Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6110
Peer-review started: October 10, 2021
First decision: December 10, 2021
Revised: December 24, 2021
Accepted: April 30, 2022
Article in press: April 30, 2022
Published online: June 26, 2022
Processing time: 249 Days and 16.9 Hours
For advanced lung squamous cell carcinoma, immune checkpoint inhibitors (ICIs) have been regarded as one of the optimal therapies. While immune-related adverse events (irAEs) are common in ICI treatment, cutaneous toxicities are among the most common irAEs. Most immune-related skin toxicity grades are low, and the prognosis is good. However, Stevens-Johnson syndrome (SJS) is a rare but extremely severe cutaneous adverse drug reaction with high mortality.
We report a rare case of SJS induced by pembrolizumab. The case involved a 68-year-old female who was diagnosed with advanced squamous cell carcinoma of the lung. SJS appeared after one cycle of immunotherapy combined with chemotherapy. After treatment with prednisone hormone symptoms, anti-infection, gamma globulin, and antipruritic agents, the skin toxicity of the patients gradually decreased and eventually disappeared. Although the antitumor treatment was stopped due to serious adverse reactions, the tumor of the patient remained stable for nearly half a year after one cycle of immune therapy combined with chemotherapy, which also corroborates the delayed effect of immunotherapy.
We believe our report can provide some references for the treatment of SJS and the treatment of immune-related adverse reactions.
Core Tip: Stevens-Johnson syndrome (SJS) is a rare but extremely severe cutaneous adverse drug reaction with high mortality. The case involved a 68-year-old female who was diagnosed with advanced squamous cell carcinoma of the lung. SJS syndrome appeared after one cycle of immunotherapy. After the optimal supportive treatment, skin toxicity disappeared.
- Citation: Wu JY, Kang K, Yi J, Yang B. Pembrolizumab-induced Stevens-Johnson syndrome in advanced squamous cell carcinoma of the lung: A case report and review of literature. World J Clin Cases 2022; 10(18): 6110-6118
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6110.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6110
Pembrolizumab is an anti-PD-1 (programmed death 1) humanized IgG4 monoclonal antibody that blocks the PD-1 receptor to enable T cell killing. Pembrolizumab, combined with chemotherapy, has shown improved efficacy in patients with advanced squamous cell carcinoma of the lung[1], with drug-related adverse events reported in 64% of patients[2]. However, adverse events of grade 3 or higher were reported in less than 10% of patients and included cutaneous side-effect cases.
Stevens-Johnson syndrome (SJS) is a severe type of pleomorphic erythema and a rare adverse mucocutaneous reaction with a mortality rate of up to 35%[3]. While SJS is characterized by maculopapular rash – pruritus – and is often related to adverse drug reactions[4,5], the mechanism of SJS has not been determined. It has been reported that at least 200 kinds of drug reactions are related to SJS. However, cases in which anti-PD-1/anti-PD-L1 drugs contribute to SJS are rare[3]. There have been several reports of anti-PD-1/anti-PD-L1 therapy inducing SJS[6-14,15]. Here, we present our report of a rare case of pembrolizumab-associated SJS in a female patient with advanced squamous cell carcinoma of the lung.
The patient was a 68-year-old female without a history of smoking. On October 1st, 2020, she was admitted due to repeated cough and breathlessness for 1 mo.
