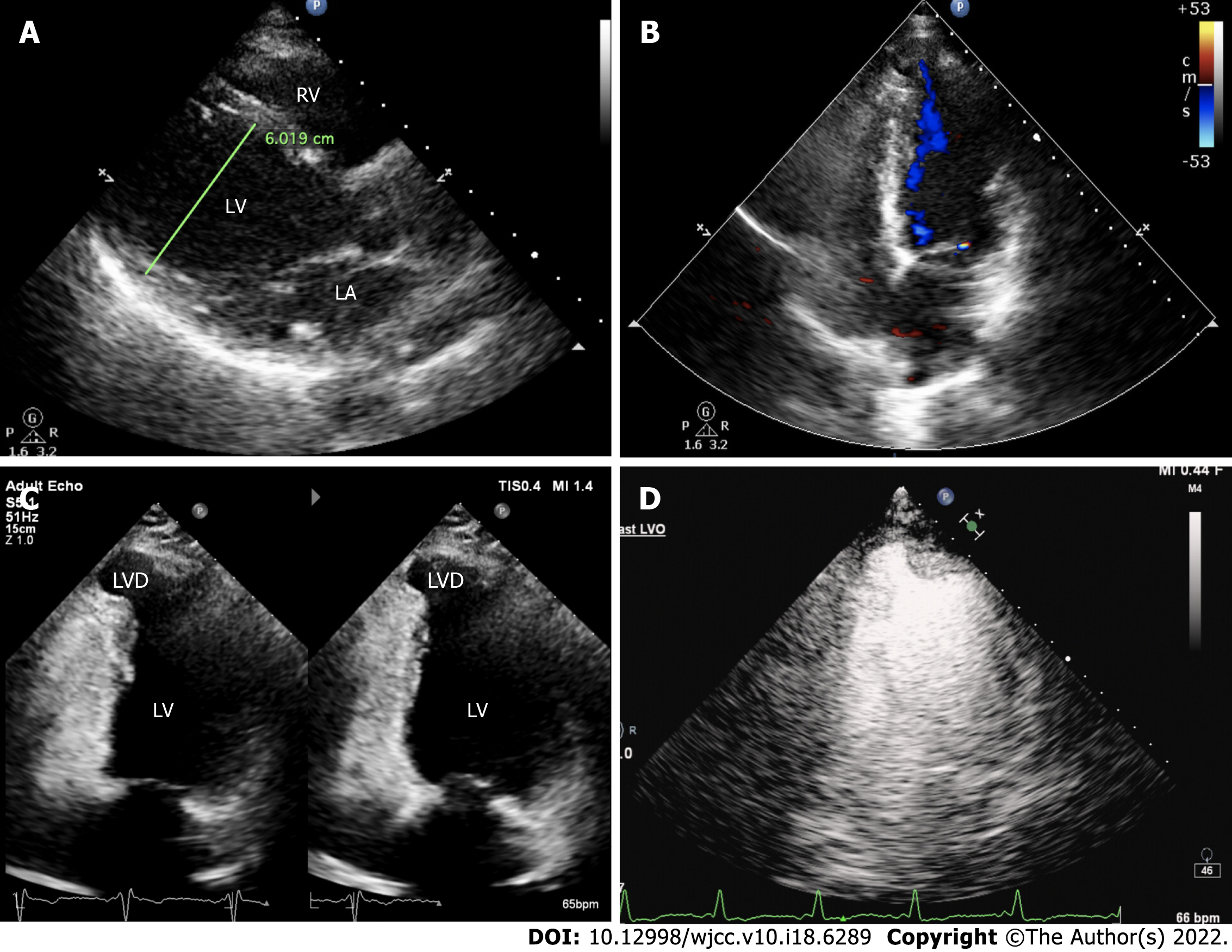
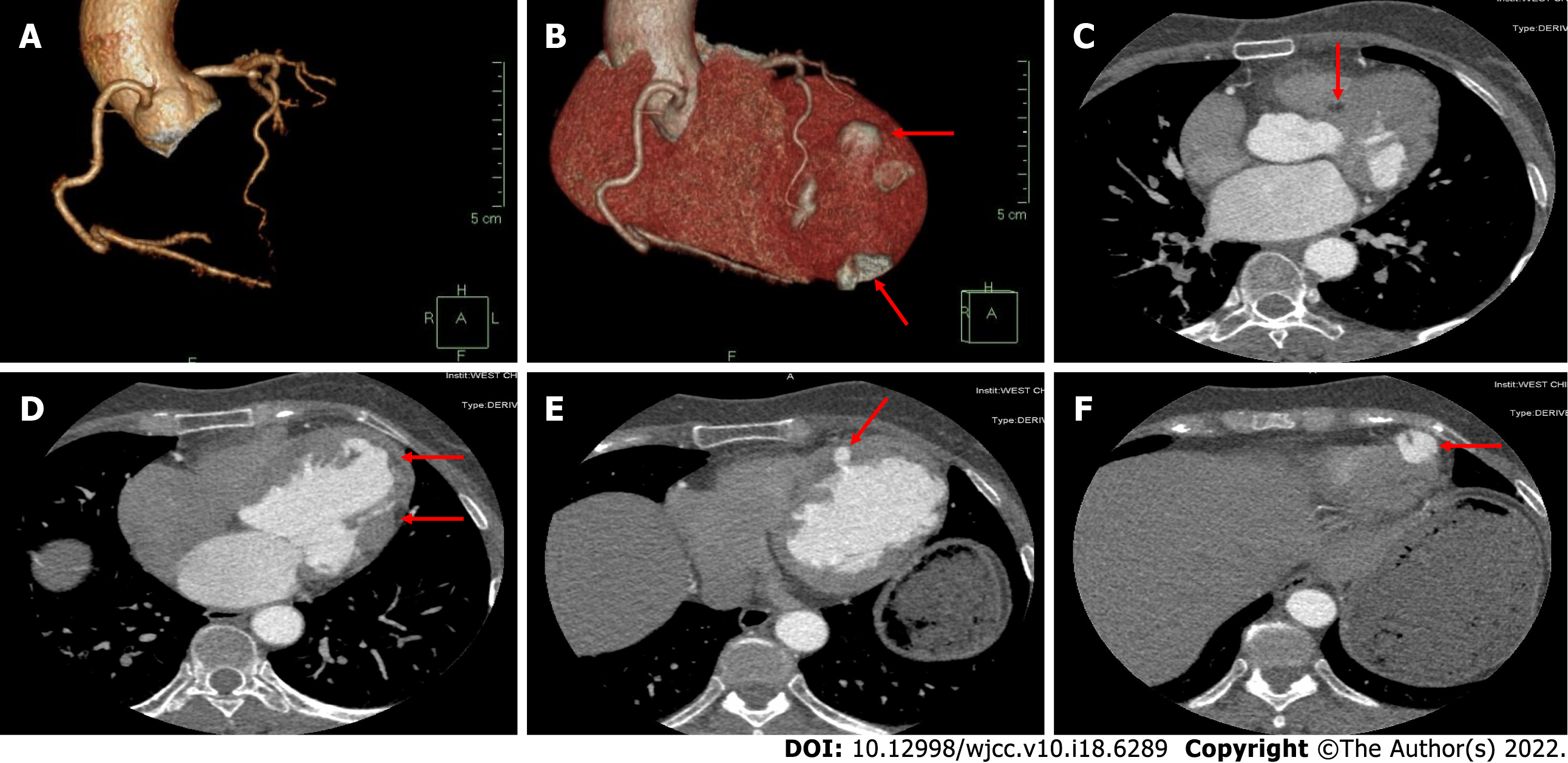
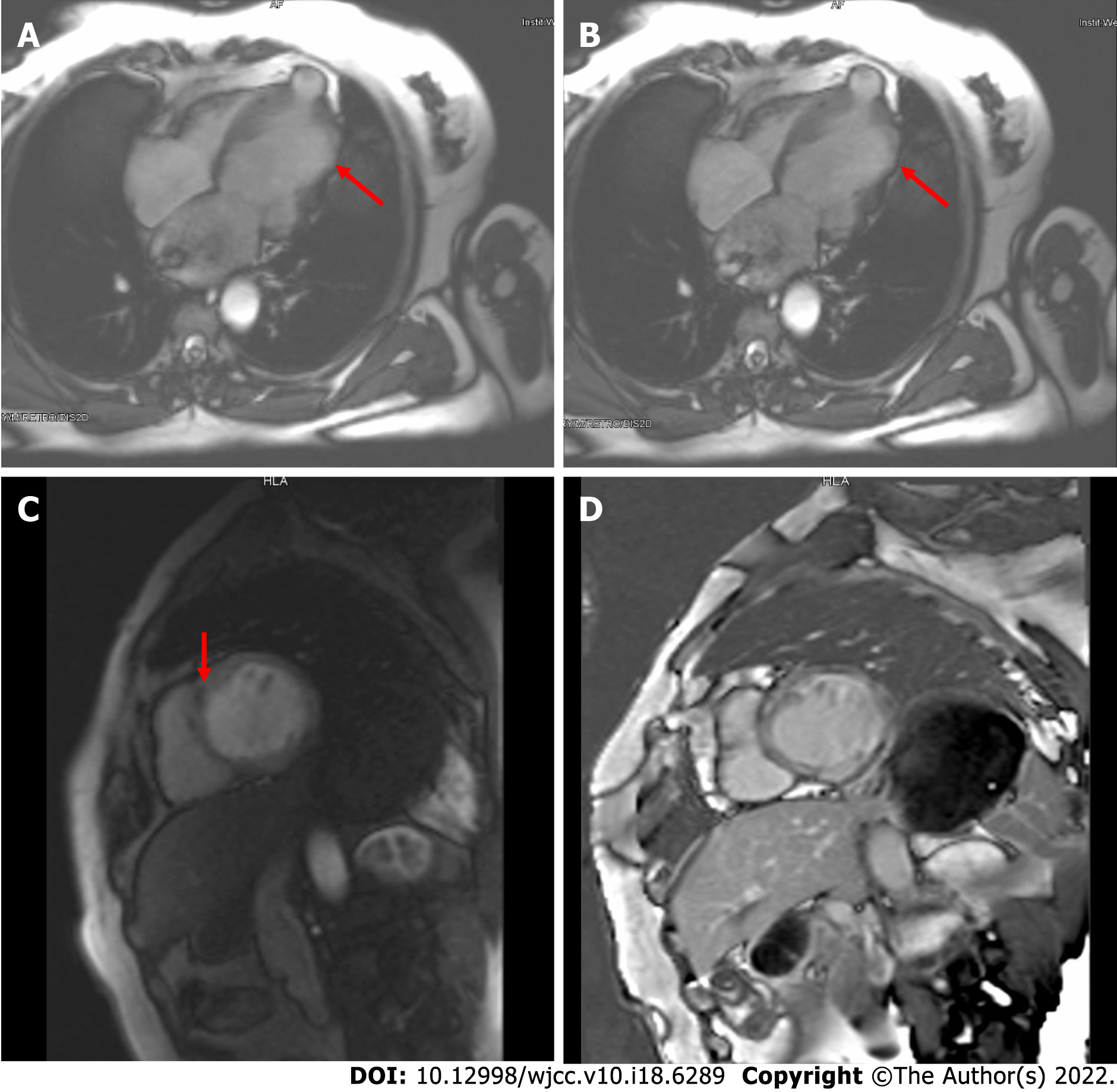
The electrocardiogram (Figure 1) revealed left anterior branch block, complete right bundle branch block (RBBB), high sidewall abnormal Q wave, and left chest leads (V4-V6) low voltage with poor R wave progression. On the other hand, Holter monitoring (Figures 2 and 3) revealed sinus arrest with a 1.74 s R-R interval, multisource premature ventricular beats, non-sustained ventricular tachycardia with an RBBB pattern, and f-QRS in leads V3-V6. Transthoracic echocardiography (TTE) showed a dilated LV with a diameter of 60 mm as well as a reduced LV ejection fraction of 35% and a left ventricular apex cystic outpouching (12 mm × 13 mm) that displayed synchronous contractility (Figure 4A-C). Myocardial contrast echocardiography revealed contractile outpouching without obvious filling defects (Figure 4D). Coronary computerized tomography angiography (CCTA) revealed right dominant coronary artery circulation without obvious stenosis, LV multiple outpouchings, uneven thickness of the LV wall (Figure 5A, B, E, and F), hypodense region (CT value -90~-114 HU) at localized myocardium of LV septum (Figure 5C), and free wall (Figure 5D). These findings were consistent with profiles of fatty tissue infiltration. In addition, we used cardiac magnetic resonance imaging (CMRI) to evaluate the cardiac structure, bilateral ventricular function, segmental movement, and tissue characterization in the patient. CMRI results revealed LV dilatation, abnormal activity of the LV wall (Figure 6A and B), an outpouching at the LV apex, and a low signal in the septum myocardium midwall after contrast injection (Figure 6C). Moreover, late gadolinium enhancement in the midwall of the left ventricular septum and free wall myocardium were also evident (Figure 6D).
High-throughput sequencing revealed no genetic variation with high clinical phenotype correlation and sufficient evidence of pathogenicity.
These findings highly pointed to LDAC.
After comprehensive evaluation of the patient, we prescribed sacubitril valsartan sodium tablets (50 mg bid) and spironolactone (20 mg qd). We did not administer a beta-blocker in this case, owing to multiple atrioventricular conduction abnormalities. In addition, she was given low-dose thiazide diuretics when needed to relieve edema and congestion symptoms. Three months later, her left ventricular size and systolic function had not changed compared to baseline. Consequently, she was subjected to an implantable cardioverter-defibrillator after full discussion in our department.
Two years later, we re-evaluated her symptoms and clinical indexes and found that the symptoms improved after taking standard oral medication for ejection fraction reduced heart failure. The pacemaker program did not record sustained ventricular tachycardia or ventricular fibrillation. Echocardiography revealed that left ventricular size and systolic function were almost similar to 2 years prior to treatment.
This case affirms the need for clinicians to be aware of LDAC in patients with localized left ventricular lesions and multiple electrocardiographic abnormalities. Notably, multimodality cardiovascular imaging and electrocardiogram (ECG) should be considered in this situation.
The patient in the present case revealed various ECG abnormalities, including sinus node dysfunction, chronic bifascicular block, abnormal Q wave, left chest leads low voltage (V4-6), poor R wave progression in leads V4-V6, and f-QRS in leads V3-V6. These were all indicative of left ventricular myocardial abnormality and extensive conduction system disorder. f-QRS, which has been defined as the presence of additional R’ waves or a notch in the R or S wave in two contiguous leads[3], indicates myocardial scarring and represents distortion of signal conduction as well as depolarization processes within ventricles[4]. Previous studies have shown that f-QRS is an independent predictor for cardiac events, ventricular arrhythmias, and sudden cardiac death[5]. Notably, it has been detected in patients with various structural heart or primary electrical diseases, such as Brugada syndrome, arrhythmogenic right ventricular dysplasia, and acquired long QT syndrome[6]. Therefore, careful differential diagnosis for cardiomyopathy was imperative for the patient in the present case. Furthermore, there is a need to consider multimodality cardiovascular imaging.
CMRI combined with CCTA is effective in identification of multiple types of cardiomyopathy and cardiac inner structures. Initially, we considered the left ventricular apex outpouching with a thick wall, narrow communication, and synchronous contractility to be a diverticulum based on evidence from echocardiography and myocardial contrast echocardiography. However, CCTA and CMRI generated more details for differential diagnosis. Previous studies have shown that CCTA, a noninvasive approach, can effectively distinguish outpouchings caused by myocardial infarction and ischemic cardiomyopathy related to occlusion (or lack thereof) of the coronary arteries[7,8]. Furthermore, CMRI has a unique advantage in identifying cardiomyopathy. Black blood with T1 and T2 sequences as well as dynamic bring blood were used to evaluate the cardiac structure, tissue characterization, bilateral ventricular function, and segmental movement. In addition, delayed enhancement imaging following administration of gadolinium can result in more information on fibrosis, scarring, and fat infiltration in the local myocardium[9]. In the present case, CCTA and CMRI results revealed LV septum and free wall local myocardium replaced by fatty tissue as well as LV midwall late gadolinium enhancement, multiple LV outpouchings, and uneven thickness of the LV wall, without stenosis of coronary arteries. These abnormalities were accompanied by non-sustained ventricular tachycardia with an RBBB pattern and f-QRS in the left chest leads. Consequently, we considered that this patient had arrhythmogenic cardiomyopathy.
Arrhythmogenic cardiomyopathy refers to a category of non-hypertrophic, non-hypertensive, and non-valvular progressive cardiomyopathy with fibrofatty myocardium infiltration[10]. Previous studies have classified arrhythmogenic cardiomyopathy into classical arrhythmogenic right ventricular cardiomyopathy, LDAC, and biventricular involvement categories[11]. The patient in the present study was eventually diagnosed with LDAC. LDAC, which was first described by Sen-Chowdhry et al[1] in 2008, has been easily overlooked or misdiagnosed as myocardial infarction, myocarditis, and dilated cardiomyopathy in clinical practice. Notably, LDAC is a relatively rare disease. For example, it accounted for less than 0.15% of 35845 consecutive patients who were referred for CMRI examinations in Fuwai Hospital (Beijing, China), National Center for Cardiovascular Diseases[12].
Clinically, LDAC patients mainly manifest palpitations, presyncope, exertional dyspnea, and chest pain with normal coronary angiography, with only a handful of cases found to be asymptomatic[12,13]. Patients with LDAC have poor prognosis. For example, Feliu et al[13] found that 32.4% of all LDAC patients studied manifested major adverse cardiovascular events, which were mainly accompanied by sudden cardiac death and ventricular arrhythmias, during a mean follow-up of 3.74 years.
At present, no specific diagnostic criteria exist for LDAC. In 2008, Dr. Chowdhry established the following initial diagnostic features of LDAC: (1) Arrhythmia: sustained or non-sustained ventricular tachycardia; (2) Imaging: (a) LV aneurysms; and (b) mild LV dilation and/or systolic impairment; (3) Biopsy/CMRI: (a) cardiomyocyte loss with fibrofatty replacement on histology; and (b) extensive late gadolinium enhancement of LV myocardium (with subepicardial/midmyocardial distribution); and (4) Unexplained T-wave inversion in V5, V6 ± V4, I, and AVL[1]. Recently, Corrado et al[11] suggested that the following elements should be considered as LDAC: (1) ECG changes, such as low QRS voltages in limb leads and inverted T waves in the inferolateral leads; (2) Ventricular arrhythmias with an RBBB pattern; and (3) Structural and functional imaging features consistent with ‘hypokinetic and fibrotic LV.’ Interestingly, the ultrasonographer initially misdiagnosed the patient in the present study as congenital ventricular diverticulum (CVD), according to the left ventricular apex cystic outpouching displaying synchronous contractility with the corresponding cardiac chamber.
CVD, first described in 1816, was often asymptomatic and incidentally detected during a regular physical check-up. Generally, the left ventricular diverticulum was in a thick wall, comprising endocardium, myocardium, and pericardium, with a narrow communication between the cavity and ventricular and displayed synchronous contractility with the LV[14]. This was likened to an appendix originating from the ventricle. The left ventricular diverticulum has an average size that varies from 0.5 cm to as large as 8.0-9.0 cm[15]. Notably, the left ventricular diverticulum not only has low prevalence, as evidenced by 0.4%-2.2% across different studies[16], but has also been associated with occurrence of other congenital abnormalities, including septal defects, dextrocardia, and pulmonary stenosis. Generally, CVD combined with midline thoraco-abdominal congenital abnormalities, diaphragmatic and sternal defects, and partial absence of diaphragmatic pericardium is referred to as Cantrell’s syndrome[17].
CVD has the atypical and nonspecific clinical manifestations at an early stage, namely arrhythmias, cardiac rupture, heart failure, and embolism[18,19], which make it easily confused with LDAC. However, some of these features can be adopted during the differential diagnosis between CVD and LDAC. First, most CVD are single and located at the cardiac apex[20,21]. Second, the left ventricular wall exhibits neither signal alterations nor signs of necrosis or fibrous tissue in CVD cases[22]. Third, CVD patients exhibit more frequent extracardiac anomalies than those with LDAC[19]. Fourth, the size of CVD does not change over time, suggesting a benign course[23]. However, the patient in the present study not only manifested multiple left ventricular outpouchings but also exhibited uneven-thickness left ventricular wall with multiple flaky fatty infiltrations. This interesting case indicates that clinicians should not ignore LDAC upon detecting left ventricle outpouching on TTE.
Although myocardial biopsy is the gold standard diagnostic criterion, this patient refused this invasive examination. After comprehensively analyzing a combination of the medical history and positive clues on auxiliary examinations, including ECG, TTE, CCTA, and CMRI, the specialists in our department unanimously diagnosed the patient with LDAC. She was subsequently administered with standard oral therapy for heart failure with reduced ejection fraction and implantable cardioverter-defibrillator. The patient was very satisfied with the process of diagnosis, treatment, and follow-up.
LDAC is a relatively rare disease, which requires multimodality cardiovascular imaging for diagnosis. CMRI combined with CCTA is an excellent approach for identification of multiple types of cardiomyopathy and cardiac inner structures. From the present case, clinicians are advised to consider LDAC in patients with localized left ventricular lesions and multiple electrocardiographic abnormalities.
The authors thank the radiology department of West China Hospital, Sichuan University for technical support. Second, we thank Dr. Jerry, PhD from the Home for researchers editorial team for editing the language of this manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mishra AK, United States; Wang H, United States S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Sen-Chowdhry S, Syrris P, Prasad SK, Hughes SE, Merrifield R, Ward D, Pennell DJ, McKenna WJ. Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J Am Coll Cardiol. 2008;52:2175-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 483] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 2. | Andreini D, Conte E, Casella M, Mushtaq S, Pontone G, Dello Russo A, Nicoli F, Carità P, Catto V, Vettor G, Gasperetti A, Sommariva E, Rizzo S, Basso C, Tondo C, Pepi M. Cardiac magnetic resonance features of left dominant arrhythmogenic cardiomyopathy: differential diagnosis with myocarditis. Int J Cardiovasc Imaging. 2022;38:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Jain R, Singh R, Yamini S, Das MK. Fragmented ECG as a risk marker in cardiovascular diseases. Curr Cardiol Rev. 2014;10:277-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Brohet C. Fragmentation of the QRS complex: the latest electrocardiographic craze? Acta Cardiol. 2019;74:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Haukilahti MA, Eranti A, Kenttä T, Huikuri HV. QRS Fragmentation Patterns Representing Myocardial Scar Need to Be Separated from Benign Normal Variants: Hypotheses and Proposal for Morphology based Classification. Front Physiol. 2016;7:653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Ratheendran AC, Subramanian M, Bhanu DK, Prabhu MA, Kannan R, Natarajan KU, Saritha Sekhar S, Thachathodiyil R, Harikrishnan MS, Pai PG. Fragmented QRS on electrocardiography as a predictor of myocardial scar in patients with hypertrophic cardiomyopathy. Acta Cardiol. 2020;75:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Al'Aref SJ, Min JK. Cardiac CT: current practice and emerging applications. Heart. 2019;105:1597-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Ramsey BC, Fentanes E, Choi AD, Branch KR, Thomas DM. Myocardial Assessment with Cardiac CT: Ischemic Heart Disease and Beyond. Curr Cardiovasc Imaging Rep. 2018;11:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Yoneyama K, Kitanaka Y, Tanaka O, Akashi YJ. Cardiovascular magnetic resonance imaging in heart failure. Expert Rev Cardiovasc Ther. 2018;16:237-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, Daubert JP, de Chillou C, DePasquale EC, Desai MY, Estes NAM 3rd, Hua W, Indik JH, Ingles J, James CA, John RM, Judge DP, Keegan R, Krahn AD, Link MS, Marcus FI, McLeod CJ, Mestroni L, Priori SG, Saffitz JE, Sanatani S, Shimizu W, van Tintelen JP, Wilde AAM, Zareba W. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16:e301-e372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 516] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 11. | Corrado D, van Tintelen PJ, McKenna WJ, Hauer RNW, Anastastakis A, Asimaki A, Basso C, Bauce B, Brunckhorst C, Bucciarelli-Ducci C, Duru F, Elliott P, Hamilton RM, Haugaa KH, James CA, Judge D, Link MS, Marchlinski FE, Mazzanti A, Mestroni L, Pantazis A, Pelliccia A, Marra MP, Pilichou K, Platonov PGA, Protonotarios A, Rampazzo A, Saffitz JE, Saguner AM, Schmied C, Sharma S, Tandri H, Te Riele ASJM, Thiene G, Tsatsopoulou A, Zareba W, Zorzi A, Wichter T, Marcus FI, Calkins H; International Experts. Arrhythmogenic right ventricular cardiomyopathy: evaluation of the current diagnostic criteria and differential diagnosis. Eur Heart J. 2020;41:1414-1429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 189] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 12. | He J, Xu J, Li G, Zhou D, Li S, Zhuang B, Chen X, Duan X, Li L, Fan X, Huang J, Yin G, Jiang Y, Wang Y, Zhao S, Lu M. Arrhythmogenic Left Ventricular Cardiomyopathy: A Clinical and CMR Study. Sci Rep. 2020;10:533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Feliu E, Moscicki R, Carrillo L, García-Fernández A, Martínez Martínez JG, Ruiz-Nodar JM. Importance of cardiac magnetic resonance findings in the diagnosis of left dominant arrythmogenic cardiomyopathy. Rev Esp Cardiol (Engl Ed). 2020;73:885-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Srichai MB, Hecht EM, Kim DC, Jacobs JE. Ventricular diverticula on cardiac CT: more common than previously thought. AJR Am J Roentgenol. 2007;189:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Ohlow MA. Congenital left ventricular aneurysms and diverticula: definition, pathophysiology, clinical relevance and treatment. Cardiology. 2006;106:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Sharma A, Kumar S. Overview of left ventricular outpouchings on cardiac magnetic resonance imaging. Cardiovasc Diagn Ther. 2015;5:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 17. | Vazquez-Jimenez JF, Muehler EG, Daebritz S, Keutel J, Nishigaki K, Huegel W, Messmer BJ. Cantrell's syndrome: a challenge to the surgeon. Ann Thorac Surg. 1998;65:1178-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Ohlow MA. Congenital left ventricular aneurysms and diverticula: an entity in search of an identity. J Geriatr Cardiol. 2017;14:750-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 19. | Ohlow MA, von Korn H, Lauer B. Characteristics and outcome of congenital left ventricular aneurysm and diverticulum: Analysis of 809 cases published since 1816. Int J Cardiol. 2015;185:34-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Restrepo CS, Lane MJ, Murillo H. Cardiac aneurysms, pseudoaneurysms, and diverticula. Semin Roentgenol. 2012;47:262-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Cresti A, Cannarile P, Aldi E, Solari M, Sposato B, Franci L, Limbruno U. Multimodality Imaging and Clinical Significance of Congenital Ventricular Outpouchings: Recesses, Diverticula, Aneurysms, Clefts, and Crypts. J Cardiovasc Echogr. 2018;28:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Romagnoli A, Ricci A, Morosetti D, Fusco A, Citraro D, Simonetti G. Congenital left ventricular diverticulum: Multimodality imaging evaluation and literature review. J Saudi Heart Assoc. 2015;27:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Scagliola R, Rosa GM, Seitun S. Cardiac Outpouchings: Definitions, Differential Diagnosis, and Therapeutic Approach. Cardiol Res Pract. 2021;2021:6792643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |