Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.5984
Peer-review started: February 21, 2022
First decision: March 23, 2022
Revised: March 30, 2022
Accepted: April 29, 2022
Article in press: April 29, 2022
Published online: June 26, 2022
Processing time: 116 Days and 1.6 Hours
Many factors have an aberrant effect on the overall survival of lung cancer (LC) patients. In recent years, remarkable progress has been made in immunotherapy, targeted treatment, and promising biomarkers. However, the available treatments and diagnostic methods are not specific for all patients.
To establish a system for predicting poor survival in patients with LC.
The expression matrix and clinical information for this study were obtained from The Cancer Genome Atlas and Gene Expression Omnibus databases. After the differential analysis of all screened genes, weighted gene coexpression network analysis was performed to analyze hub genes related to patient survival. A logistic regression model was used to construct the scoring system. The expression of the hub genes was verified by performing quantitative reverse transcription-polymerase chain reaction.
A total of 5007 differentially expressed genes were selected for the Weighted Gene Co-expression Network Analysis algorithm. We found that the turquoise module showed the highest correlation with patient prognosis. The gene module with the greatest positive correlation with patient survival was located in the turquoise area. The Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analyses performed for the genes contained in the turquoise module indicated the potential roles of the selected genes in the regulation of LC development. In addition, protein–protein interaction analysis was performed to screen hub genes, which identified 100 hub genes located in the core area of the network. We then intersected the 100 hub genes with 75 key genes sorted by module members to identify real hub genes associated with prognosis. Forty-one genes were finally selected. We then used a logistic regression model to determine 11 independent risk genes, namely CCNB2, CDC20, CENPO, FOXM1, HJURP, NEK2, OIP5, PLK1, PRC1, SKA1, UBE2C and SPARC.
We constructed a predictive model based on 11 independent risk genes to establish a system predicting the survival status of patients with non-small-cell lung carcinoma.
Core Tip: This was a bioinformatics-based study aimed at identifying a novel system for predicting overall survival in lung cancer patients. We constructed a predictive model using Weighted Gene Co-expression Network Analysis, protein-protein interaction network, and least absolute contraction and selection operator-logistic regression analysis. And the expression of hub genes was verified by polymerase chain reaction, immunohistochemistry in lung cancer cell lines, and patient samples.
- Citation: Zhong C, Liang Y, Wang Q, Tan HW, Liang Y. Construction and validation of a novel prediction system for detection of overall survival in lung cancer patients. World J Clin Cases 2022; 10(18): 5984-6000
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/5984.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.5984
Lung cancer (LC) is one of the most common malignant tumors and one of the leading causes of cancer-related deaths worldwide. In 2012, the deaths caused by LC were approximately 1.6 million, accounting for 19% of the total global cancer deaths[1,2]. Despite advancement in its treatment, surgery is the primary therapy for patients with non-small-cell lung carcinoma. However, the overall survival rate of LC patients remains low.
Many factors have an aberrant effect on the overall survival of LC patients. The main reason is that patients who are diagnosed with advanced and metastasis LC cannot undergo radical surgery. Therefore, the development of more advanced diagnosis and predictive biomarkers is a promising direction for cancer diagnosis and treatment[3, 4].
In recent years, remarkable progress has been made in immunotherapy, targeted treatment, and promising biomarkers. However, the available treatments and diagnostic methods are not specific for all patients[5]. A high recurrence rate is observed after such treatment because of the complexity of cancer. Identification of new diagnostic and therapeutic biomarkers for cancer treatment is urgent[6].
The development of high-throughput technology has made important contributions to the identification of a large number of target genes in various diseases[7]. At the same time, as an emerging cross-discipline, bioinformatics analysis is widely used in the discovery of disease-related genes, new drug molecular targets, drug design, and functional analysis, which is helpful for the discovery of disease mechanisms[8]. Xie et al[9] performed bioinformatics analysis to analyze tumorigenesis-related genes and their target miRNAs in colon cancer, which facilitated the exploration of the potential targets for diagnosis, prognosis and treatment of colon carcinoma. Using RNA-Seq and bioinformatics methods, several key genes including ID1, ID3 and SMAD9 were identified in esophageal squamous cell carcinoma[10]. Many genes associated with LC progression and invasion have been identified by a combination of bioinformatics analysis and high-throughput sequencing[11-13].
The identification of differentially expressed genes (DEGs) has garnered considerable scientific attention. However, this method does not consider genes with similar expression patterns. Weighted Gene Co-expression Network Analysis (WGCNA) is a new algorithm that evaluates the correlation between gene modules and clinical features by constructing a scale-free gene coexpression network. In this study, we combined the WGCNA algorithm with DEGs to identify pivotal genes associated with clinicopathological characteristics and to provide insights into targeted therapy of LC.
The clinical and expression data of LC patients were derived from the Gene Expression Omnibus (GEO) and The Cancer Genome Atlas (TCGA) databases (https://portal.gdc.cancer.gov/; http://www.ncbi.nlm.nih.gov/geo/). GEO data contains two cohorts (GSE30129 and GSE50081). The sva package was used to normalize the Meta-GEO data. Next, we used the TCGA data (LogFC > 0.5, P < 0.05) to identify the DEGs and combined these DEGs with all GEO genes. Finally, 5007 genes were selected for the subsequent analyses.
WGCNA R package was used to analyze the coexpression networks. We determined the threshold of β = 5 to establish the optimal weighted network by Pearson’s correlational analysis. The adjacent matrix was transformed into a topological overlap measure matrix via topological overlapping dissimilarity to estimate its connectivity property in the network. We set the minimum number of module genes to 100, and the threshold for merging similar modules was set to 0.25. P < 0.05 was considered to indicate statistical significance. After the modules of interest were selected, the key genes were selected according to the gene signature (GS) and module membership (MM) of each module.
The functional analysis of core genes was performed using the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG). Through the R language clusterProfiler and ggplot2 packages, several important pathways have been discovered so far. The cut-off criteria were defined as count > 2 and P < 0.05.
We employed the STRING database to analyze the interaction between the module genes and set the confidence score to ≥ 0.9. The Cytohubba plug-in of Cytoscape software (version 3.7.0) was used to identify the core genes in the network.
All patients were assigned to training and validation sets in the ratio of 6:4. The least absolute contraction and selection operator (LASSO) reduced the data dimensionality, and Cox regression analysis was applied to construct a patient prognostic evaluation model. The predictive efficacy of the model was evaluated by the receiver operating characteristic (ROC) curve. A nomogram was used to visualize the scoring system through the rms package in the R software.
A549 and H1299 LC cells, and human lung fibroblasts were purchased from the Cancer Cell Repository (Shanghai Cell Bank, Shanghai, China). The medium used for cell culture was 10% Dulbecco’s modified eagle’s medium (supplemented with fetal bovine serum and penicillin/streptomycin). The cells were cultured in an incubator under 5% CO2 at 37 ℃.
We use 1 mL TRIzol (Invitrogen, Grand Island, NY, United States) and 200 μL chloroform to extract the total RNA from 2 × 106 cells in LC cells. Total RNA was reverse transcribed into cDNA (TaKaRa Bio, Shiga, Japan). The cDNA, primers, and the SYBR Green PCR Master Mix (TOYOBO, Osaka, Japan) were quantitatively detected by PCR. The primer sequences of all genes are depicted in Table 1. The gene expression level was evaluated by the 2-ΔΔCt method. All experiments were repeated three times.
| Gene | Forward primer sequence (5’-3’) | Reverse primer sequence (5’-3’) |
| CCNB2 | CCGACGGTGTCCAGTGATTT | TGTTGTTTTGGTGGGTTGAACT |
| CDC20 | GCACAGTTCGCGTTCGAGA | CTGGATTTGCCAGGAGTTCGG |
| CENPO | AGTGAGCAGATCCCGTAAACA | GGTTGGGTTCTACATTGGCAATA |
| FOXM1 | CGTCGGCCACTGATTCTCAAA | GGCAGGGGATCTCTTAGGTTC |
| HJURP | CCACGCTGACCTACGAGAC | CTCACCGCTTTTTGAATCGGC |
| GADPH | ACAACTTTGGTATCGTGGAAGG | GCCATCACGCCACAGTTTC |
| NEK2 | TGCTTCGTGAACTGAAACATCC | CCAGAGTCAACTGAGTCATCACT |
| OIP5 | TGAGAGGGCGATTGACCAAG | AGCACTGCGTGACACTGTG |
| PLK1 | AAAGAGATCCCGGAGGTCCTA | GGCTGCGGTGAATGGATATTTC |
| PRC1 | ATCACCTTCGGGAAATATGGGA | TCTTTCTGACAGACGGATATGCT |
| SKA1 | CCTGAACCCGTAAAGAAGCCT | TCATGTACGAAGGAACACCATTG |
| UBE2C | GACCTGAGGTATAAGCTCTCGC | TTACCCTGGGTGTCCACGTT |
All data in this study were analyzed using GraphPad Prism 5 and R software. The data were expressed as mean ± SD. A two-tailed t test was applied for quantitative real-time polymerase chain reaction (qRT-PCR) analysis among different groups. The results were considered to be significant at P < 0.05.
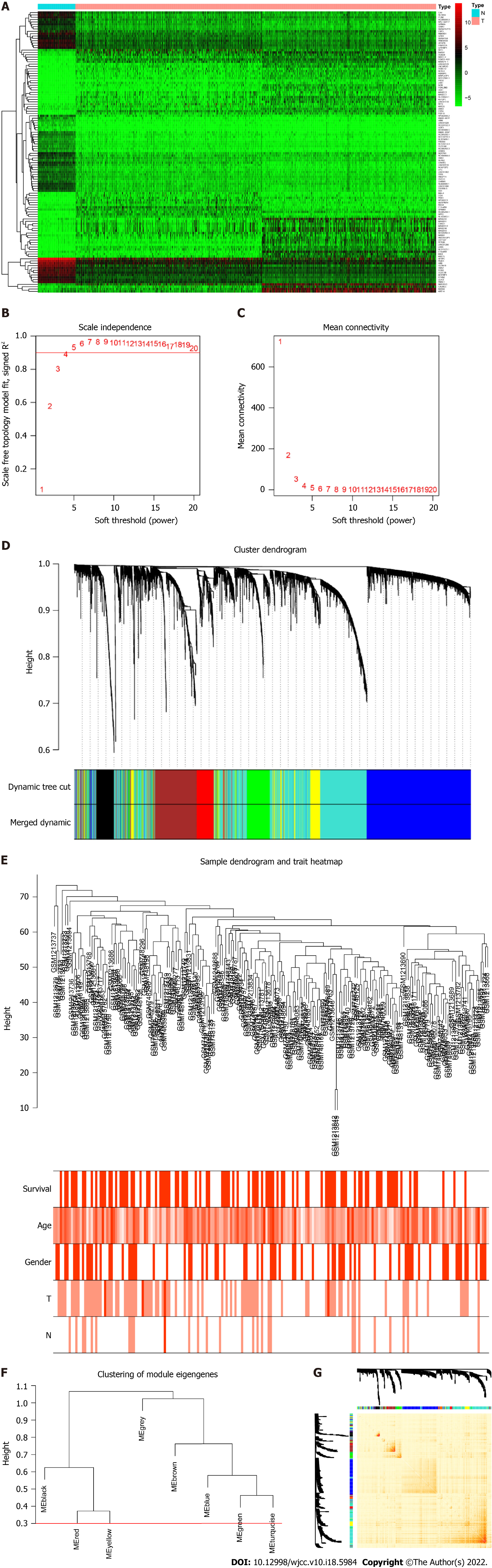
We screened DEGs based on the TCGA dataset by including 1037tumor/108normal samples. A total of 10 970 DEGs were selected based on the criteria of P < 0.05 and |log 2 FC| > 1. The top 30 up- and downregulated genes are shown in Figure 1A. We then intersected DEGs of TCGA with all genes in the GEO dataset and found 5007 common genes for further WGCNA.
To determine the roles of common DEGs associated with prognosis and other clinicopathological characteristics of LC patients, WGCNA was performed to construct a coexpression network. As shown in Figure 1B and C, the correlation coefficient was converted to the adjacent coefficient according to the optimal parameter (β = 5). Thereafter, highly correlated samples and delete discrete samples were clustered (Figure 1E). A threshold of 0.25 and a minimum gene number of 150 were considered to merge similar modules. Figure 1D shows eight modules that were finally selected on the basis of the filter criteria. The hierarchical clustering of module hub genes is shown in Figure 1F.
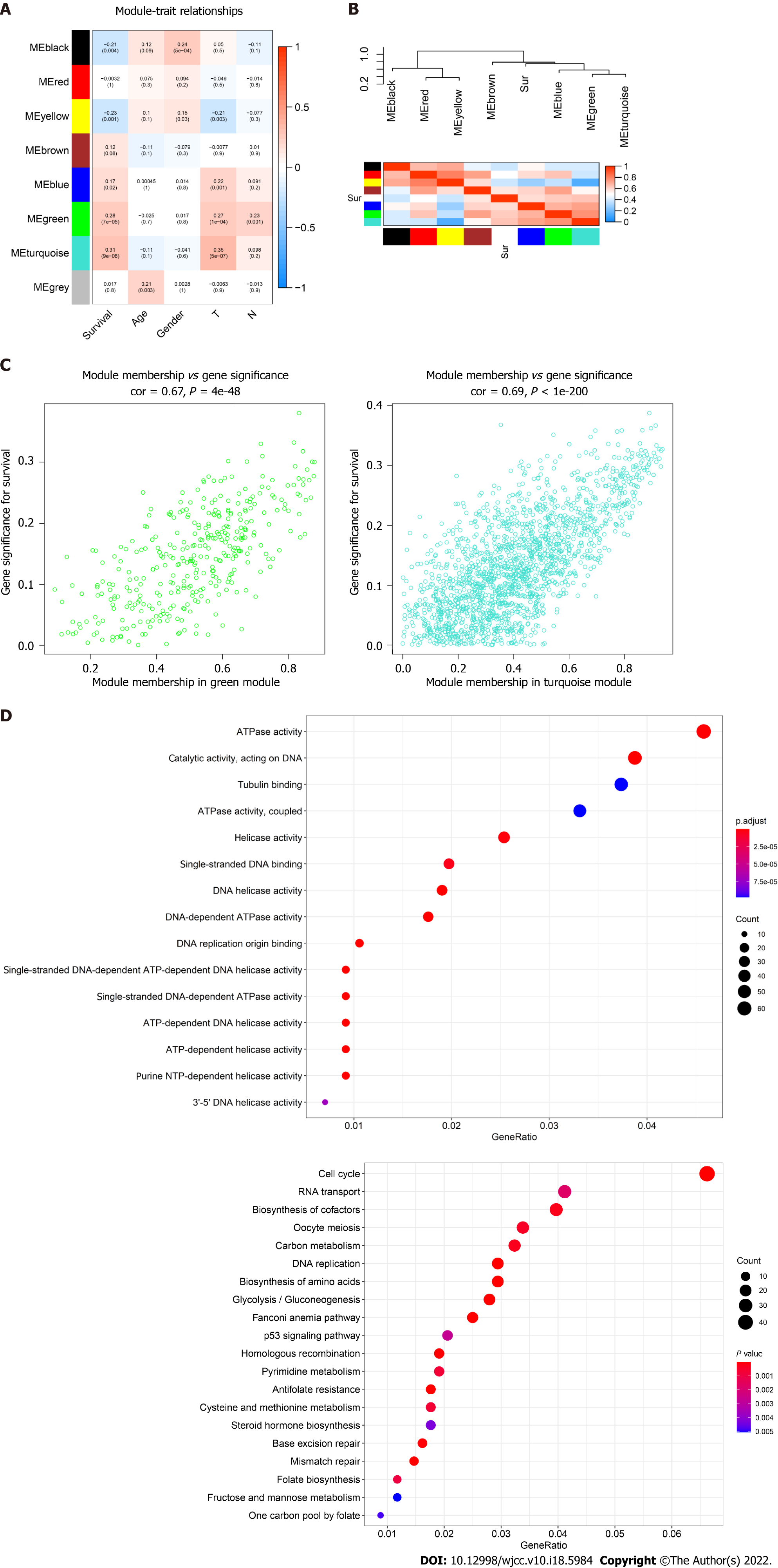
The topological overlap matrix plot (Figure 1G) indicated the correlation between the genes of the eight modules sorted using the clustering tree. The turquoise module showed a positive correlation of about 0.31 with LC patient survival, followed by the green module (0.28) and yellow module (0.23) (Figure 2A and B). The turquoise module contained 1673 genes. We then selected 75 key genes from the turquoise module with MM > 0.8 (Figure 2C). Taken together, the turquoise module was finally selected for further analysis.
GO enrichment analysis experiments showed that the turquoise module genes mainly encoded for ATPase, helicase, 3′-5′ DNA helicase, DNA-dependent ATPase, DNA helicase, ATP-dependent DNA helicase, and ATP-dependent helicase and associated with the binding of many molecules including DNA replication origin, single-stranded DNA, and tubulin. KEGG analysis indicated that many signaling pathways involved in Fanconi anemia and the p53 signaling pathway were correlated with turquoise module genes. In addition, other important pathways such as metabolism of carbon, pyrimidine, cysteine, and methionine, cell cycle, and DNA repair were also found in the turquoise module. These results indicated that the mechanism that affects the survival of LC patients may be closely related to the molecular binding mechanism and several important signaling pathways (Figure 2D).
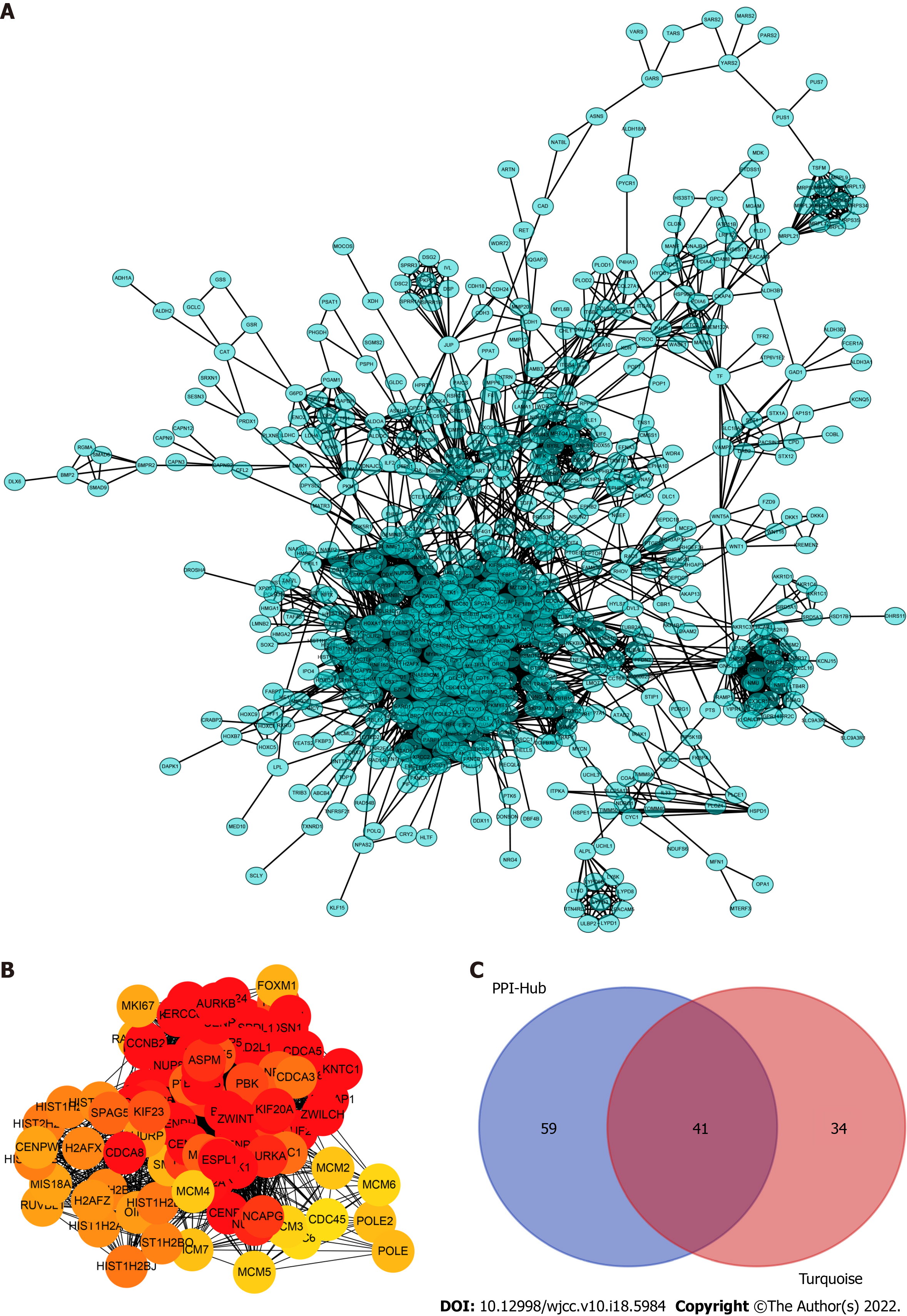
To determine which cluster of genes in the turquoise module have a pivotal effect on the prognosis of LC, we constructed protein–protein interaction networks using the STRING database (Figure 3A) and Cytoscape software and found 100 hub genes located in the core area of the network (Figure 3B). We then intersected the 100 hub genes with 75 key genes sorted by MM to identify real hub genes associated with prognosis (Figure 3C).
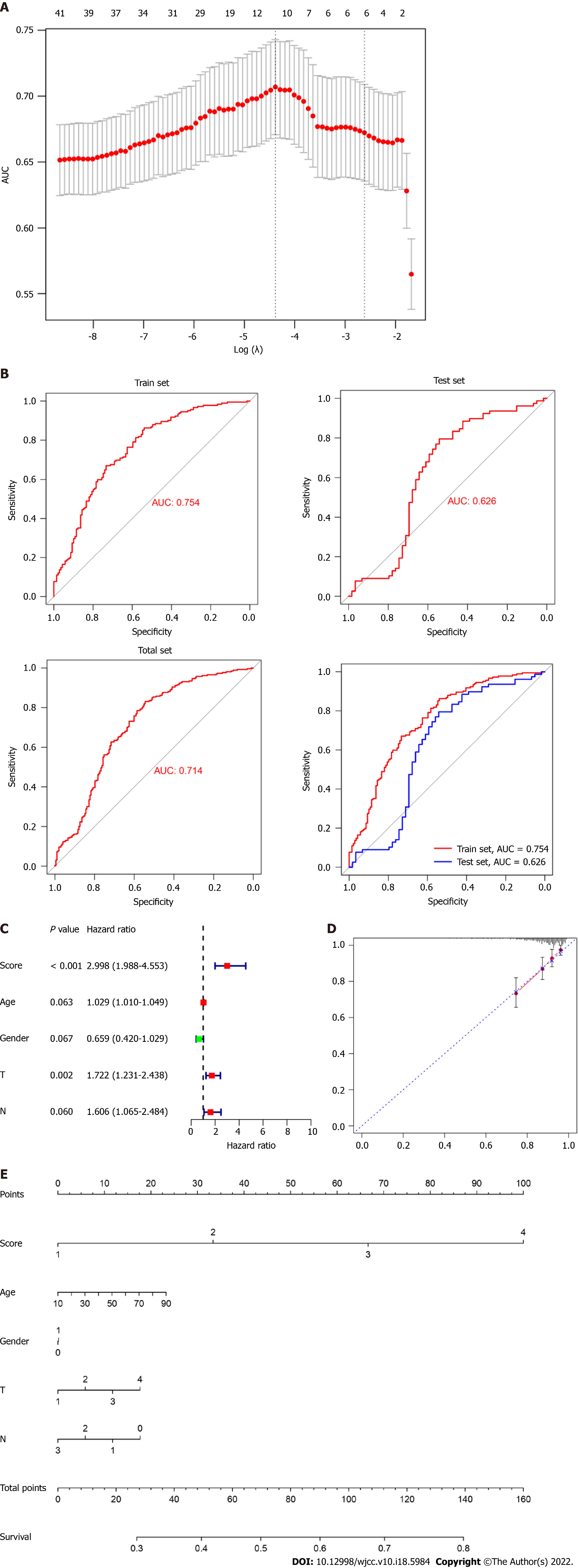
Subsequently, we performed a LASSO-logistic analysis of real hub genes to establish a prognostic evaluation model. Finally, 11 prognostic genes were selected in the predictive model in the training dataset, namely CCNB2, CDC20, CENPO, FOXM1, HJURP, NEK2, OIP5, PLK1, PRC1, SKA1, UBE2C and SPARC (Figure 4A). The risk score = 4.43 (Intercept) + CCNB2-expression × 0.552 + CDC20-expression × 0.037 CENPO-expression × 0.287 + FOXM1-expression × 0.106 + HJURP-expression × 0.229 + NEK2-expression × 0.083 OIP5-expression × 0.020 PLK1-expression × 0.520 + PRC1-expression × 0.192 SKA1-expression × 0.110 + UBE2C-expression × 0.263. Subsequently, we evaluated the reliability of the model by the ROC curve. The results showed that the area under the curve of the training set and test set were 0.754 and 0.626, respectively (Figure 4B). Cox regression analysis showed that risk score was an independent risk factor for predicting the poor prognosis of LC patients (Figure 4C). To further evaluate the prognosis of LC, we constructed a nomogram based on risk factors (Figure 4D and E).
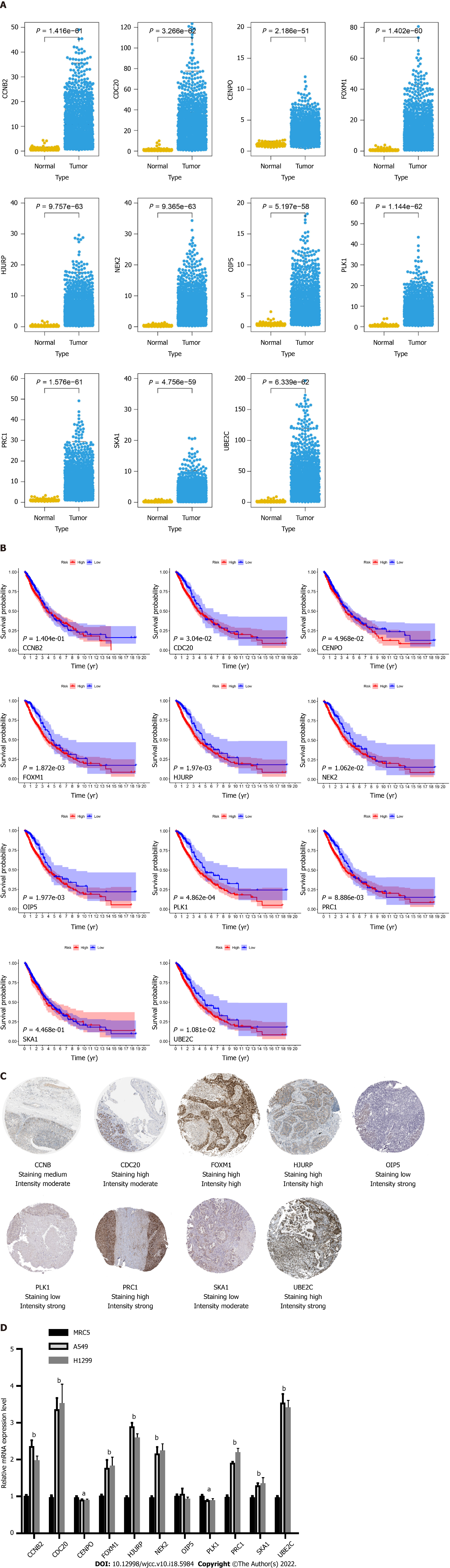
After the construction of the scoring system in the GEO dataset, we determined the effect of 11 genes in the TCGA dataset. As shown in Figure 5A, all genes were differentially expressed in LC patients compared with normal samples. Moreover, all genes were significantly correlated with patient prognosis except for CCNB2 and SKA1 (Figure 5B).
To validate the expression of the 11 hub genes, we performed an immunohistochemistry experiment obtained from the Protein Atlas database. Immunohistochemistry indicated that the protein levels of CDC20, FOXM1, HJURP, PRC1, UBE2C and CCNB2 were increased in the LC samples compared with the normal samples, whereas those of OIP5, PLK1 and SKA1 were decreased (Figure 5C). To explore the mRNA expression levels of these genes, we performed qRT-PCR analysis and found that the mRNA levels of CCNB2, CDC20, FOXM1, HJURP, NEK2, PRC1, SKA1 and UBE2C were increased in the LC cell lines, whereas those of CENPO, OIP5 and PLK1 was decreased (Figure 5D).
LC treatments include a combination of radical surgery, radiation therapy, chemotherapy, and precise targeted therapy[14]. Despite advancement in LC treatment and diagnosis, the 5-year overall survival rate remains low[15]. The main reason is that patients who are diagnosed with advanced and metastasis LC cannot undergo radical surgery. Therefore, more specific and sensitive biomarkers are needed to facilitate early diagnosis and prediction of overall survival.
Recently, several therapeutic targets and prognostic biomarkers have been identified using advanced high-throughput sequencing technology and integrative bioinformatics analysis. Previous studies have reported many prognostic biomarkers in LC by performing a combined analysis using TCGA and GEO datasets and validated by in vitro experiments. Sun et al[16] reported the role of C-type lectin domain family 3 member B (CLEC3B) in tumor progression, prognosis, and immune responses in LC by performing RNA-Seq and bioinformatics analysis; the expression and methylation of CLEC3B were also validated by qRT-PCR analysis. miRNA-144-3p, an important noncoding RNA, was identified and validated as an independent risk factor for LC prognosis by performing bioinformatics analysis and qRT-PCR[17]. However, because of the insufficient sample size, biological heterogeneity, and different statistical methods, highly effective genes are not found in clinical practice. Moreover, the prediction efficiency in tumor patients could be limited by simply using a single GS instead of a multi-GS. Therefore, more biological markers and more effective prediction models are required for the prevention and treatment of LC.
In the present study, we initially detected DEGs based on the TCGA dataset and intersected these DEGs with all GEO cohort genes to obtain an expression profile. We used the WGCNA algorithm to identify core genes in GEO expression data and that were highly related to clinical features. WGCNA classified eight modules and subsequently correlated the modules with clinical characteristics. In these modules, the turquoise module contained 1673 genes that showed the highest correlation with LC patient prognosis. We then performed GO enrichment and KEGG pathway analyses of the turquoise module genes and found that the function of these genes was mainly related to activation of enzymes including ATPase, helicase, 3′-5′ DNA helicase, DNA-dependent ATPase, DNA helicase, ATP-dependent DNA helicase, and ATP-dependent helicase and binding of many molecules including DNA replication origin, single-stranded DNA, and tubulin and activation of signaling pathways involved in Fanconi anemia and p53 signaling pathway. Subsequently, we performed a protein–protein interaction network analysis of the genes contained in the yellow module and intersected the network hub genes with MM > 0.8. Forty-one genes were selected and subjected to LASSO-logistic regression. We finally identified 11 prognostic genes, namely CCNB2, CDC20, CENPO, FOXM1, HJURP, NEK2, OIP5, PLK1, PRC1, SKA1, UBE2C and SPARC. Among these genes, FOXM1 and PLK1 are the most studied genes in LC. FOXM1, an important family member of the FOX family, plays a pivotal role in a series of biological processes, including facilitating cell proliferation, differentiation, and organ development[18]. FOXM1 level is significantly increased in LC cells and could be regulated by miR-216b, which promotes cancer progression and epithelial–mesenchymal transition in LC cells[19]. Moreover, FOXM1 could directly regulate the radiosensitivity of LC cells via interacting with KIF20A, suggesting that FOXM1 might be a novel therapeutic target for LC treatment. FOXM1 could also be regulated by other important molecules. The family with sequence similarity 188-member B is a member of the novel putative deubiquitinase family and directly binds to FOXM1, which promotes LC progression[20]. PLK1 is highly correlated with LC progression. PLK1 can target and regulate the transforming growth factor β signaling pathway, and then amplify its metastatic activity by positive feedback[20]. PLK1 is also regulated by long noncoding RNAs. For instance, miR-296-5p decreases the ability of cell invasion and migration by directly targeting PLK1 in LC cells[21]. However, other genes have not been actively researched, especially with regard to the mechanism of progression in LC. Therefore, in-depth knowledge of these genes will help develop new biomarkers for early LC diagnosis and prediction of prognosis.
After the scoring system was constructed, we further evaluated the performance of the model in LC patients. The ROC curve showed that the model had an excellent predictive performance. In addition, the risk predictor of the model can be considered an independent risk factor for predicting LC prognosis. To conclude, this study showed the potential of prognostic genes in LC patients using WGCNA combined with the established predictive model. However, this study also had some limitations. Firstly, LC patients were from public databases, thus the number of samples was limited. In future studies, we will collect samples from our hospital to expand the sample size to validate the predictive model. Second, the molecular biological mechanism by which the hub gene affects the prognosis of patient needs to be further explored.
This study used the WGCNA algorithm to identify functional modules highly correlated with LC prognosis. After construction of the predictive model, we screened and validated 11 prognostic genes, which might be considered new therapeutic targets for the diagnosis and treatment of LC. This study also had some limitations. The mechanisms of the effect of the 11 prognostic genes on cancer progression need to be studied in the future.
Many factors have an aberrant effect on the overall survival of lung cancer (LC) patients. In recent years, remarkable progress has been made in immunotherapy, targeted treatment, and promising biomarkers. However, the available treatments and diagnostic methods are not specific for all patients.
Identifying new diagnostic and therapeutic biomarkers for cancer treatment is urgent.
We aimed to establish a system for predicting poor survival in patients with LC.
Weighted Gene Co-expression Network Analysis (WGCNA), functional enrichment analysis, quantitative real-time polymerase chain reaction, and other bioinformatics analysis were used in this study.
A total of 5007 differentially expressed genes were selected for the WGCNA algorithm. The turquoise module showed the highest correlation with patient prognosis. The gene module with the greatest positive correlation with patient survival was located in the turquoise area. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analyses performed for the genes contained in the turquoise module indicated the potential roles of the selected genes in the regulation of LC development. In addition, protein–protein interaction analysis was performed to screen hub genes, which identified 100 hub genes located in the core area of the network. We intersected the 100 hub genes with 75 key genes sorted by module members to identify real hub genes associated with prognosis. Forty-one genes were finally selected. We used a logistic regression model to determine 11 independent risk genes, namely CCNB2, CDC20, CENPO, FOXM1, HJURP, NEK2, OIP5, PLK1, PRC1, SKA1, UBE2C and SPARC.
We constructed a model based on 11 independent risk genes to establish a system to predict the survival status of patients with non-small-cell lung carcinoma.
The new predictive model could play a role in overall survival.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Miranda LA, Brazil; Mizuno N, Japan S-Editor: Gong ZM L-Editor: Kerr C P-Editor: Gong ZM
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [PubMed] [DOI] [Full Text] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [PubMed] [DOI] [Full Text] |
| 3. | Villalobos P, Wistuba II. Lung Cancer Biomarkers. Hematol Oncol Clin North Am. 2017;31:13-29. [PubMed] [DOI] [Full Text] |
| 4. | Di X, Jin X, Li R, Zhao M, Wang K. CircRNAs and lung cancer: Biomarkers and master regulators. Life Sci. 2019;220:177-185. [PubMed] [DOI] [Full Text] |
| 5. | Ciliberto D, Staropoli N, Caglioti F, Gualtieri S, Fiorillo L, Chiellino S, De Angelis AM, Mendicino F, Botta C, Caraglia M, Tassone P, Tagliaferri P. A systematic review and meta-analysis of randomized trials on the role of targeted therapy in the management of advanced gastric cancer: Evidence does not translate? Cancer Biol Ther. 2015;16:1148-1159. [PubMed] [DOI] [Full Text] |
| 6. | Tsoukalas N, Kiakou M, Tsapakidis K, Tolia M, Aravantinou-Fatorou E, Baxevanos P, Kyrgias G, Theocharis S. PD-1 and PD-L1 as immunotherapy targets and biomarkers in non-small cell lung cancer. J BUON. 2019;24:883-888. [PubMed] |
| 7. | Moncho-Amor V, Pintado-Berninches L, Ibañez de Cáceres I, Martín-Villar E, Quintanilla M, Chakravarty P, Cortes-Sempere M, Fernández-Varas B, Rodriguez-Antolín C, de Castro J, Sastre L, Perona R. Role of Dusp6 Phosphatase as a Tumor Suppressor in Non-Small Cell Lung Cancer. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Full Text] |
| 8. | Kulasingam V, Diamandis EP. Strategies for discovering novel cancer biomarkers through utilization of emerging technologies. Nat Clin Pract Oncol. 2008;5:588-599. [PubMed] [DOI] [Full Text] |
| 9. | Xie B, Zhao R, Bai B, Wu Y, Xu Y, Lu S, Fang Y, Wang Z, Maswikiti EP, Zhou X, Pan H, Han W. Identification of key tumorigenesisrelated genes and their microRNAs in colon cancer. Oncol Rep. 2018;40:3551-3560. [PubMed] [DOI] [Full Text] |
| 10. | Zhao Y, Zhu J, Shi B, Wang X, Lu Q, Li C, Chen H. The transcription factor LEF1 promotes tumorigenicity and activates the TGF-β signaling pathway in esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2019;38:304. [PubMed] [DOI] [Full Text] |
| 11. | Zhao T, Khadka VS, Deng Y. Identification of lncRNA biomarkers for lung cancer through integrative cross-platform data analyses. Aging (Albany NY). 2020;12:14506-14527. [PubMed] [DOI] [Full Text] |
| 12. | Xue L, Xie L, Song X. Identification of potential tumor-educated platelets RNA biomarkers in non-small-cell lung cancer by integrated bioinformatical analysis. J Clin Lab Anal. 2018;32:e22450. [PubMed] [DOI] [Full Text] |
| 13. | Liu L, Ahmed T, Petty WJ, Grant S, Ruiz J, Lycan TW, Topaloglu U, Chou PC, Miller LD, Hawkins GA, Alexander-Miller MA, O'Neill SS, Powell BL, D'Agostino RB Jr, Munden RF, Pasche B, Zhang W. SMARCA4 mutations in KRAS-mutant lung adenocarcinoma: a multi-cohort analysis. Mol Oncol. 2021;15:462-472. [PubMed] [DOI] [Full Text] |
| 14. | Hensing T, Chawla A, Batra R, Salgia R. A personalized treatment for lung cancer: molecular pathways, targeted therapies, and genomic characterization. Adv Exp Med Biol. 2014;799:85-117. [PubMed] [DOI] [Full Text] |
| 15. | Li F, He H, Qiu B, Ji Y, Sun K, Xue Q, Guo W, Wang D, Zhao J, Mao Y, Mu J, Gao S. Clinicopathological characteristics and prognosis of lung cancer in young patients aged 30 years and younger. J Thorac Dis. 2019;11:4282-4291. [PubMed] [DOI] [Full Text] |
| 16. | Sun J, Xie T, Jamal M, Tu Z, Li X, Wu Y, Li J, Zhang Q, Huang X. CLEC3B as a potential diagnostic and prognostic biomarker in lung cancer and association with the immune microenvironment. Cancer Cell Int. 2020;20:106. [PubMed] [DOI] [Full Text] |
| 17. | Chen YJ, Guo YN, Shi K, Huang HM, Huang SP, Xu WQ, Li ZY, Wei KL, Gan TQ, Chen G. Down-regulation of microRNA-144-3p and its clinical value in non-small cell lung cancer: a comprehensive analysis based on microarray, miRNA-sequencing, and quantitative real-time PCR data. Respir Res. 2019;20:48. [PubMed] [DOI] [Full Text] |
| 18. | Zhang Y, Qiao WB, Shan L. Expression and functional characterization of FOXM1 in non-small cell lung cancer. Onco Targets Ther. 2018;11:3385-3393. [PubMed] [DOI] [Full Text] |
| 19. | Wang L, Wang Y, Du X, Yao Y, Wang L, Jia Y. MiR-216b suppresses cell proliferation, migration, invasion, and epithelial-mesenchymal transition by regulating FOXM1 expression in human non-small cell lung cancer. Onco Targets Ther. 2019;12:2999-3009. [PubMed] [DOI] [Full Text] |
| 20. | Choi YE, Madhi H, Kim H, Lee JS, Kim MH, Kim YN, Goh SH. FAM188B Expression Is Critical for Cell Growth via FOXM1 Regulation in Lung Cancer. Biomedicines. 2020;8. [PubMed] [DOI] [Full Text] |