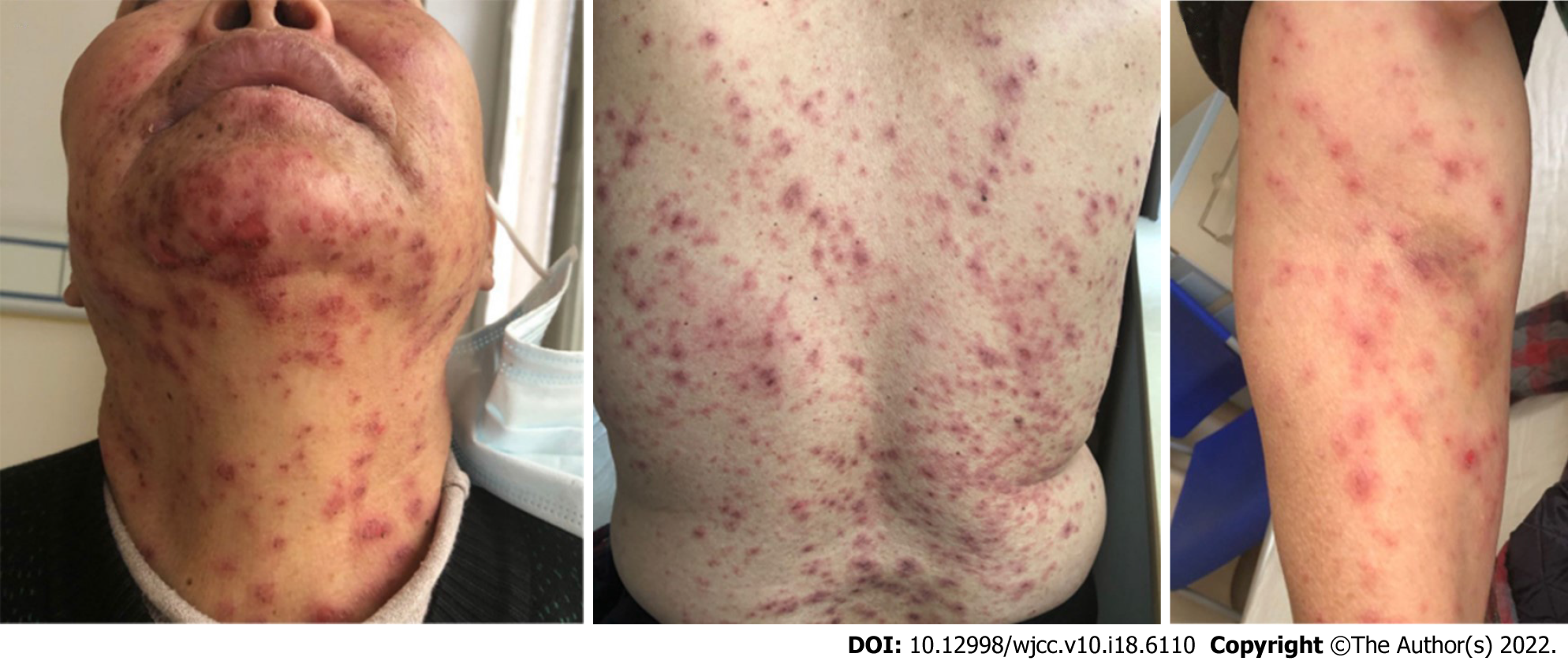
Systemic examinations, including chest computed tomography (CT), whole abdominal CT, brain magnetic resonance imaging (MRI), bone scintigraphy, and blood tests, were performed. The test results showed that the levels of tumor markers were clearly elevated, and CT indicated a lung mass in the right lobe, several bilateral nodules, multiple mediastinal lymph nodes, and a solitary liver metastasis. Squamous cell carcinoma of the lung was diagnosed through CT-guided percutaneous needle lung biopsy, and polymerase chain reaction (PCR) revealed no epidermal growth factor receptor, anaplastic lymphoma kinase, or receptor tyrosine kinase mutations. According to the American Joint Commission on Cancer 8th edition staging system, she was clinically diagnosed with stage IVA lung squamous cell carcinoma (cT4N2M1b). According to the 2020 National Comprehensive Cancer Network guidelines, the combination of immunotherapy and chemotherapy is the best optional treatment for patients with advanced lung squamous cell carcinoma. On October 14, 2020, the patient was treated with one cycle of paclitaxel 270 mg d1 + cisplatin 120 mg d1 chemotherapy combined with pembrolizumab therapy. On November 4, 2021, which was nearly three weeks after one cycle of chemotherapy, the patient started with low fever, sore throat, and severe fatigue. Then, the patient was considered to be related to cold exposure. Penicillin treatment was used in the hospital nearby, but the patient's symptoms did not improve, which lasted for almost 5 d. On November 9th, small papules and typical erythema, accompanied by severe itchiness and general discomfort, gradually appeared on the patient's skin and were mainly distributed in the anterior chest and face. Considering the severity of the patient's symptoms, the patient visited our hospital on November 12th. The patient’s temperature was normal. The pain in her throat persisted. The physical examination of the patient showed that multiple erythematous papules could be detected on the patient’s head, neck, chest, and back (covering 30% of the total body skin) (Figure 1), most of which fused to form blisters. The patient reported that these papules felt mild itchiness but painful. Eyelid edema was obvious. There were some ulcers around the lip. The patient’s oral ulcers were too painful to allow her to eat anything.
There was no remarkable past medical history with no alcohol consumption or history of smoking.
Family and personal history were unremarkable. She had no significant medical history or drug allergy.
A poor general condition, SCORTEN (severity-of-illness score for TEN) score of 4 points. Nikolsky’s sign was positive. Koebner phenomenon was negative. Erythematous papules could be detected on the patient’s head, neck, chest, and back (covering 30% of the total body skin) with mild itchy but painful symptoms. Eyelid edema was obvious. There were some ulcers around the lip. Her oral ulcers were too painful to allow her to eat anything. No other apparently positive signs were found.
She was admitted to our hospital, and the relevant blood tests after admission are shown in Table 1 below.
| Time | November 18, 2021 | November 26, 2021 | December 1, 2021 | December 7, 2020 |
| White blood cell | 10.3×108/L (3.5-9.5) | 10.01×108/L (3.5-9.5) | 9.28×108/L (3.5-9.5) | 10.76×108/L (3.5-9.5) |
| Red blood cell | 2.68×1012/L (3.8-5.1) | 3.01×1012/L (3.8-5.1) | 2.86×1012/L (3.8-5.1) | 2.63×1012/L (3.8-5.1) |
| Hemoglobin | 78 g/L (115-150) | 98 g/L (115-150) | 97.9 g/L (115-150) | 89.3 g/L (115-150) |
| Thrombocyte | 115×109/L (125-350) | 124×109/L (125-350) | 135×109/L (125-350) | 133×109/L (125-350) |
| C-reactive protein | 22.07 mg/L (0-10) | 60.5 mg/L (0-10) | ||
| Erythrocyte sedimentation rate | 44 mm/h (0-22) | 51 mm/h (0-22) | ||
| Procalcitonin | 0.05 ng/mL (< 0.05) | 0.05 ng/mL (< 0.05) | 0.09 ng/mL (< 0.05) | |
| D-dimer | 0.83 ng/mL (< 0.5 mg/L) | 646 ng/mL (< 0.5 mg/L) | ||
| Immunoglobulin G (IgG) | 22.2 g/L (7.0-12.6) | |||
| ANA reaction | Positive | |||
| ANA drop degree | 0.486 | |||
| Anti-SSA/Ro antibody | Strongly positive (+++) | |||
| Anti-SSB | Positive (++) | |||
| Interleukin-6 | 39.26 pg/mL (0-7) |
Chest and abdominal CT scans showed that the primary lung lesions and liver metastases were significantly reduced, and the overall response evaluation was PR according to RECIST 1.1 (Response Evaluation Criteria in Solid Tumors).
We arranged an urgent consultation with a dermatologist. According to the skin and mucous membrane performance of the patient during these days, the rapid development of the disease, and history of PD-1 inhibitor use, pembrolizumab-induced SJS was diagnosed.
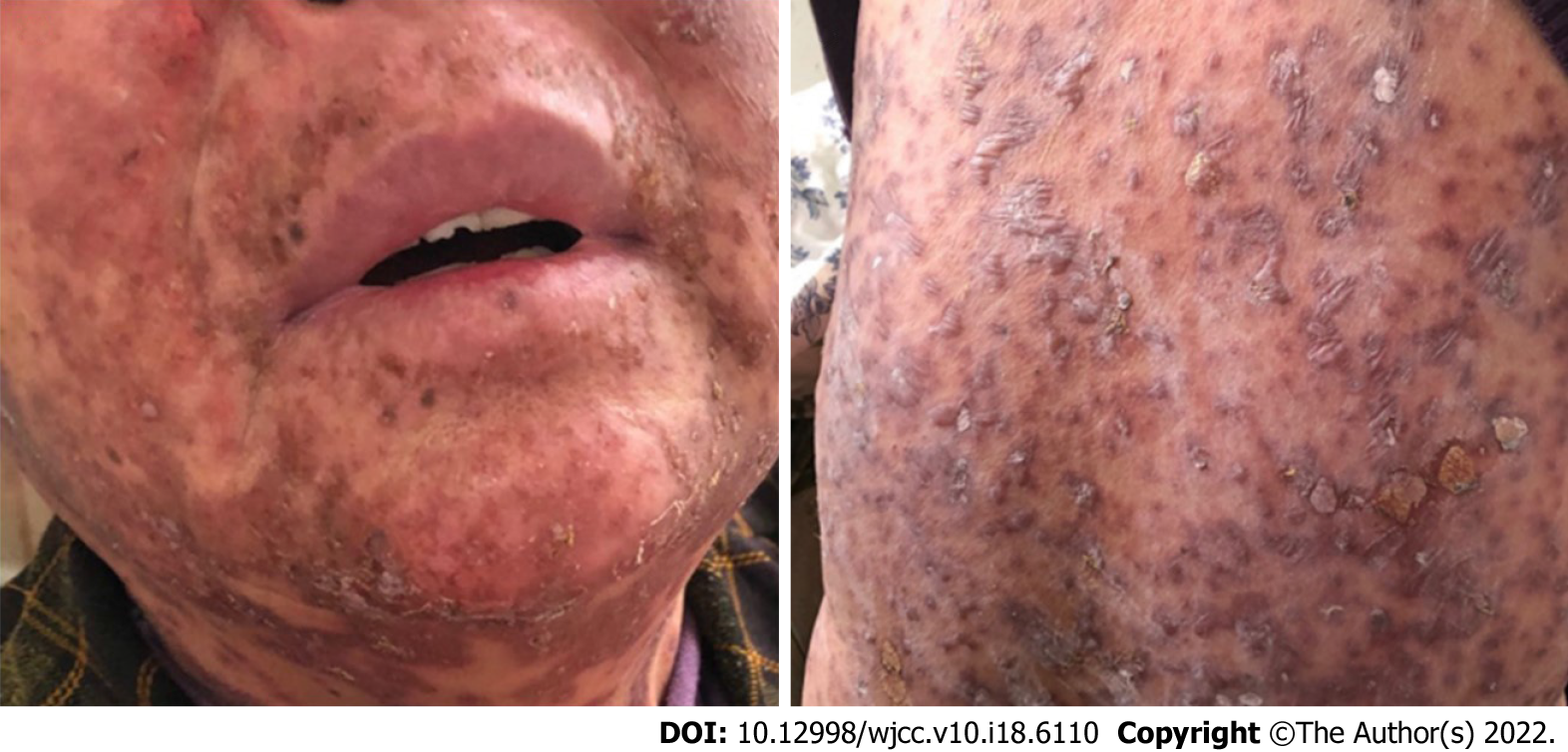
Therefore, we immediately administered moderate- to high-potency topical steroids to treat the affected areas, oral antihistamines for pruritus and oral prednisone at 40 mg/d. After three days of treatment, the dermatologic toxicities were clearly aggravated. On November 18 (Figure 2), the rash began to spread to almost the entire body (covering more than 45% of the total body skin). The blisters were formed superficially in the epidermis with skin ulceration, and most of them had blood and fluid oozing. Part of the epidermis was peeled off from the surface of the body, exposing a moist, painful, flushed erosive surface. The oral ulcers continued to be aggravated. Both the itchiness and pain worsened, and the patient became severe (G3-4). At this point, we suspended immunotherapy, administered high potency topical steroids to the affected areas and prophylactically used antibiotics; additionally, we increased the prednisone dose to 100 mg. After three days of treatment, the cutaneous toxicities continued to worsen (Figure 3).
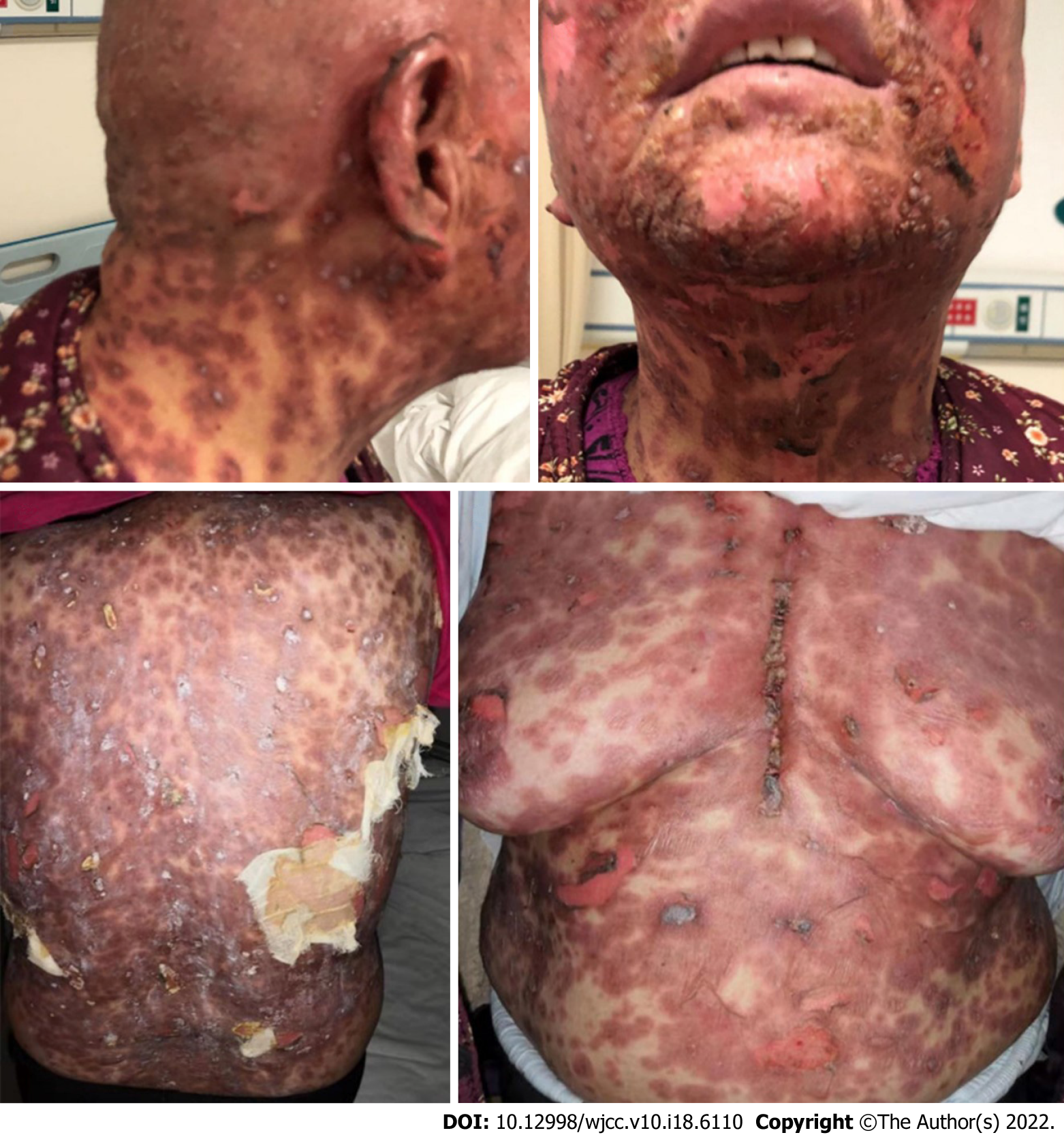
By referring to the opinions from the consultation, intravenous methylprednisolone 120 mg/d (2 mg/kg/d), gamma globulin 20 g/d, topical gentamicin, and diluted potassium permanganate were administered. Sepprayi 25 mg (recombinant human type II tumor necrosis factor receptor-antibody fusion protein, rhTNFR:Fc) was injected subcutaneously twice a week, and oral antihistamine was administered for pruritus. After a week of treatment, the dermatologic toxicities were gradually alleviated (Figure 4). Then, oral prednisone was gradually reduced, and topical drug administration continued. The treatment lasted for 3 mo, and the skin toxicity eventually disappeared (Figure 5). In terms of lung carcinoma, the pulmonary nodule was smaller than the baseline and remained stable during the 6-mo evaluation. In May 2021, the latest re-examination showed that although the patient did not receive any antitumor treatment, the lesion remained stable.
Since the 21st century, the introduction of immunotherapy treatments has dramatically revolutionized the treatment paradigm of non-small-cell lung cancer (NSCLC)[16]. However, immune checkpoint inhibition usually leads to systemic adverse reactions, which are immune-related adverse events, mainly encompassing rash, colitis, pneumonitis, hepatitis, and thyroiditis[17]. Dermatologic toxicities seem to be the most frequently reported adverse events. SJS is a rare and severe dermatologic toxicity with high mortality[4]. The first SJS case induced by pembrolizumab in NSCLC was reported in a Japanese case[14]. Our case report is the first Chinese case of pembrolizumab-associated SJS in NSCLC.
At present, the exact mechanism of SJS remains undefined. The currently recognized theory is the T cell-mediated type IV delayed hypersensitivity reaction[18,19]. The drug triggering SJS binds the T cell receptor and MHC class I, and as a result, it leads to the massive replication of cytotoxic T cells, which directly kill keratinocytes, and the release of granulysin, which destroys cells in the skin and the mucous membrane[20]. Yun-Shiuan’s research showed that the blockade of PD-1/PD-L1 may contribute to the imbalance of the immune system, manifesting the enhancement of the T cell response and increasing the incidence of hypersensitivity[21]. During the treatment of SJS induced by ipilimumab and nivolumab in a melanoma patient[22], an increase in CD8+ T cells in the dermal epidermal junction and an increase in PD-L1 expression in keratinocytes were noted. Unfortunately, the biopsy analysis of our case has not been finished, and therefore, this viewpoint cannot be further confirmed. Overall, the mechanism of SJS caused by immunosuppressive drugs requires further research.
Cutaneous toxicities of immune checkpoint inhibitors might result in a longer PFS (progression-free survival) and a higher OS (overall survival) rate[23]. Bairavi and his colleagues demonstrated that NSCLC patients with one irAE and multisystem irAEs incrementally improved OS and PFS. Longer immune checkpoint inhibitor durations were an independent risk factor for the development of irAEs. In our case, it was rare that such severe AEs occurred after just one cycle of immunotherapy[24]. Susana also indicated that the median PFS was 9.49 mo in the group with irAEs vs 1.99 mo in the group without irAEs (P < 0.0001) in NSCLC treated with nivolumab[25]. In our case, the condition of the patient was stable for up to 6 mo just after one cycle of the treatment. Thus, we speculate that skin toxicities and delayed immunological effects both contributed to such a long progression-free survival time.
There is no standard treatment regimen for SJS[26], and multidisciplinary care, best supportive care, and corticosteroids are currently the most important components of its therapy[20]. By applying high-dose corticosteroids early, we can rapidly arrest SJS, while the optimal cutoff time of corticosteroids remains controversial because of its adverse drug reaction[27]. From the author’s perspective, the appropriate duration of high-dose corticosteroids is within 4 wk. The combination of IVIG and steroids seems to bring better outcomes to patients with SJS[28].
In addition to corticosteroids and IVIG, drugs that suppress the immune response or inflammatory factors are also being tried in the treatment of SJS. Over the last several years, several retrospective trials have advocated the benefits of cyclosporine in the treatment of SJS/TEN[29,30]. Cyclosporine inhibits the activation of CD4+ and CD8+ T cells in the early phase, subsequently inhibiting the secretion of granulysin, granzyme, and perforin[31]. Despite a lack of randomized control trials, cyclosporine has proven to have a mortality benefit in the treatment of SJS/TEN without a low risk of side effects. In our case, we did not use cyclosporine due to the lack of experience in the early phase of treatment for SJS. In the late phase, we used recombinant human tumor necrosis factor receptor type II-Fc fusion protein antibody as recommended by the dermatologist. The reason we used Sepprayi is that it can reduce the level of inflammatory factors, such as tumor necrosis factor-α (TNF-α), inhibiting the occurrence of hypersensitivity[32]. In our case, Sepprayi had a clear effect on the improvement of the patient's inflammatory response. However, more clinical practice and data support are needed due to limited trials.
Immunotherapy, as a new treatment in the 2010s, has a definite effect on the treatment of advanced lung cancer. However, there remain many difficulties to be overcome in the treatment of serious adverse reactions related to immunotherapy. The combination of high-dose corticosteroid shock therapy, IVIG, cyclosporine, and best supportive care might reduce mortality in the treatment of SJS. The incidence of serious immune-related skin toxicity, such as SJS, is low, but the lethality is still very high. However, there is still a long way to go for immune-related adverse events, such as predictors for adverse events and ways to prevent them in advance. In future studies, we might focus more on the prediction, prevention, and treatment of irAEs, although immunotherapy is in full swing.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Bos S, United Kingdom; Deshwal H, United States; Ng QX, Singapore S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L; KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4095] [Cited by in RCA: 4862] [Article Influence: 486.2] [Reference Citation Analysis (0)] |
| 2. | Kamińska-Winciorek G, Cybulska-Stopa B, Lugowska I, Ziobro M, Rutkowski P. Principles of prophylactic and therapeutic management of skin toxicity during treatment with checkpoint inhibitors. Postepy Dermatol Alergol. 2019;36:382-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Micheletti RG, Chiesa-Fuxench Z, Noe MH, Stephen S, Aleshin M, Agarwal A, Boggs J, Cardones AR, Chen JK, Cotliar J, Davis MDP, Dominguez A, Fox LP, Gordon S, Hamrick R, Ho B, Hughey LC, Jones LM, Kaffenberger BH, Kindley K, Kroshinsky D, Kwong BY, Miller DD, Mostaghimi A, Musiek A, Ortega-Loayza AG, Patel R, Posligua A, Rani M, Saluja S, Sharon VR, Shinkai K, John JS, Strickland N, Summers EM, Sun N, Wanat KA, Wetter DA, Worswick S, Yang C, Margolis DJ, Gelfand JM, Rosenbach M. Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Multicenter Retrospective Study of 377 Adult Patients from the United States. J Invest Dermatol. 2018;138:2315-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 4. | Ma DH, Tsai TY, Pan LY, Chen SY, Hsiao CH, Yeh LK, Tan HY, Lu CW, Chen CB, Chung WH. Clinical Aspects of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis With Severe Ocular Complications in Taiwan. Front Med (Lausanne). 2021;8:661891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Xu Z, Shen J, Yang Y, Yuan R, Xiang LF, Zhang C. Severe Cutaneous Adverse Reactions: A Single-Center Retrospective Study of 173 Patients in China. Ann Dermatol. 2019;31:545-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Gracia-Cazaña T, Padgett E, Calderero V, Oncins R. Nivolumab-associated Stevens-Johnson syndrome in a patient with lung cancer. Dermatol Online J. 2021;27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Logan IT, Zaman S, Hussein L, Perrett CM. Combination Therapy of Ipilimumab and Nivolumab-associated Toxic Epidermal Necrolysis (TEN) in a Patient With Metastatic Melanoma: A Case Report and Literature Review. J Immunother. 2020;43:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Riano I, Cristancho C, Treadwell T. Stevens-Johnson Syndrome-Like Reaction After Exposure to Pembrolizumab and Recombinant Zoster Vaccine in a Patient With Metastatic Lung Cancer. J Investig Med High Impact Case Rep. 2020;8:2324709620914796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Robinson S, Saleh J, Curry J, Mudaliar K. Pembrolizumab-Induced Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis in a Patient With Metastatic Cervical Squamous Cell Carcinoma: A Case Report. Am J Dermatopathol. 2020;42:292-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Ryu S, Jun I, Kim TI, Seo KY, Kim EK. Pembrolizumab-induced Stevens-Johnson Syndrome with Severe Ocular Complications. Ocul Immunol Inflamm. 2021;1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Salati M, Pifferi M, Baldessari C, Bertolini F, Tomasello C, Cascinu S, Barbieri F. Stevens-Johnson syndrome during nivolumab treatment of NSCLC. Ann Oncol. 2018;29:283-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Saw S, Lee HY, Ng QS. Pembrolizumab-induced Stevens-Johnson syndrome in non-melanoma patients. Eur J Cancer. 2017;81:237-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Shibata A, Yoshikawa T, Makita S, Nakagawa S, Ueda Y, Akiyama M. A case of recurrent Stevens-Johnson syndrome caused by nivolumab therapy. Eur J Dermatol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Haratake N, Tagawa T, Hirai F, Toyokawa G, Miyazaki R, Maehara Y. Stevens-Johnson Syndrome Induced by Pembrolizumab in a Lung Cancer Patient. J Thorac Oncol. 2018;13:1798-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Maloney NJ, Ravi V, Cheng K, Bach DQ, Worswick S. Stevens-Johnson syndrome and toxic epidermal necrolysis-like reactions to checkpoint inhibitors: a systematic review. Int J Dermatol. 2020;59:e183-e188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 16. | Horinouchi H, Nogami N, Saka H, Nishio M, Tokito T, Takahashi T, Kasahara K, Hattori Y, Ichihara E, Adachi N, Noguchi K, Souza F, Kurata T. Pembrolizumab plus pemetrexed-platinum for metastatic nonsquamous non-small-cell lung cancer: KEYNOTE-189 Japan Study. Cancer Sci. 2021;112:3255-3265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Si X, He C, Zhang L, Liu X, Li Y, Wang H, Guo X, Zhou J, Duan L, Wang M. Management of immune checkpoint inhibitor-related dermatologic adverse events. Thorac Cancer. 2020;11:488-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Ford M, Sahbudin I, Filer A, Steven N, Fisher BA. High proportion of drug hypersensitivity reactions to sulfasalazine following its use in anti-PD-1-associated inflammatory arthritis. Rheumatology (Oxford). 2018;57:2244-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Venkateswaran N, Khianey R, Generoso A. Stevens Johnson Syndrome in a Patient with Giant Cell Arteritis During Short Term Tocilizumab Therapy. Cureus. 2020;12:e7662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Charlton OA, Harris V, Phan K, Mewton E, Jackson C, Cooper A. Toxic Epidermal Necrolysis and Steven-Johnson Syndrome: A Comprehensive Review. Adv Wound Care (New Rochelle). 2020;9:426-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Hsu YO, Lu KL, Fu Y, Wang CW, Lu CW, Lin YF, Chang WC, Yeh KY, Hung SI, Chung WH, Chen CB. The Roles of Immunoregulatory Networks in Severe Drug Hypersensitivity. Front Immunol. 2021;12:597761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Vivar KL, Deschaine M, Messina J, Divine JM, Rabionet A, Patel N, Harrington MA, Seminario-Vidal L. Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J Cutan Pathol. 2017;44:381-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 23. | Wu Y, Wu H, Lin M, Liu T, Li J. Factors associated with immunotherapy respond and survival in advanced non-small cell lung cancer patients. Transl Oncol. 2022;15:101268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | de Zegher F, Jaeken J. Endocrinology of the carbohydrate-deficient glycoprotein syndrome type 1 from birth through adolescence. Pediatr Res. 1995;37:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 332] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 25. | Cortijo-Cascajares S, Cercós-Lletí AC, Ortiz-Pérez S, Caro-Teller JM, Ferrari-Piquero JM. Analysis of immune-mediated reactions in patients with non-small cell lung cancer treated with nivolumab and its association with effectiveness. J Oncol Pharm Pract. 2021;10781552211067429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Park SY, Oh IY, Kim JH, Kim HJ, Seo B, Kwon OY, Song WJ, Kwon HS, Cho YS, Moon HB, Kim TB. Therapeutic Effects of Mesenchymal Stem Cells on a Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis Model. J Korean Med Sci. 2020;35:e130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Lehloenya RJ, Isaacs T, Nyika T, Dhana A, Knight L, Veenstra S, Peter J. Early high-dose intravenous corticosteroids rapidly arrest Stevens Johnson syndrome and drug reaction with eosinophilia and systemic symptoms recurrence on drug re-exposure. J Allergy Clin Immunol Pract. 2021;9:582-584.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Liotti L, Caimmi S, Bottau P, Bernardini R, Cardinale F, Saretta F, Mori F, Crisafulli G, Franceschini F, Caffarelli C. Clinical features, outcomes and treatment in children with drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis. Acta Biomed. 2019;90:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 30. | Gilbert M, Scherrer LA. Efficacy and safety of cyclosporine in Stevens-Johnson syndrome and toxic epidermal necrolysis. Dermatol Ther. 2019;32:e12758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | González-Herrada C, Rodríguez-Martín S, Cachafeiro L, Lerma V, González O, Lorente JA, Rodríguez-Miguel A, González-Ramos J, Roustan G, Ramírez E, Bellón T, de Abajo FJ; PIELenRed Therapeutic Management Working Group. Cyclosporine Use in Epidermal Necrolysis Is Associated with an Important Mortality Reduction: Evidence from Three Different Approaches. J Invest Dermatol. 2017;137:2092-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 32. | Li L, Zhang X, Cui Y, Lu YY, Wang SX, Dong GF, Shi YZ, Luo RQ, Lei YX. [Effect of recombinant human tumor necrosis factor receptor type II-Fc fusion protein antibody on cytokines and bone metabolism in patients with juvenile idiopathic arthritis]. Zhonghua Yi Xue Za Zhi. 2010;90:2205-2208. [PubMed] |