Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6277
Peer-review started: January 13, 2022
First decision: March 23, 2022
Revised: April 1, 2022
Accepted: April 27, 2022
Article in press: April 27, 2022
Published online: June 26, 2022
Processing time: 154 Days and 12.1 Hours
Brain arteriovenous malformation (AVM), an aberrant vascular development during the intrauterine period, is traditionally considered a congenital disease. Sporadic reports of cases of de novo AVM formation in children and adults have challenged the traditional view of its congenital origin.
In this report, we have presented the case of a child with a de novo brain AVM. Magnetic resonance imaging and magnetic resonance angiography of the brain showed no AVM at the age of 5 years and 2 mo. Brain AVM was first detected in this child at the age of 7 years and 4 mo. The brain AVM was significantly advanced, and hemorrhage was seen for the first time at the age of 12 years and 8 mo. There was further progression in the AVM, and hemorrhage occurred again at the age of 13 years and 5 mo. Genetic analysis of this patient revealed a mutation in the NOTCH2 (p.Asp473Val) gene.
In short, our case has once again confirmed the view that brain AVM is an acquired disease and is the result of the interaction of genes, environment, and molecules.
Core Tip: Brain arteriovenous malformation (AVM) is one of the main causes of spontaneous cerebral hemorrhage in children. At present, the mechanism of the occurrence and development of AVM is not clear, and there have been very few case reports that have documented the progression of a de novo brain AVM. In this report, we present the case of a child with a de novo brain AVM. Brain AVM was first detected in this child at the age of 7 years and 4 mo and was significantly advanced and hemorrhaging at the age of 12 years and 8 mo. Genetic analysis of this patient revealed a mutation in the NOTCH2 (p.Asp473Val) gene.
- Citation: Huang H, Wang X, Guo AN, Li W, Duan RH, Fang JH, Yin B, Li DD. De novo brain arteriovenous malformation formation and development: A case report. World J Clin Cases 2022; 10(18): 6277-6282
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6277.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6277
Brain arteriovenous malformation (AVM) is one of the main causes of spontaneous cerebral hemorrhage in children[1] and accounts for 30%-50% of spontaneous cerebral hemorrhage in children[1,2]. It is generally believed that AVM is a kind of congenital vascular disease[3]. The abnormality is formed in the third to fourth week of embryonic development, when the length is about 40-80 mm, during the process of cerebrovascular development and manifests as abnormal coiled and interlaced vascular clusters[4]. Arteries and veins are directly connected by malformed vascular clusters. At present, the mechanism of the occurrence and development of AVM is not clear, and there have been very few case reports that have documented the progression of a de novo brain AVM[5-7]. Here, we have documented for the first time a case of a child with a de novo brain AVM that progressed and ruptured twice within 6 years. We have also reported for the first time the potential role of heterozygous mutations in the NOTCH2 gene in the pathogenesis of AVM.
A 15-year-old boy was admitted to the hospital in November 2019 with complaints of an acute headache for 3 h.
The child presented with a sudden severe headache for 3 h with no inducing factors. He was conscious and had no history of nausea, dizziness, or fever.
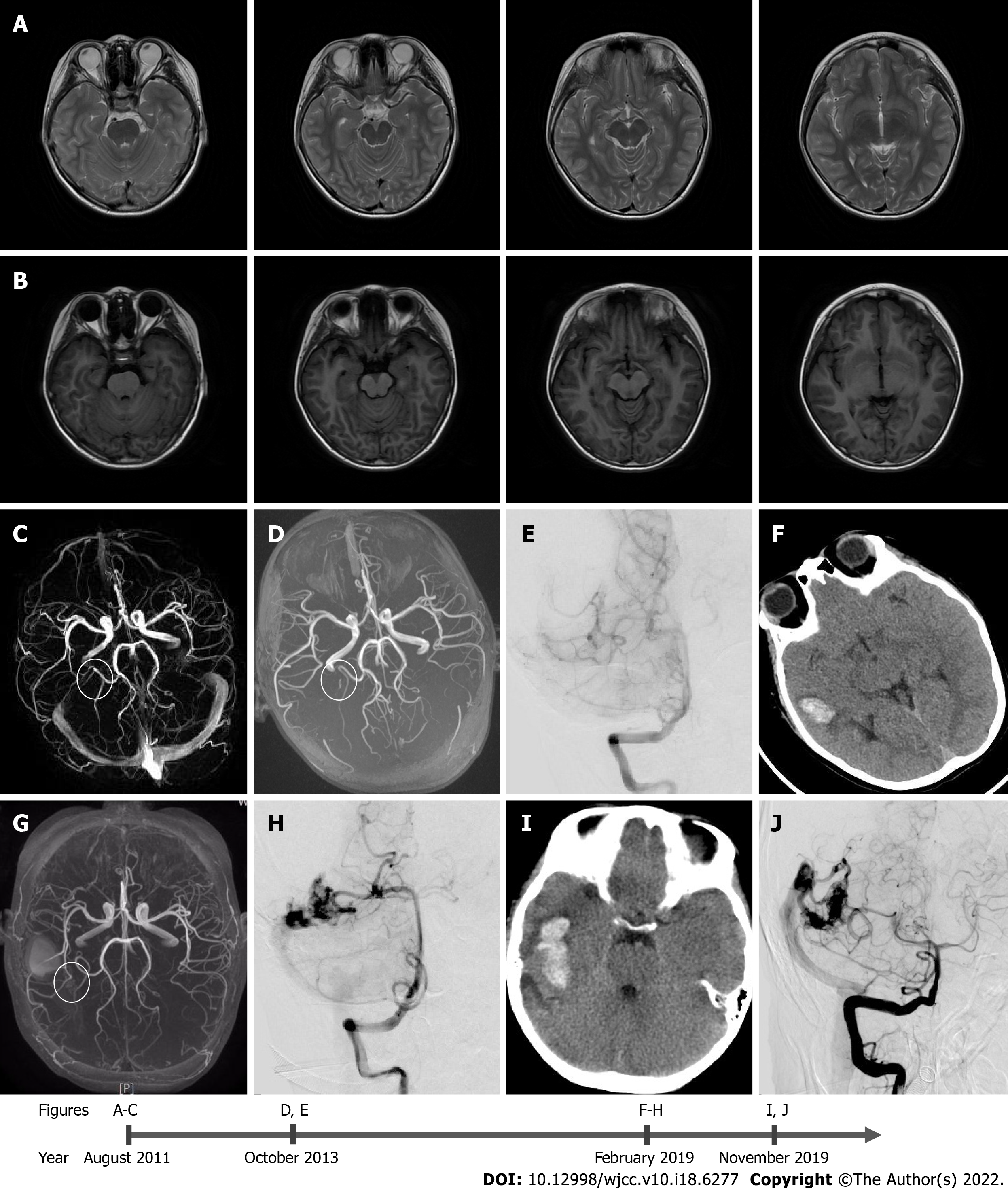
The boy had normal growth and development and no history of other special diseases. In August 2011, the child presented at another hospital with complaints of a headache for more than 1 wk. At that time, magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), and magnetic resonance venography (MRV) were performed. The scans revealed that there was no vascular abnormality, abnormal signal intensity, or any other findings suggestive of an AVM (Figure 1A-C). At that time, the doctor did not administer any treatment, and the headache was ameliorated by itself. In October 2013, the child again presented at another hospital with headache and nausea for 2 d. His MRA scan revealed a small right temporal AVM, the size of which was 6.2 mm × 1.9 mm (Figure 1C). Digital subtraction angiography revealed the presence of a suspicious lesion in the right temporal lobe (Figure 1D). The boy was treated for headaches and was not examined further. In February 2019, the boy presented at our hospital complaining of a sudden headache and vomiting for 2 h. He was conscious when he came to our hospital. His Glasgow coma scale score was 14, and National Institutes of Health Stroke Scale score was 1. Computed tomography of the head demonstrated acute intracerebral hemorrhage in the right temporal lobe. The MRA revealed a small AVM in the right temporal lobe (Figure 1E). Symptomatic treatment was given to the child during hospitalization, and gamma knife radiosurgery was suggested. Unfortunately, the child’s parents rejected radiotherapy.
The patient did not have any personal or family history of brain AVM.
The patient was conscious when examined at our hospital in November 2019. His Glasgow coma scale score was 15 and his National Institutes of Health Stroke Scale score was 1.
Laboratory examinations showed no significant abnormality.
A small AVM within the right temporal lobe was visualized in the MRA (Figure 1G), and cerebral angiogram revealed the presence of a 21.6 mm × 21.2 mm right temporal AVM, which was supplied by the inferior temporal branches of the right posterior cerebral artery. Venous drainage occurred through superficial cerebellar veins into the right sigmoid sinus. It was classified as Spetzler-Martin grade 1 (Figure 1H).
In November 2019, computed tomography of the head demonstrated the presence of an acute intracerebral hemorrhage in the right temporal lobe (Figure 1I). Digital subtraction angiography displayed abnormal vascular clusters, which were slightly larger than those seen 10 mo ago. The size of these clusters was 24.3 mm × 22.8 mm. The AVM was classified as a Spetzler-Martin grade 1 (Figure 1J).
After receiving consent from the child’s parents, we conducted genetic analysis for the child and his parents. We discovered a mutation that might be related to the incidence of AVM, NOTCH2 heterozygous mutation [NM_024408.3; c.1418A>T; p.Asp473Val]. The mutation was only found in the child.
Brain AVM, Spetzler-Martin grade 1.
The parents refused to conduct further interventional treatment. Supportive treatment was administered during the hospitalization period, and the boy was discharged after 10 d in good clinical condition.
The clinical condition of the child was good during the final follow-up, which was performed on January 2021. The child was in good general condition at the time. After January 2021, he was lost to follow-up.
The patient underwent MRI, MRA, and MRV scans in August 2011, at the age of 5 years and 2 mo for nonspecific headaches. No abnormal vascular lesions or secondary signs of brain AVM were observed. A brain AVM was first identified in October 2013. Bleeding occurred for the first time in February 2019 and occurred again in November 2019. The malformed vascular clusters progressed significantly during this period. No risk factors were associated with AVM in this case. Literature review revealed that most of the reported AVM cases had underlying pathology, and only a few of them had no cause of de novo AVM[5,8]. Our case report is the first to define the progression and eventual rupture within 6 years following its primary discovery. We also report for the first time that heterozygous mutations in the NOTCH2 gene might be playing a role in the pathogenesis of AVM.
Currently, the pathogenesis of brain AVM is not clearly understood. The imbalance of some signal molecules during embryonic development is believed to be the most likely reason for no capillary formation between the arteries and veins[9]. This balance is dependent on the strict regulation of various angiogenic factors, and any interference for these automatic regulation factors may lead to the formation of AVM[10,11]. There was no high-risk factor for AVM in this case, and the only high-risk factor that may have a relation in the development of AVM was the heterozygous mutation of the NOTCH2 gene. The NOTCH2 protein is the receptor of the Notch signaling pathway, and its activity can directly affect Notch signaling[12]. However, the Notch signaling pathway has complex and context-dependent effects on angiogenesis[13,14]. Studies have demonstrated that abnormal activation of Notch signaling in human brain AVM was associated with AVM bleeding[15]. The inhibition and activation of Notch signaling were both associated with AVM formation. The mutation in this case (p.Asp473Val) was first described by Gilbert et al[16]. The mutation is located in exon 8, and exon 8 encodes the epidermal growth factor-12 domain of NOTCH2[16,17]. Unfortunately, there has been no research on the effect of this mutation on the function of the NOTCH2 protein.
We report for the first time a case of de novo AVM formation in a child, which progressed and eventually ruptured within 6 years. Our case contests the traditional view that brain AVM is congenital, and our case once again confirms the view that brain AVM is an acquired disease that is the result of an interaction of genes, environment, and molecules.
We thank the Department of Neurosurgery and the Department of Radiology of The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University for the diagnosis and treatment of the patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Almzeogi MA, Serbia; Medina SM, Bolivia S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Sison V, Stackhouse T, Breeze R, Hall T, McKenzie P, Tartaglia N. Arteriovenous Malformation in a Youth with Atypical Autism Symptoms. J Child Dev Disord. 2017;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Jimenez JE, Gersey ZC, Wagner J, Snelling B, Ambekar S, Peterson EC. Role of follow-up imaging after resection of brain arteriovenous malformations in pediatric patients: a systematic review of the literature. J Neurosurg Pediatr. 2017;19:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Leblanc GG, Golanov E, Awad IA, Young WL; Biology of Vascular Malformations of the Brain NINDS Workshop Collaborators. Biology of vascular malformations of the brain. Stroke. 2009;40:e694-e702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Mullan S, Mojtahedi S, Johnson DL, Macdonald RL. Embryological basis of some aspects of cerebral vascular fistulas and malformations. J Neurosurg. 1996;85:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 145] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Santos R, Aguilar-Salinas P, Entwistle JJ, Aldana PR, Beier AD, Hanel RA. De Novo Arteriovenous Malformation in a Pediatric Patient: Case Report and Review of the Literature. World Neurosurg. 2018;111:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Chen W, Choi EJ, McDougall CM, Su H. Brain arteriovenous malformation modeling, pathogenesis, and novel therapeutic targets. Transl Stroke Res. 2014;5:316-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Florian IA, Beni L, Moisoiu V, Timis TL, Florian IS, Balașa A, Berindan-Neagoe I. 'De Novo' Brain AVMs-Hypotheses for Development and a Systematic Review of Reported Cases. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Yeo JJ, Low SY, Seow WT, Low DC. Pediatric de novo cerebral AVM: report of two cases and review of literature. Childs Nerv Syst. 2015;31:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Storkebaum E, Quaegebeur A, Vikkula M, Carmeliet P. Cerebrovascular disorders: molecular insights and therapeutic opportunities. Nat Neurosci. 2011;14:1390-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Achrol AS, Guzman R, Varga M, Adler JR, Steinberg GK, Chang SD. Pathogenesis and radiobiology of brain arteriovenous malformations: implications for risk stratification in natural history and posttreatment course. Neurosurg Focus. 2009;26:E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Ma L, Guo Y, Zhao YL, Su H. The Role of Macrophage in the Pathogenesis of Brain Arteriovenous Malformation. Int J Hematol Res. 2015;1:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Wang MM. Notch signaling and Notch signaling modifiers. Int J Biochem Cell Biol. 2011;43:1550-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709-2718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 325] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 14. | Murphy PA, Lu G, Shiah S, Bollen AW, Wang RA. Endothelial Notch signaling is upregulated in human brain arteriovenous malformations and a mouse model of the disease. Lab Invest. 2009;89:971-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Wang LJ, Xue Y, Huo R, Yan Z, Xu H, Li H, Wang J, Zhang Q, Cao Y, Zhao JZ. N6-methyladenosine methyltransferase METTL3 affects the phenotype of cerebral arteriovenous malformation via modulating Notch signaling pathway. J Biomed Sci. 2020;27:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Gilbert MA, Bauer RC, Rajagopalan R, Grochowski CM, Chao G, McEldrew D, Nassur JA, Rand EB, Krock BL, Kamath BM, Krantz ID, Piccoli DA, Loomes KM, Spinner NB. Alagille syndrome mutation update: Comprehensive overview of JAG1 and NOTCH2 mutation frequencies and insight into missense variant classification. Hum Mutat. 2019;40:2197-2220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 17. | Duan Z, Li FQ, Wechsler J, Meade-White K, Williams K, Benson KF, Horwitz M. A novel notch protein, N2N, targeted by neutrophil elastase and implicated in hereditary neutropenia. Mol Cell Biol. 2004;24:58-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |