Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5446
Peer-review started: October 10, 2021
First decision: January 13, 2022
Revised: January 21, 2022
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: June 6, 2022
Processing time: 235 Days and 7.8 Hours
Chromosome i(17)(q10) abnormality is mainly associated with chronic myeloid leukemia (CML), myelodysplastic syndrome/myeloproliferative tumors (MDS/MPD), and acute myeloid leukemia (AML). The role of i(17)(q10) in AML is still unknown, the differences between AML and acute promyelocytic leukemia (APL)-like AML with i(17)(q10) need more research. This study aimed to investigate the clinical characteristics and laboratory evidence of 2 AML cases with i(17)(q10), similar to APL phenotype.
Both pediatric patients were males; case 1 had newly diagnosed AML, and case 2 showed relapsed tumor after 1 year of drug withdrawal. Bone marrow cell morphology, chromosome karyotype analysis, Fully-instrumented submersible housing test, immunological assays, molecular biological methods, and blood tumor panoramic gene test were performed. All-trans retinoic acid (ATRA) combined with arsenic acid (As2O3) were used in the first course of treatment. Bone marrow was dominated by abnormal promyelocytic granulocytes. Karyotype test revealed i(17)(q10) isochromosome. Immunological phenotype mainly included positive expressions of CD9, CD13, CD33, and CD38. Case 1 suffered intracranial hemorrhage after re-chemotherapy and died on D162. For case 2, on D145 and D265, bone marrow promyelocytic granulocytes accounted for 2%. Flow cytometric residual lesion detection showed no abnormal immuno
ATRA, As2O3, and chemotherapy may be ineffective in treating APL-like AML with i(17)(q10) but without t(15;17) and PML-RARA fusion gene.
Core Tip: Herein we reported two cases of acute myeloid leukemia (AML) mimicking APL after the same treatment protocols. A rare chromosomal abnormality, i(17)(q10), was observed in two pediatric patients, which mimicked acute promyelocytic leukemia (APL) phenotype. Both patients showed no responses to all-trans retinoic acid and arsenic trioxide induction therapy. One patient with i(17)(q10) died after 5 mo, and the other patient with i(17)(q10) add (14)14 had been medication free more than 10 mo and achieved complete tumor remission for 3 years since drugs were withdrawn. Pediatric AML mimicking APL is difficult to treat and additional cases should be studied to provide better treatment strategies for these patients.
- Citation: Yan HX, Zhang WH, Wen JQ, Liu YH, Zhang BJ, Ji AD. Pediatric acute myeloid leukemia patients with i(17)(q10) mimicking acute promyelocytic leukemia: Two case reports. World J Clin Cases 2022; 10(16): 5446-5455
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5446.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5446
Chromosome i(17)(q10) abnormality is described as any unreasonable damage or breakage of the centromeres of chromosome 17, resulting in absence of the short arm and an iso-arm of the long arm[1]. Isochromosome 17 i(17)(q10) is mainly associated with chronic myeloid leukemia (CML)[2,3], myelodysplastic syndrome/myeloproliferative tumors (MDS/MPD)[4-6], and acute myeloid leukemia (AML)[6,7]. Genetic mutation analysis showed that 95% of patients with chromosome karyotype i(17)(q10) carried at least one mutation, and on average three mutations. The three most commonly mutated genes were ASXL1(66%), SRSF2(65%), and SETBP1 (48%)[8,9]. In acute promyelocytic leukemia (APL), chromosome karyotype i(17)(q10) was often accompanied by t(15;17) and PML-RARa fusion gene with an incidence of 1.9% and 4.1%, respectively[10,11]. APL children with i(17)(q10) have poor prognosis[12]. In a group study of 478 children with AML, chromosome karyotype analysis showed only one i(17)(q10) abnormality case, without morphological description and prognostic evaluation[13]. A 10-year-old African Black APL child carrying i(17)(q10) karyotype but without t(15;17) abnormality, who was in serious condition at admission, did not get tumor remission after treatment, and died within 2 wk[14]. A Chinese i(17)(q10) AML adult with a similar phenotype to APL was reported[15]. In the present case study, we treated 1 AML child with i(17)(q10) and 1 AML child with i(17)(q10) and (14)(p11) who had a phenotype similar to APL in our department. These two cases were investigated and followed up, and their clinical significance was discussed.
Case 1: A 3-year-old boy of Han nationality, was admitted to the Pediatric Hematology Department of Xianyang Caihong Hospital, China on December 19, 2016. The boy had paleness and fever for more than half a month, as well as exophthalmos and pain in the right knee joint due to unknown reasons for two weeks.
Case 2: A 12-year-old Han boy was admitted to our hospital with a history of a pale complexion for one month and skin bleeding for 10 d.
Case 1: He had a fever of 38.2 °C, moderate anemia, scattered red bleeding spots on the skin, protruded eyeballs, and no swelling of the superficial lymph nodes.Initial blood tests showed the following: Hb 75 g/L, white blood cell (WBC) count 5.82 × 109/L, and platelet (PLT) count 73 × 109/L.
Case 2: He had moderate anemia and bleeding spots on the skin and mucosa throughout the body. Initial blood test results showed the following: Hb 68 g/L, WBC count 17.53 × 109/L, and PLT count 60 × 109/L.
Case 1: There is no history of past illness.
Case 2: He was diagnosed with APL 3 years ago in a local hospital based on bone marrow morphology and immunological classification, with negative PML-RARa fusion gene at the time of diagnosis.The patient received all-trans retinoic acid (ARAT) and arsenic trioxide (ATO) as induction therapy, and bone marrow examination showed no tumor remission on D29. He then received three cycles of consolidation therapy (DA, HA, and MA regimen) and maintenance therapy, bone marrow evaluation showed complete remission, and the treatment was stopped.
Case 1: He had been in good health condition, with no family history of inherited blood disorder, no history of tumor-associated genetic abnormalities, and no history of drug or food allergies.
Case 2: There is no personal and family history.
Case 1: Upon examination, he had a fever of 38.2 °C, moderate anemia, scattered red bleeding spots on the skin, protruded eyeballs, and no swelling of the superficial lymph nodes. On auscultation, his heart and lung were normal, and the liver and spleen were not examined.
Case 2: He had moderate anemia and bleeding spots on the skin and mucosa throughout the body. Auscultation of the heart and lung showed no abnormalities. Subcostal areas of the liver and spleen were not examined.
A volume of 0.1 mL bone marrow fluid was extracted from the posterior superior iliac spine (sampling was very difficult), a bone marrow smear was prepared and submitted for examination. Chromosome G-banding karyotype analysis: 3 mL of sterile bone marrow fluid was taken from the patient, and g-banding technique was employed to detect the chromosomes in trypsin-digested short-term cell culture. Karyotype results were analyzed according to the international system for human cytogenetic nomenclature (ISCN, 1991).
Immunophenotype: 2 mL of bone marrow fluid with heparin anticoagulant was obtained, 5x105-5×106/mL cells were isolated using FACSort flow cytometry (BD Biosciences) and analyzed with CellQuest software > The expression levels of leukemia related antigens in the cell population were analyzed and calculated. Monoclonal antibodies used included HLA-DR, CD2, CD3, CD4, CD7, CD8, CD9, CD11b, CD13, CD14, CD15, CD16, CD19, CD22, CD33, and CD34 Labeled by FITC, PE, and PerCP, or APC- CD38, CD56, CD64, CD71, CD117, CD123, and MPO. All antibodies were purchased from BD Biosciences.
PML-RARa fusion gene was detected by real-time quantitative PCR: A volume of 2 mL bone marrow fluid with heparin anticoagulant was collected to isolate the mononuclear cells, and total DNA of mononuclear cells was extracted.
There is no imaging examinations.
Acute promyelocytic leukemia -like acute myeloid leukemia with i(17)(q10).
Phase Ⅰ: Two children were treated with ATAR combined with ATO to induce remission: ATAR (30 mg/M2/d), divided 3 times, orally, D1-30; ATO (0.02 mg/Kg), 1 time/day, intravenous infusion, D1-28. Then they were treated with low molecular weight heparin anticoagulant correction therapy based on the coagulation test. Bone marrow cell morphology and leukocyte residual lesions were detected on D29. Blood WBC count was 27.53 × 109/L. Considering the possibility of retinoic acid syndrome, dexamethasone tablets were administered orally at 1.5 mg/time, 3 times a day. With fever regression, WBC was reduced to 12.27 × 109/L on D7. Case 2 showed fever on D2 of treatment, with a temperature of 38.5 ℃, and still had a fever on D3. Blood routine test showed a WBC count of 27.53 × 109/L. Considering the possibility of the retinoic acid syndrome, dexamethasone tablet was taken 1.5 mg/time, 3 times a day. With fever regression, WBC decreased to 12.27 × 109/L in D7.
Phase Ⅱ: On D33, case 1 was treated with the DAE regimen, which including the following: DNR (40 mg/M2/d), D1, 3 and 5, intravenous infusion, once a day; Ara-c (200mg/M2/d), D 1-7, q12h, subcutaneous injection; and Vp-16 /E (100mg/M2/d), D 5, 6, 7, intravenous infusion, once a day. Reexamination of bone marrow cell morphology on D 65 showed no remission. The pediatric patient gave up treatment and discharged themselves. On D154, he came to the hospital again with fatigue, sallow complexion, skin hemorrhagic spots, bone pain, and eyeball herniation. Bone marrow examination showed 85% abnormal promyelocytic granulocytes. D156 Chemotherapy with MAH protocol: M (10 mg/M2/d), D1, 2 and 3; A (200 mg/M2/d), D1-7, q12h, subcutaneous injection; and H (3 mg/M2/d),D 1-7, subcutaneous injection. Case 2 was treated with the MAH regimen on D 47 and D80. The doses and methods were as above. On D115, he was treated with IDA (10mg/M2/ D1-3); intravenous infusion; once a day. The dosage and usage of the ara-C and H were the same as before. On D145, bone marrow cell morphology was evaluated, residual lesions were detected by flow cytometry, and WT1 gene copy number was detected by molecular biological techniques (See Methods). On D175, he was treated with HD ara-C (2.0 g/M2/d); D1, 3, 5 and 7; q12h. The dose and usage of HD ara-C were the same as above. On D205, HD ara-C dose and usage were the same as above: Vp-16 (100 mg/M2/d), D1-5; intravenous infusion. On D 235, he received HD ara-C (3.0g/M2/d); D1, 3, 5, 7; q12h; the dose and usage were the same as above. During D265-730, 6-MP [50 mg/M2/d (D1-21)] plus low-dose ara-C (40mg/M2) were given. D1-4 (D22-28) maintenance therapy: Bone marrow cell morphology was returned one year after drug discontinuation, residual lesions were detected using flow cytometry, and WT1 gene copy number was detected by molecular biological tools (See methods).
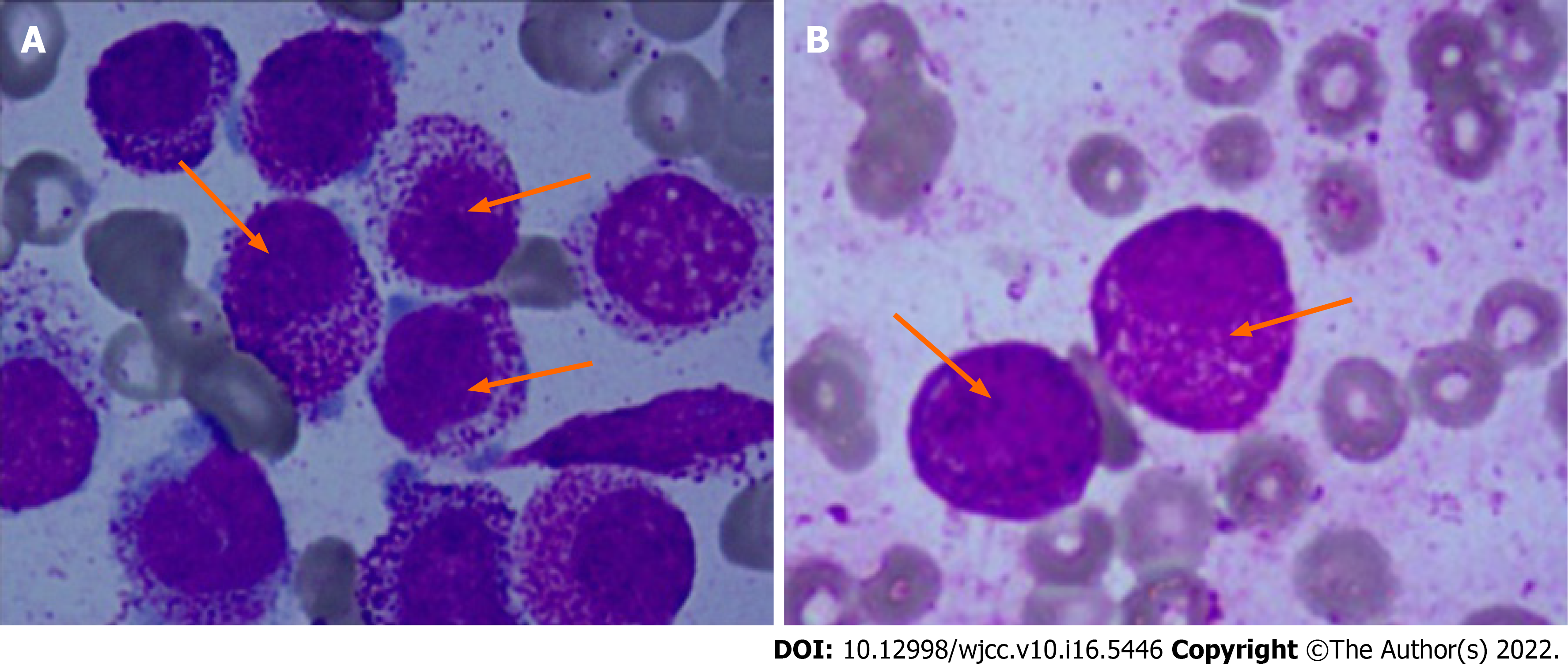
Bone marrow smear: Case 1 showed active bone marrow with nucleated cell hyperplasia. Case 2 originally had granulocyte at 1.0%, and abnormal early young granulocyte at 83.0% and 85.0%, respectively. The cytoplasm was bulky and filled with azure particles. Plasma particles were visible both inside and outside some cells, with less outside the cells. Round or oval nucleus, coarse chromatin, and indistinct nucleoli were observed. Acute promyelocytic leukemia is shown in Figure 1.
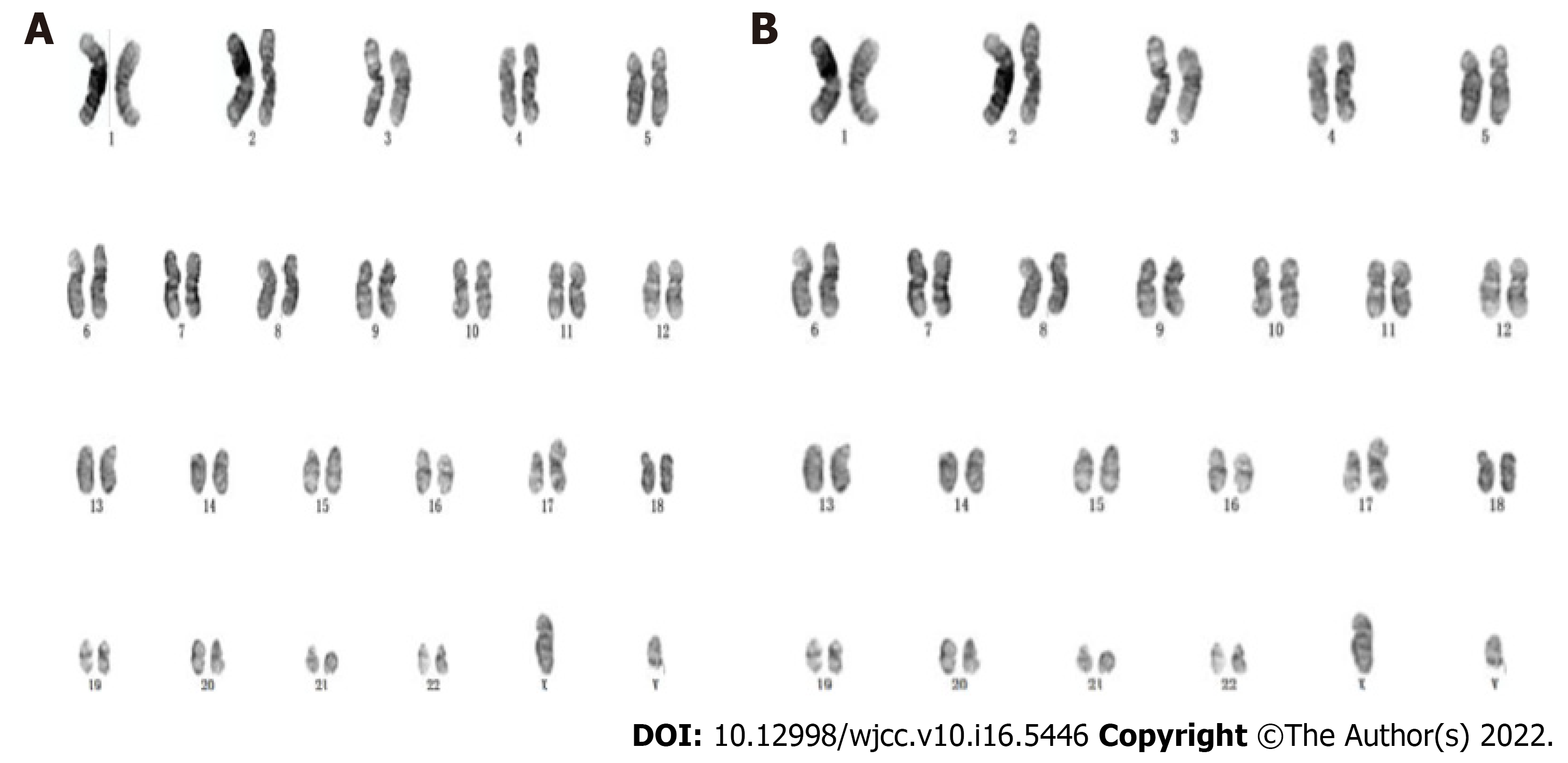
The following results were revealed: Case 1: 46XY, i(17)(q10)[9]/46, XY[11], long equi arm of chromosome 17; Cases 2: 46XY, Add(14)(P11), i(17)(q10)[4]/46, XY[1], a short arm of chromosome 14 with an additional fragment of unknown origin and a long arm of chromosome 17, as shown in Figure 2.
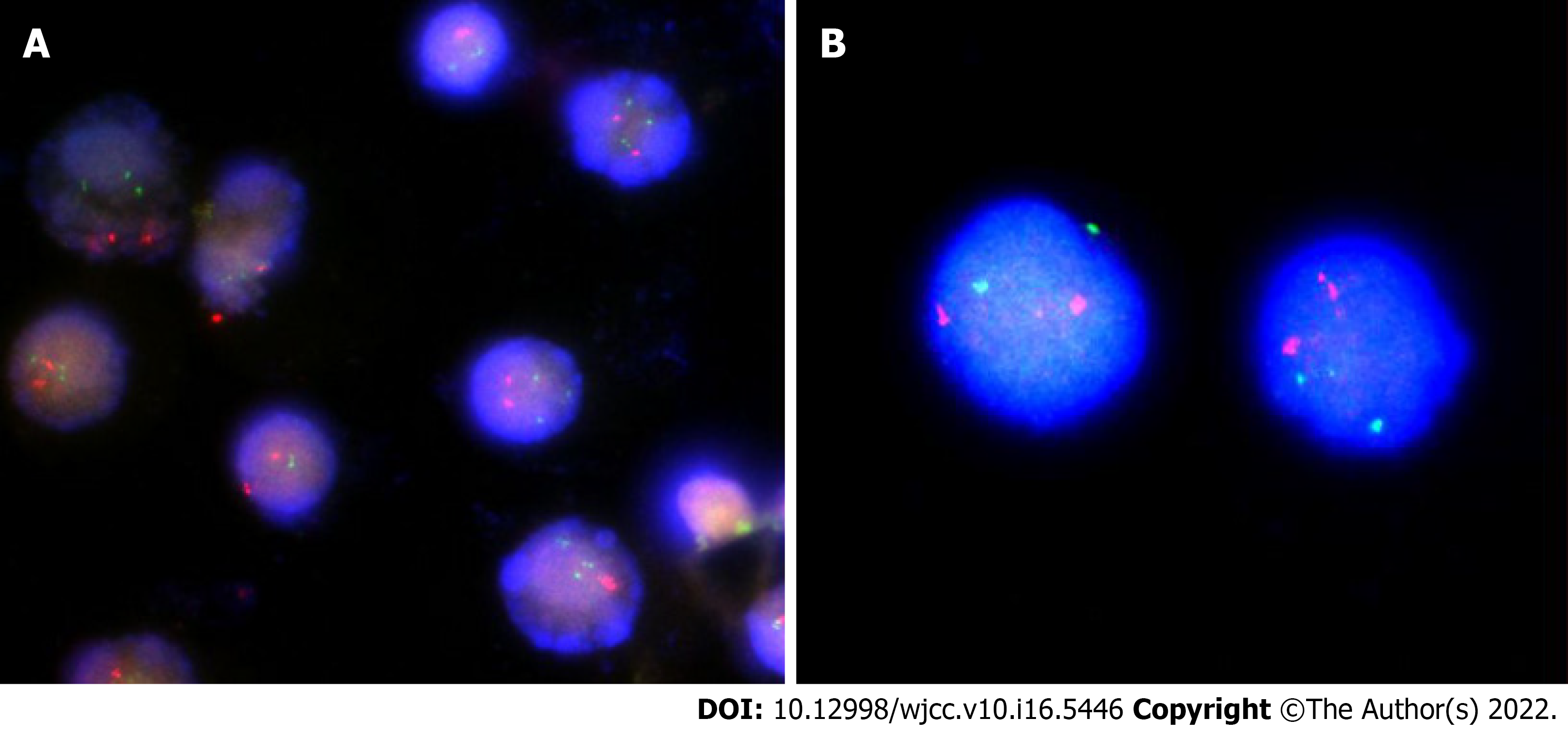
Case 1: The nuclear in situ hybridization (nuc ish) (PML × 2, RARA × 3) (180/400) showed no fusion signal by PML/RARA translocation probe, and the copy number of RARA (located at 17q21) site increased, accounting for about 45%.
Case 2: The nuc ish (PML × 2, RARA × 2) showed no abnormal signal in the PML/RARA locus, and the detection result was negative as shown in Figure 3.
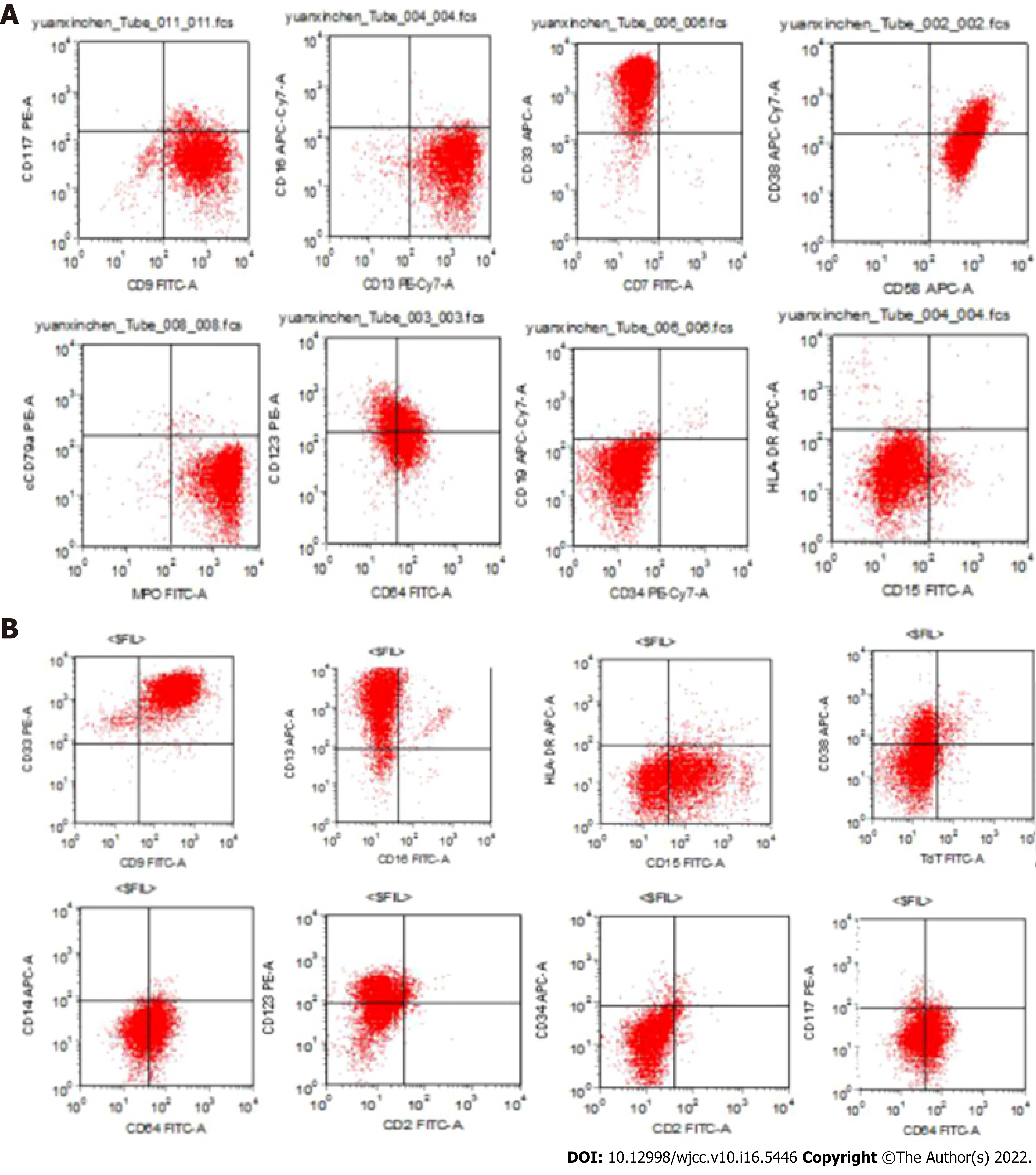
Abnormal cells were accounted for 88% in Case 1 and 78% in Case 2. Flow cytometric analysis on CD45/SSC dot plot showed that CD9, CD13, CD33, and CD38 were mainly expressed in all analyses, while CD64, CD123, and MPO were only expressed in some analyses. CD58 was expressed in case 1 and CD15 was expressed in case 2, as shown in Figure 4. Molecular biological detection: PML/RARa, PLZF/RARa, NPM/RARa, STAT5b/RARa, NuMA1/RARa, PRKARIA/RARa, and FIPIL1/RARa fusion-gene tests showed negative results.
Detection of gene (exon) variation related to myeloid and gonorrhea hematologic malignancies was performed by targeted capture method. Mutation sites were clearly associated with the disease, and all of them had mutations of WT1 (Wilms Tumor 11). Case 1 WT1: NM_024426: exon9:c.G1367C:p.C456s; mutation frequency 49.2%. Case 2 WT1: NM_024426:exon1:c.410_413del:p.137_138del; mutation frequency 100%. Case 1 with EP300 (E1A Binding Protein p300) mutations: NM_001429: exon31:c.C5449T:p.Q1817X; mutation frequency 72.1%.
For case 1, the following mutation sites might be associated with the disease: (1) USP6 (NM_004505:exon12:c.854delG:p.W285fs) was a frameshift mutation, with a mutation frequency of 32.6%; (2) NUTM2G (NM_001170741:exon7:c.C2102T:p.701L) was a missense mutation, with a mutation frequency of 78.8%.
For case 2, the following mutation sites might be associated with the disease: (1) TAL1 (T-Cell Acute Lymphocytic Leukemia genemutation (NM_003189:exon6:c.821_822insGGGGGGGGG
Case 1 review of bone marrow on D29 and D65: It was still very difficult to collect bone marrow; myelodysplastic hyperplasia was pronounced; abnormal promyelocytic granulocytes were 33% and 78%, respectively; the treatment was ineffective on D156; MAH regimen was used for chemotherapy; the patient died of intracranial hemorrhage on D162.
Case 2 review of bone marrow on D29: It was still very difficult to obtain bone marrow samples; myelodysplasia decreased and promyelocytic granulocytes accounted for 32%. On repeated examination of bone marrow on D46, D145, D265 to D730, it was still very difficult to obtain bone marrow samples; reduced myelodysplasia was observed; abnormal morphology of promyelocytic granulocytes accounted for 2%; cytoplasm contained a large number of arrocysts; nuclei had lumps and no nucleolus. Residual leukemia detection revealed no immunophenotypic abnormal cell population (residual leukemia cells < 104); complete remission occurred. Up to now, the drug has been discontinued for 1 year, and the clinical, morphological, and flow cytometry results continued to show complete remission. However, the copy number of the WT1 gene was 1010-1087, and the expression rate was 51.95%-55.29%, which indicated the risk of recurrence, and allogeneic hematopoietic stem cell transplantation was necessary.
APL is a rare subtype of AML and has different morphological and immunological characteristics compared with other myeloid leukemia cells. Karyotype t (15;17) is a unique chromosome translocation in APL. At the molecular level, PML/RARα fusion gene is formed by translocation of PML gene at 15q and RARα gene at 17q.Therefore, it is a highly specific cytogenetic marker for this type of leukemia. In this case study, the phenotype of 2 children showed typical APL characteristics, especially some cells had inner and outer plasma membrane, with thick azinophilus granules (Figure 1). Immunophenotypic markers mainly included CD9, CD13, CD33, CD38 (Figure 2), and CD64, CD123, MPO were also expressed in case 1. In addition, case 1 also had the expression of CD15, which was consistent with the immunophenotype of APL[16,17] and the isolated i(17)(q10) AML with similar APL morphology.
Previously, 4 isolated i(17) (q10) cases were reported, including 2 children[13,14], 1 adult[15], 1 case without age information[18], and 3 cases with M3 (APL) FAB classification. There were no t(15;17) and PML-RARa fusion gene detected and patients had no responses to ATRA treatment[15]. One case did not respond to chemotherapy and the survival time was less than 1 mo[14]. The other 2 cases did not mention prognosis[13,19]. In this study, there were 2 cases examined, case 1 was isolated i(17)(q10), case 2 was isolated i(17)(q10) add(14)(p11). The t(15;17) was not present, and PML-RARa fusion gene was not detected by Fully-instrumented submersible housing and second-generation sequencing, which rendered ATRA and As2O3 combined chemotherapy ineffective. Case 1 survived 5.5 mo. Case 2 achieved sustained complete remission after intensive chemotherapy with acute non-eluting regimen. The difference might not be related to isolated i(17)(q10) add(14)(P11), which was speculated to contribute to the transport of chemotherapeutic drugs. Similar studies have not been reported on leukemia patients, and the underlying specific mechanism needs further exploration. However, with the high expression of WT1 gene, the risk of recurrence is still very high[20]. Further clinical follow-up is required, and hematopoietic stem cell transplantation is necessary. However, this case was different from occult APL and APL with i(17)(q10) and PML-RARa fusion gene, for which the ATRA and As2O3 combined chemotherapy was effective[2,18].
Patients with myeloid tumor i(17)(q10) are mostly MDS/MPO+ patients with a chronic history, and often have pathological hematopoiesis in granulocyte, erythrocyte, and megakaryocyte lines, with an average of 3 gene mutations, mainly ASXL1, SRF2, and SETP1[8,9]. In this study, 2 patients had a short course of the disease, with no history of MDS/MPO+, and no erythrocyte or megakaryocyte pathological hematopoiesis except granulocyte lineage, which was similar to a 27-year-old female APL-like AML patient with a short course of the disease, having no chronic history and multi-family pathological hematopoiesis[15].
Gene mutations in these APL-like AML cases were reported for the first time, and there were 5 mutations in case 1, including WT EP300 c.854delg: P w285fs frame-shift mutation and C.C2102T: P 701L missense mutation. WT1, TAL1, TTN, and DDX11 mutations were found in case 2. Both cases had WT1 gene mutations, which were consistent with the characteristics of i(17)(q10) gene mutations (0-6) in MDS/MPO+ patients, but the gene mutation points were completely different. Therefore, case with i(17)(q10) was clinically diagnosed. The morphological diversity was probably due to different mutation patterns, and the number and order of the mutations might play a key role[8]. Therefore, both morphologic and immunological manifestations of APL were found in 2 children without t(15;17) and PML-RARa fusion-gene expression. Though preliminary diagnosis of AML morphologically similar to APL[15] were made for both children, the treatment failed in case 1, and case 2 with add(14)(p11) achieved sustained complete remission after chemotherapy, which might be related to the different gene mutation points.
Through literature review, 6 patients with i(17)(q10) have been known (including 2 in this group), 5 morphologically similar to AML, 1 without FAB classification mentioned[13], 5 with isolated TYPE i(17)(q10), 1 with add(14)(p11), and 3 patients (including 2 in this group) had CD33 immunophenotype with CD3 MPO expression[15]. Using existing genetic and molecular biological techniques, t(15;17) among the 6 patients with PML-RARa fusion gene were not detected, 3 patients failed to respond toATRA-As2O3 and chemotherapy and died (survival shorter than 5.5 mo), 1 patient achieved sustained complete response after chemotherapy, and 2 patients did not show prognosis[13,18].
Morphology, immunology, chromosomal karyotype, and molecular biological genotyping of 6 cases with i(17)(q10) in different periods and their prognosis are shown in Table 1.
| Number | Age | Sex | FABtyping | Karyotype | Immunological phenotype | Genemutation | Therapeutic reaction | Prognosis | Ref. |
| 1 | 27 | F | M3 | i(17)(q10) | CD33+/CD13+/ MPO | PML-RARa (-) | Retinoic acid (-) | Die (survival 1 mo) | Yan et al[15], China |
| 2 | 10 | M | M3 | i(17)(q10) | - | - | NR | Die (survival < 1 mo) | Bernstein et al[14], South Africa |
| 3 | Child | F | - | i(17)(q10) | - | - | - | - | Raimondi et al[13], Mexico |
| 4 | - | - | M3 | i(17)(q10) | - | - | - | - | Becher et al[19] |
| 5 | 3 (Case 1) | M | M3 | i(17)(q10) | CD9/CD13/CD33/ CD38/CD64/CD123/CD58/MPO | WT1/EP300/C.854delG: P w285fs Frame shiftC.C2102T: P.701LMissense | - | Wen JQ, China | |
| 6 | 12 (Case 2) | M | M3 | i(17)(q10) Add(14) (p11) | CD9/CD13/CD33/ CD38/CD64/CD123/CD15/MPO | WT1/TAL1/TTN/DDX11 | + | CR 36 months, WT1 copy: 1010-1087 | Wen JQ, China |
APL-like AML with i(17)(q10) has morphological and immunological characteristics similar to APL, without t(15;17) and PML-RARa fusion gene expression. ATRA-As2O3 and chemotherapy were not effective in treating the patients, with short survival period. If a chromosomal addition occurred, a sustained complete remission should be achieved and related clinical manifestations should be revealed. It is necessary to further strengthen the molecular biological study and collect a large number of cases to provide better treatment strategies.
We thank the Hematological Tumor Molecular Special Detection Technology Research Center of Kindstar Global (Wuhan) for its technological support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jabbarpour Z, Iran S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2981] [Cited by in RCA: 3182] [Article Influence: 198.9] [Reference Citation Analysis (0)] |
| 2. | Meza Espinoza JP, Judith Picos Cárdenas V, Gutiérrez-Angulo M, González García JR. Secondary chromosomal changes in 34 Philadelphia-chromosome-positive chronic myelocytic leukemia patients from the Mexican West. Cancer Genet Cytogenet. 2004;148:166-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Wang W, Tang G, Cortes JE, Liu H, Ai D, Yin CC, Li S, Khoury JD, Bueso-Ramos C, Medeiros LJ, Hu S. Chromosomal rearrangement involving 11q23 locus in chronic myelogenous leukemia: a rare phenomenon frequently associated with disease progression and poor prognosis. J Hematol Oncol. 2015;8:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Sheth FJ, Sheth JJ, Desai C. Case of near triploidy with i(17)(q10) in blast crisis CML. Cancer Genet Cytogenet. 2006;164:177-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Garay CA, Al-Saleem T, Testa JR, Smith MR. Coexisting myelodysplasia and myeloproliferative features in a single clone containing 5q-, Ph and i(17q). Leuk Res. 1999;23:965-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 6. | Nishida H, Ueno H, Park JW, Yano T. Isochromosome i(17q) as a sole cytogenetic abnormality in a case of leukemic transformation from myelodysplastic syndrome (MDS)/myeloproliferative diseases (MPD). Leuk Res. 2008;32:1325-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Kanagal-Shamanna R, Bueso-Ramos CE, Barkoh B, Lu G, Wang S, Garcia-Manero G, Vadhan-Raj S, Hoehn D, Medeiros LJ, Yin CC. Myeloid neoplasms with isolated isochromosome 17q represent a clinicopathologic entity associated with myelodysplastic/myeloproliferative features, a high risk of leukemic transformation, and wild-type TP53. Cancer. 2012;118:2879-2888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Meggendorfer M, Haferlach C, Zenger M, Macijewski K, Kern W, Haferlach T. The landscape of myeloid neoplasms with isochromosome 17q discloses a specific mutation profile and is characterized by an accumulation of prognostically adverse molecular markers. Leukemia. 2016;30:1624-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Meggendorfer M, Bacher U, Alpermann T, Haferlach C, Kern W, Gambacorti-Passerini C, Haferlach T, Schnittger S. SETBP1 mutations occur in 9% of MDS/MPN and in 4% of MPN cases and are strongly associated with atypical CML, monosomy 7, isochromosome i(17)(q10), ASXL1 and CBL mutations. Leukemia. 2013;27:1852-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Berger R, Le Coniat M, Derré J, Vecchione D, Jonveaux P. Cytogenetic studies in acute promyelocytic leukemia: a survey of secondary chromosomal abnormalities. Genes Chromosomes Cancer. 1991;3:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Heim S, Mitelman F. Secondary chromosome aberrations in the acute leukemias. Cancer Genet Cytogenet. 1986;22:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Kim MJ, Yoon HS, Cho SY, Lee HJ, Suh JT, Lee J, Yoon HJ, Lee WI, Park TS. ider(17)(q10)t(15;17) associated with relapse and poor prognosis in a pediatric patient with acute promyelocytic leukemia. Cancer Genet Cytogenet. 2010;201:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Raimondi SC, Chang MN, Ravindranath Y, Behm FG, Gresik MV, Steuber CP, Weinstein HJ, Carroll AJ. Chromosomal abnormalities in 478 children with acute myeloid leukemia: clinical characteristics and treatment outcome in a cooperative pediatric oncology group study-POG 8821. Blood. 1999;94:3707-3716. [PubMed] |
| 14. | Bernstein R, Macdougall LG, Pinto MR. Chromosome patterns in 26 South African children with acute nonlymphocytic leukemia (ANLL). Cancer Genet Cytogenet. 1984;11:199-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | YC D, ZR Z, Y L. Clinical significance of isolated isochromosome 17q in hematologic tumors.. |
| 16. | Ren F, Zhang N, Xu Z, Xu J, Zhang Y, Chen X, Tan Y, Chang J, Wang H. The CD9+ CD11b- HLA-DR- immunophenotype can be used to diagnose acute promyelocytic leukemia. Int J Lab Hematol. 2019;41:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Gong JY, Li YY, Li CW, Wang YS, Liu Y, Wang C, Ru K, Mi YC, Wang JX, Wang HJ. [Application of immunophenotypic analysis and molecular genetics in the diagnosis of acute promyelocytic leukemia]. Zhonghua Xue Ye Xue Za Zhi. 2019;40:288-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Lee GY, Christina S, Tien SL, Ghafar AB, Hwang W, Lim LC, Lim TH. Acute promyelocytic leukemia with PML-RARA fusion on i(17q) and therapy-related acute myeloid leukemia. Cancer Genet Cytogenet. 2005;159:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Becher R, Carbonell F, Bartram CR. Isochromosome 17q in Ph1-negative leukemia: a clinical, cytogenetic, and molecular study. Blood. 1990;75:1679-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Krauth MT, Alpermann T, Bacher U, Eder C, Dicker F, Ulke M, Kuznia S, Nadarajah N, Kern W, Haferlach C, Haferlach T, Schnittger S. WT1 mutations are secondary events in AML, show varying frequencies and impact on prognosis between genetic subgroups. Leukemia. 2015;29:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |