Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5428
Peer-review started: September 19, 2021
First decision: October 25, 2021
Revised: November 12, 2021
Accepted: April 24, 2022
Article in press: April 24, 2022
Published online: June 6, 2022
Processing time: 256 Days and 8.6 Hours
There is limited information on ipsilateral synchronous papillary renal cell carcinoma (PRCC) and clear cell renal cell carcinoma (CCRCC). Therefore, these rare tumors are often misdiagnosed preoperatively as a single tumor with intrarenal metastasis or some other diseases. Effective management and long-term overall survival might be affected because the prognosis of the two tumors differs.
We describe a case of ipsilateral synchronous PRCC and CCRCC with two histological variants in a 72-year-old man, whose mass was found incidentally, with no other chief complaints and vital signs were normal. Initial ultrasound revealed a hypoechoic lobular mass with a volume of 7.8 cm × 4.8 cm × 2.8 cm in the middle to lower pole of the left kidney. A subsequent contrast-enhanced computed tomography scan showed a single endophytic mass of 7.5 cm in diameter. The patient underwent laparoscopic left radical nephrectomy. A final diagnosis of ipsilateral synchronous PRCC and CCRCC was confirmed by pathological examination. There was no recurrence or metastasis after 25 mo follow-up.
We report a case of ipsilateral synchronous PRCC and CCRCC, and review related literature to estimate the prevalence of similar cases. The above descri
Core Tip: Ipsilateral synchronous papillary renal cell carcinoma (PRCC) and clear cell renal cell carcinoma (CCRCC) are rare, and reports on this tumor are scarce. We describe an incidentally detected case of this rare disease. Preoperative imaging examinations revealed a single mass on both ultrasound and computed tomography. Further pathological examination confirmed two types of tumors: PRCC and CCRCC. The patient underwent laparoscopic left radical nephrectomy and there was no recurrence or metastasis after 25 mo follow-up.
- Citation: Yin J, Zheng M. Ipsilateral synchronous papillary and clear renal cell carcinoma: A case report and review of literature. World J Clin Cases 2022; 10(16): 5428-5434
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5428.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5428
As the two most common subtypes, papillary renal cell carcinoma (PRCC) and clear cell renal cell carcinoma (CCRCC) within the same kidney at the same time are very rare[1,2]. A few articles have reported ipsilateral synchronous PRCC and CCRCC by searching PubMed[3-6]. Accurate diagnosis of RCC is important for prognosis and evaluation of therapeutic response. In clinical practice, ipsilateral synchronous PRCC and CCRCC are often misdiagnosed preoperatively as an isolated mass or multiple lesions due to intrarenal metastasis. As a result, patients may have a short survival after the disease is finally confirmed postoperatively. Here, we describe a case of ipsilateral synchronous PRCC and CCRCC treated by laparoscopic left radical nephrectomy, and review the relevant literature.
A 72-year-old man was admitted to hospital because of a left renal mass found on routine physical examination. The patient had no other chief complaints, such as hematuria, fever or lumber pain.
The patient had no relevant medical history.
The patient had a long history of hypertension for 20 years, and smoking for 50 years. He denied any other previous medical history.
No associated family history was reported.
The patient’s temperature was 36.8 °C, respiratory rate was 18 breaths/min, heart rate was 72 bpm, and blood pressure was 145/92 mmHg. The patient’s height was 172 cm and weight was 86 kg. Physical examination revealed normal vital signs, especially in the lumber areas, the contour was normal, and there was no palpable mass or percussion pain.
The results of routine blood tests, routine urine tests and renal function examinations, routine fecal tests, blood biochemistry, immune indices, and tumor markers were all within the normal range.
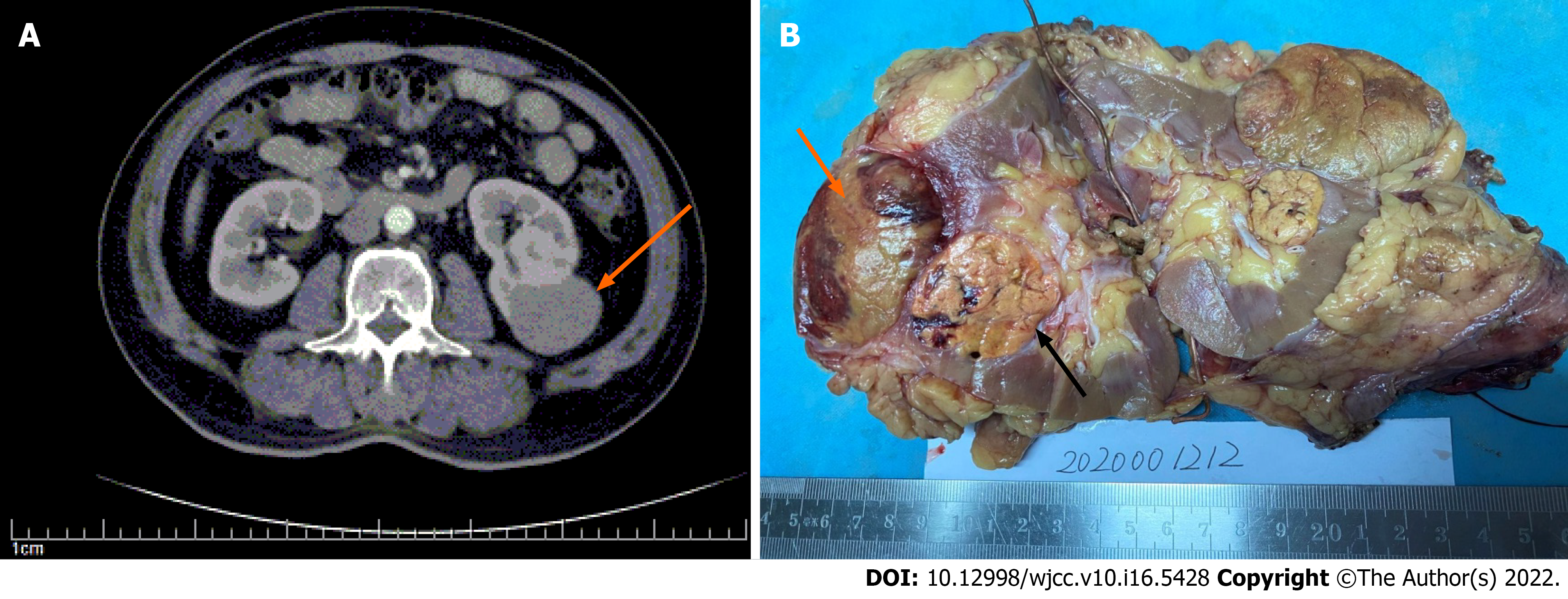
The initial impression on ultrasound revealed a hypoechoic lobular mass with a volume of 7.8 cm × 4.8 cm × 2.8 cm in the middle to lower pole of the left kidney. Subsequent computed tomography (CT) scan showed a single endophytic mass of 7.5 cm in diameter, with contrast enhancement (Figure 1A).
The final diagnosis was ipsilateral synchronous PRCC (WHO grade II/III) and CCRCC (WHO grade II/III) in the left kidney according to the imaging and pathological results.
As the tumor was in the middle to lower pole of the left kidney, a partial nephrectomy was firstly considered by the surgeons to preserve more kidney function. However, after a discussion with the family numbers and the patient, a laparoscopic radical nephrectomy was accepted, as the patient wished for a longer survival time. He finally underwent laparoscopic left radical nephrectomy. The operation went smoothly, and there were no adhesions between the left kidney and peripheral organs. He was discharged on day 6 with no postoperative complications.
After 25 mo follow-up, the patient was free from disease with no recurrence or metastasis on CT, and renal function was within the normal range. He was continuously followed up.
Renal cell carcinoma (RCC) is the most common solid mass of the kidney and comprises 2%-3% of all cancers in adults[7]. The incidence of RCC has risen over the past few years due to incidental detection[8]. RCC is divided into CCRCC and non CCRCC subtypes, while the most common subtype of non-CCRCC is PRCC. The combination of different subtypes has rarely been reported[9]. The incidence of ipsilateral synchronous PRCC and CCRCC is approximately 4.8%[10]. The rarity of the disease may contribute to its misdiagnosis in clinical practice. As in our case, an initial diagnosis of angiomyolipoma (AML) was suspected.
The underlying etiology of ipsilateral synchronous PRCC and CCRCC is still unclear, which partly affects the understanding of clinicians. The best known possible etiological factors for RCC are smoking, obesity, and hypertension[11]. Smoking is implicated as the key etiology of RCC, and heavy smoking is associated with increased risk. Ustuner et al[3] reported coexisting PRCC and CCRCC in the same kidney in 2014. That patient presented with a 40-year history of smoking and a 2.03 relative risk (RR) of renal cancer. A meta-analysis by Hunt et al[13] revealed that men who smoked 1–9, 10–20 or ≥ 21 cigarettes/d had an RR of 1.60, 1.83 and 2.03, respectively. Nonetheless, the relationships between obesity or hypertension and the disease are not yet firmly established. In our case, a history of smoking and hypertension for many years was noted, while obesity was not mentioned as an etiological factor.
Ultrasound or abdominal CT can reveal the lesion preoperatively. Most CCRCCs are hypervascular, the degree of enhancement quickly decreases in the parenchyma phase, presenting as “fast in and fast out”. In contrast, there is no predominant enhancement in the three phases of PRCC revealed by CT, as the vessels of the fibrovascular core are thin and sparse in the papilla[13]. Unfortunately, there is still a 3.5% risk of missing coexisting tumors in RCC[10]. Up to 70% of multifocal lesions are missed on preoperative imaging due to small size or adjacent location of the lesions. Our case was diagnosed preoperatively as a single mass both by ultrasound and CT. Missing the second mass was not only a radiological misdiagnosis, but also reflected a lack of awareness of the imaging features of the ipsilateral synchronous PRCC and CCRCC, and this could have had a serious effect on the treatment strategy. Excessive dependence on radiological findings to obtain an accurate preoperative diagnosis of the ipsilateral synchronous PRCC and CCRCC is undesirable.
Diagnosis and subclassification of RCC must be based primarily on pathology. Our case was initially diagnosed with AML, because of the similar gross features (Figure 1B). A cut surface color of yellow–brown could create confusion with AML, as the latter is well circumscribed, has extended borders, and contains soft yellow regions admixed with firm tan regions. Most PRCCs are bilateral and multifocal, and areas of hemorrhage and necrosis are commonly seen in PRCC; thus, confusion with other tumors is understandable.
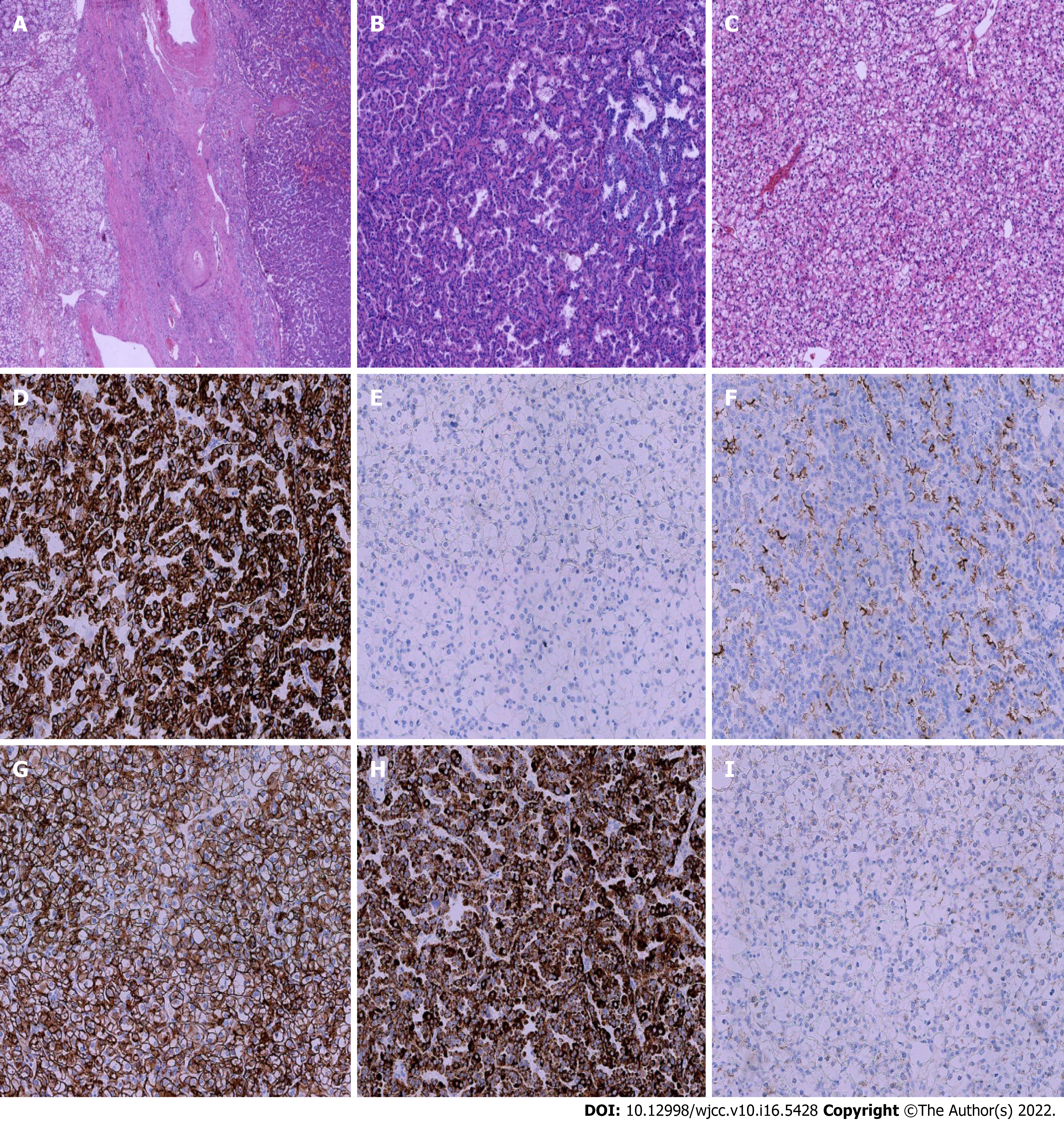
Microscopically, PRCC may be confused with CCRCC when a solid growth with clear cytoplasm is seen, and CCRCC with a papillary pattern or eosinophilic cytoplasm can be confused with PRCC. In this situation, the classic morphological features of the two tumors are helpful in distinguishing them, such as whether a pseudopapillary pattern was observed. Due to the different immunoprofiles shown in PRCC and CCRCC, immunohistochemical staining may also offer an important clue to the diagnosis. PRCC is strongly positive for CK8/18, CK 7 and P504s, while CCRCC is strongly positive for CD10, CAIX and Vimentin. CK7 may be expressed in both tumors, but PRCC often shows a diffuse cytoplasm positive for CK7, while no positivity is observed in CCRCC. Our case showed similar findings to those described in the literature, with strong positive staining for CK7 in PRCC and negative staining in CCRCC (Figure 2).
In addition, differential diagnosis is necessary between PRCC and collecting duct carcinoma, when the latter has a papillary pattern. Nevertheless, collecting duct carcinoma often presents as a high-grade tumor with predominant desmoplastic stroma, and occurs in the medulla. CK7 may be expressed in both tumors, but negativity for CEA, 34βE12 and some other biomarkers can help to exclude PRCC. When thinking back to our first gross diagnosis, if the epithelioid subtype of AML was exhibited, CCRCC might be misclassified initially as AML. CCRCC is always positive for Vimentin, CD10 and CAIX, but negative for the biomarkers of AML, such as HMB45 and SMA. Careful attention to the morphology and immunohistochemistry may help to establish the correct diagnosis.
Unfortunately, there has been little research on the molecular pathology of ipsilateral synchronous PRCC and CCRCC, even though the characteristic cytogenetic alterations of PRCC and CCRCC have been revealed, which is partly due to a lack of awareness of this rare entity. Further study should focus on these aspects using modern molecular techniques.
The aim of surgical management for multifocal renal tumors is not only to prevent recurrence and metastasis, but also to minimize the number of surgical procedures and prolong kidney function. Both laparoscopic, robotic and partial nephrectomy for multiple lesions of ipsilateral renal tumors have been reported[14-17]. Some studies have compared the two main surgical options for managing this subset of patients, and similar tumor-specific survival was observed for patients treated with nephron sparing surgery (NSS) and radical nephrectomy[18-21]. Regardless of which surgical mode is selected, the right balance between oncological control and renal function preservation should be considered. Although a single mass was diagnosed preoperatively, our patient still underwent laparoscopic radical nephrectomy with the aim of achieving a long-term survival. Different tumors have different prognoses and vary in aggressiveness[22]. If a single mass is confirmed, to preserve more renal function and improve quality of life, NSS may be performed. Dependence on preoperative imaging can lead to missing multifocal lesions[10,23]. Thus, complete mobilization and inspection of the entire kidney is justified when performing NSS to identify multifocal disease[23]. In such a situation, whether an adequate surgical range is obtained, or whether a long life-time survival is achieved, should be considered. Accurate preoperative diagnosis is also key to achieving long-term survival.
Effective adjuvant therapies are necessary to reduce recurrence andmetastasis, such as autologous tumor vaccines, carbonic anhydrase IX monoclonal antibody, tyrosine kinase inhibitors and novel immunotherapies, which have significantly improved the overall survival of patients with RCC in recent years[14,24,25]. To date, most studies have focused on the identification of an effective adjuvant therapy for CCRCC, in order to improve the outcome of patients with high-risk RCC, while the optimal treatment option for PRCC has not yet been established[26]. As most PRCCs are associated with a better outcome than CCRCCs in patients without metastases, the regular treatment strategies and follow-up employed for patients with CCRCC are sufficient for patients with nonmetastatic PRCC[27]. In addition, more research on the novel gene signatures of RCC may improve the survival of patients with PRCC and CCRCC, and should be conducted in clinical trials[28]. For patients with ipsilateral synchronous PRCC and CCRCC, a regular adjuvant therapy for CCRCC may be sufficient.
There are insufficient data to compare different types of RCC in the same kidney with unilateral multifocal tumors in terms of survival. Bilateral synchronous renal tumors have been evaluated[29,30], but there are no such data for unilateral synchronous RCC of different types. The prognosis of PRCC is more favorable than that of CCRCC, as the former is less aggressive. Capaccio et al[2] reported three patients who had unilateral synchronous PRCC and CCRCC treated by radical nephrectomy, and only one died from the disease 5 year after surgery. Ustuner et al[3] described a 67-year-old man with unilateral synchronous PRCC and CCRCC, who was disease-free at the 6 mo and 1 year follow-up. The present case was disease-free with no recurrence or metastasis after 25 mo.
As the incidence of ipsilateral synchronous PRCC and CCRCC is low, a lack of awareness or experience may exist among clinicians. If there are multifocal masses in a single kidney, intrarenal metastasis is always initially considered, and the possibility of coexisting ipsilateral synchronous tumors may be ignored. However, as the prognosis of PRCC and CCRCC differs, the therapeutic strategies may differ, such as surgery or adjuvant chemotherapy. Even though they have a low incidence rate, different histological subtypes of multiple ipsilateral synchronous RCCs need to be classified as a special entity postoperatively, due to the different therapeutic strategies performed. Further studies are still needed to make a comparison and comment on the course of the disease as this is only a single case without any controls, and the clinical implications are limited.
This was a rare case of ipsilateral synchronous PRCC and CCRCC with two histological variants. The diagnosis of ipsilateral synchronous PRCC and CCRCC can be established through detailed clinical history, imaging findings, and most importantly, pathological examination and immunohistochemical staining. As the prognosis of the two tumors differs, careful clinical decision-making and appropriate surgical management are required to manage the disease. The above descriptions are expected to help understand the disease, and improve diagnosis in the future. If optimally applied, these tactics can achieve long life expectancy and long-term preservation of renal function in patients with ipsilateral synchronous PRCC and CCRCC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghazanfar A, United Kingdom; Sachdeva S, India S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Billings B, Hamrick LC, Bueschen AJ, Kenney PJ. Coexisting angiomyolipoma and renal cell carcinoma in a kidney of an elderly woman: case report and review of the literature. ScientificWorldJournal. 2004;4 Suppl 1:27-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Capaccio E, Varca V, Simonato A, Toncini C, Carmignani G, Derchi LE. Synchronous parenchymal renal tumors of different histology in the same kidney. Acta Radiol. 2009;50:1187-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Ustuner M, Yaprak B, Teke K, Ciftci S, Kart M, Yildiz K, Culha M. Coexisting papillary and clear renal cell carcinoma in the same kidney. Case Rep Urol. 2014;2014:575181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Alhusban M, Alhamss S, Alzumaili B, Al-Daghmin A. Ipsilateral synchronous clear and papillary renal cell carcinoma: A case report and review of the literature. Urol Case Rep. 2018;16:110-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Kawecki J, Leśniak P, Wieczorek K, Budziarz P, Duda W, Poloczek R, Dutkiewicz S. A case of both clear and papillary renal cell carcinomas in the left kidney. Cent European J Urol. 2012;65:221-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Simhan J, Canter DJ, Sterious SN, Smaldone MC, Tsai KJ, Li T, Viterbo R, Chen DY, Greenberg RE, Kutikov A, Uzzo RG. Pathological concordance and surgical outcomes of sporadic synchronous unilateral multifocal renal masses treated with partial nephrectomy. J Urol. 2013;189:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55843] [Article Influence: 7977.6] [Reference Citation Analysis (132)] |
| 8. | Alasker A, Williams SK, Ghavamian R. Small renal mass: to treat or not to treat. Curr Urol Rep. 2013;14:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Sorbellini M, Bratslavsky G. Decreasing the indications for radical nephrectomy: a study of multifocal renal cell carcinoma. Front Oncol. 2012;2:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Richstone L, Scherr DS, Reuter VR, Snyder ME, Rabbani F, Kattan MW, Russo P. Multifocal renal cortical tumors: frequency, associated clinicopathological features and impact on survival. J Urol. 2004;171:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Lipworth L, Tarone RE, McLaughlin JK. The epidemiology of renal cell carcinoma. J Urol. 2006;176:2353-2358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 12. | Tsuda K, Kinouchi T, Tanikawa G, Yasuhara Y, Yanagawa M, Kakimoto K, Ono Y, Meguro N, Maeda O, Arisawa J, Usami M. Imaging characteristics of papillary renal cell carcinoma by computed tomography scan and magnetic resonance imaging. Int J Urol. 2005;12:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Hunt JD, van der Hel OL, McMillan GP, Boffetta P, Brennan P. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer. 2005;114:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 327] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | Tannir NM, Pal SK, Atkins MB. Second-Line Treatment Landscape for Renal Cell Carcinoma: A Comprehensive Review. Oncologist. 2018;23:540-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Chowdhury N, Drake CG. Kidney Cancer: An Overview of Current Therapeutic Approaches. Urol Clin North Am. 2020;47:419-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 16. | Flum AS, Wolf JS Jr. Laparoscopic partial nephrectomy for multiple ipsilateral renal tumors using a tailored surgical approach. J Endourol. 2010;24:557-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Lang H, Lindner V, Martin M, Letourneux H, Roy C, Saussine C, Jacqmin D. Prognostic value of multifocality on progression and survival in localized renal cell carcinoma. Eur Urol. 2004;45:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Cignoli D, Fallara G, Larcher A, Rosiello G, Montorsi F, Capitanio U. How to improve outcome in nephron-sparing surgery: the impact of new techniques. Curr Opin Urol. 2021;31:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Krambeck A, Iwaszko M, Leibovich B, Cheville J, Frank I, Blute M. Long-term outcome of multiple ipsilateral renal tumours found at the time of planned nephron-sparing surgery. BJU Int. 2008;101:1375-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Minervini A, Serni S, Giubilei G, Lanzi F, Vittori G, Lapini A, Carini M. Multiple ipsilateral renal tumors: retrospective analysis of surgical and oncological results of tumor enucleation vs radical nephrectomy. Eur J Surg Oncol. 2009;35:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Mano R, Kent M, Larish Y, Winer AG, Chevinsky MS, Hakimi AA, Sternberg IA, Sjoberg DD, Russo P. Partial and Radical Nephrectomy for Unilateral Synchronous Multifocal Renal Cortical Tumors. Urology. 2015;85:1404-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Harlow BL, Klaassen Z, Holzman S, Reinstatler L, Franken AA, Kavuri SK, Terris MK, Master VA, Moses KA. Multiple Discordant Histology After Nephrectomy: Descriptive Analysis and Outcomes. Clin Genitourin Cancer. 2016;14:e171-e175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Crispen PL, Lohse CM, Blute ML. Multifocal renal cell carcinoma: clinicopathologic features and outcomes for tumors </=4 cm. Adv Urol. 2008;518091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Roy AM, Briggler A, Tippit D, Dawson K, Verma R. Neoadjuvant Cabozantinib in Renal-Cell Carcinoma: A Brief Review. Clin Genitourin Cancer. 2020;18:e688-e691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Posadas EM, Limvorasak S, Figlin RA. Targeted therapies for renal cell carcinoma. Nat Rev Nephrol. 2017;13:496-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 26. | Park I, Lee SH, Lee JL. A Multicenter Phase II Trial of Axitinib in Patients With Recurrent or Metastatic Non-clear-cell Renal Cell Carcinoma Who Had Failed Prior Treatment With Temsirolimus. Clin Genitourin Cancer. 2018;16:e997-e1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Vaishampayan U. Evolving Treatment Paradigms in Non-clear Cell Kidney Cancer. Curr Treat Options Oncol. 2018;19:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Gul A, Rini BI. Adjuvant therapy in renal cell carcinoma. Cancer. 2019;125:2935-2944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Rothman J, Crispen PL, Wong YN, Al-Saleem T, Fox E, Uzzo RG. Pathologic concordance of sporadic synchronous bilateral renal masses. Urology. 2008;72:138-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Klatte T, Wunderlich H, Patard JJ, Kleid MD, Lam JS, Junker K, Schubert J, Böhm M, Allhoff EP, Kabbinavar FF, Crepel M, Cindolo L, De La Taille A, Tostain J, Mejean A, Soulie M, Bellec L, Bernhard JC, Ferriere JM, Pfister C, Albouy B, Colombel M, Zisman A, Belldegrun AS, Pantuck AJ. Clinicopathological features and prognosis of synchronous bilateral renal cell carcinoma: an international multicentre experience. BJU Int. 2007;100:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |